Genetic Variability of HUPRA Syndrome—A Case Report
Abstract
1. Introduction
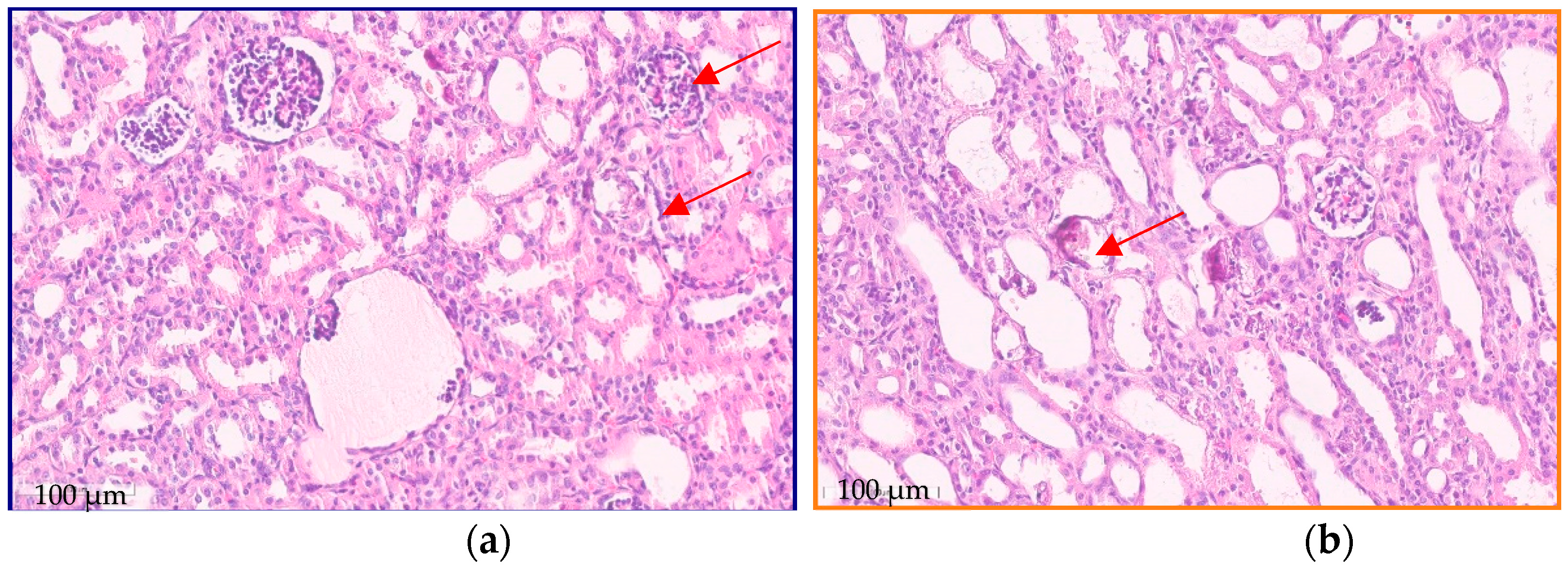
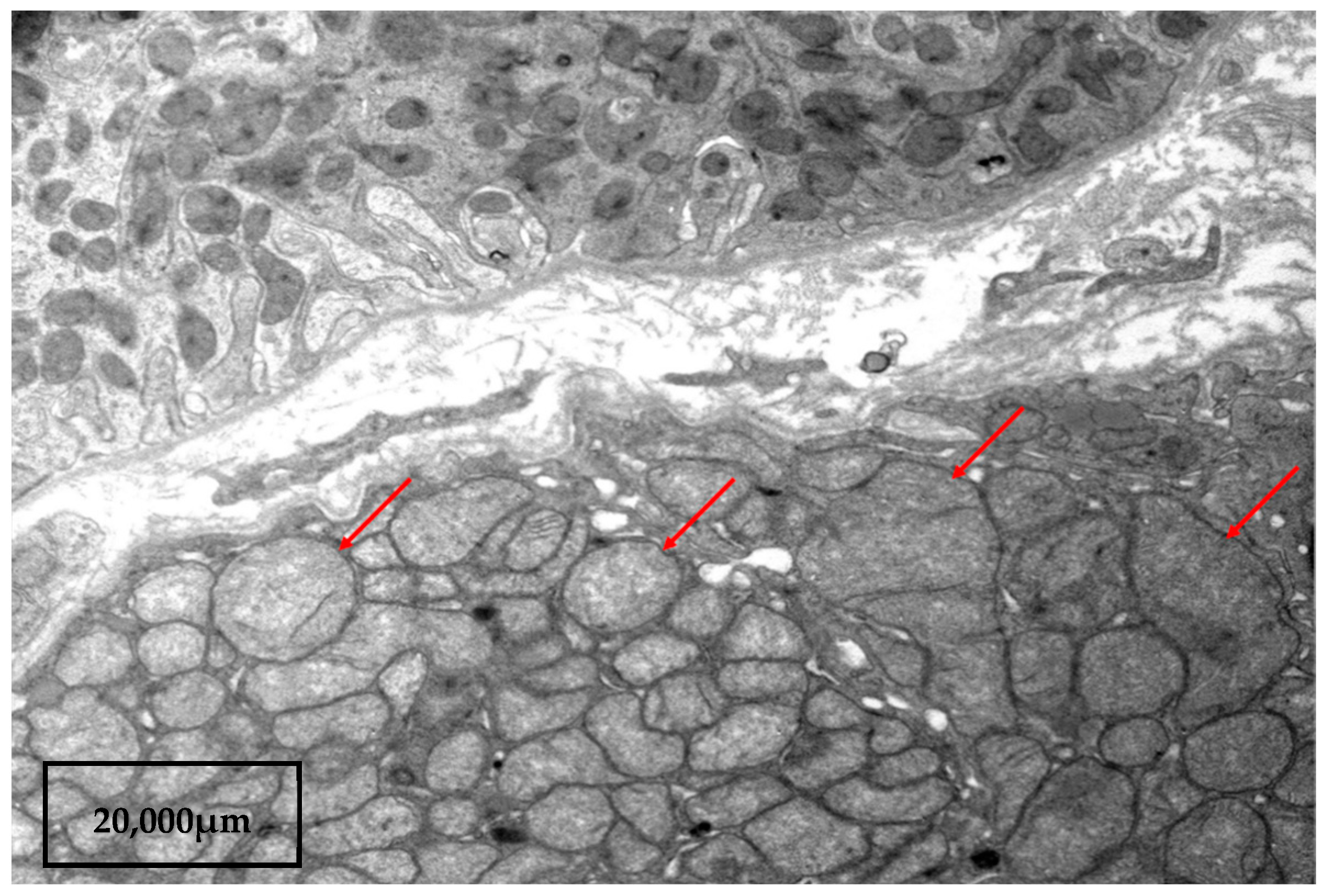
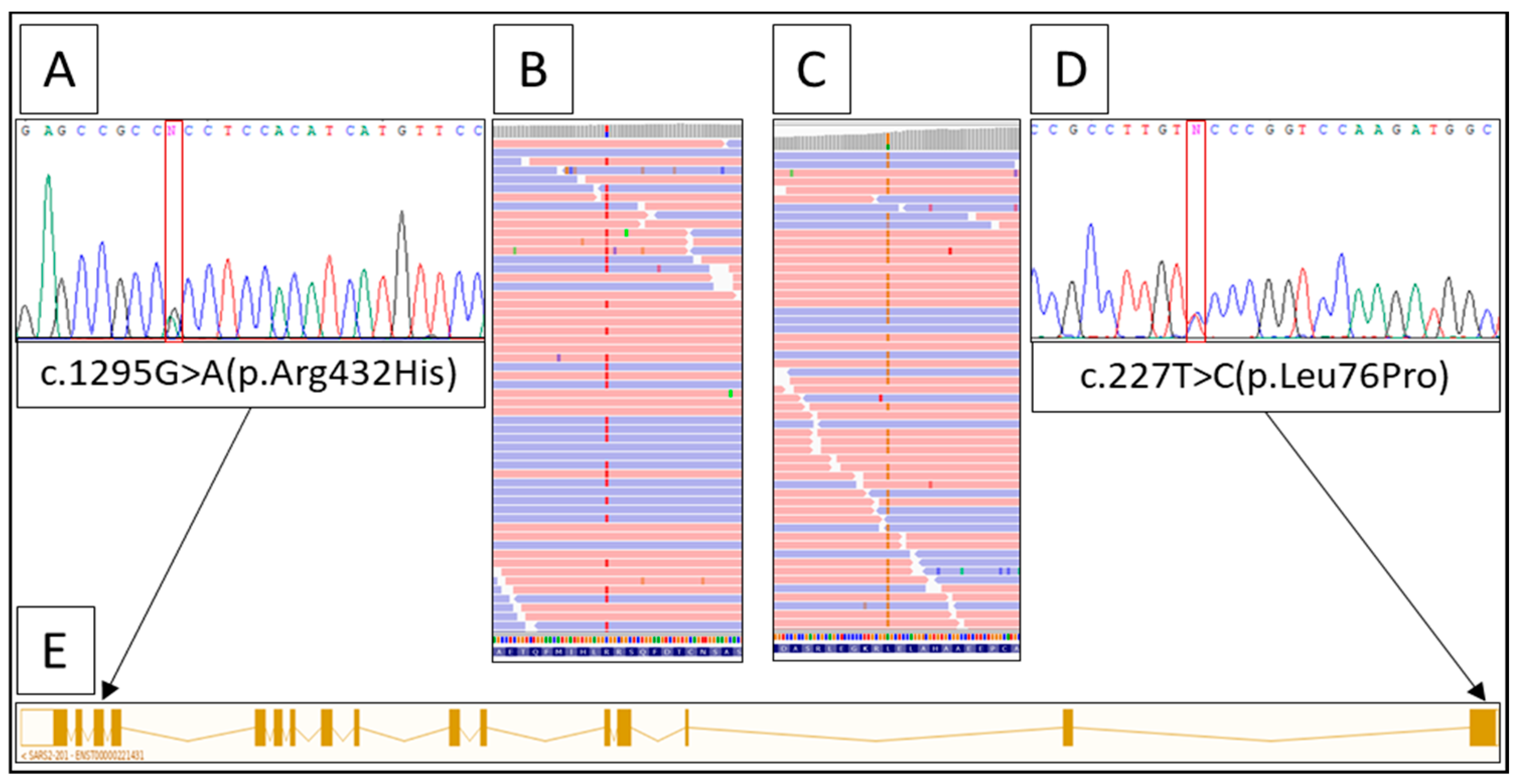
2. A Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Belostotsky, R.; Ben-Shalom, E.; Rinat, C.; Becker-Cohen, R.; Feinstein, S.; Zeligson, S.; Segel, R.; Elpeleg, O.; Nassar, S.; Frishberg, Y. Mutations in the mitochondrial seryl-tRNA synthetase cause hyperuricemia, pulmonary hypertension, renal failure in infancy and alkalosis, HUPRA Syndrome. Am. J. Hum. Genet. 2011, 88, 193–200. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Kim, S.S.; Dey, K.K.; Weissbrod, O.; Márquez-Luna, C.; Gazal, S.; Price, A.L. Improving the informativeness of Mendelian disease-derived pathogenicity scores for common disease. Nat. Commun. 2020, 11, 6258. [Google Scholar] [CrossRef] [PubMed]
- Govers, L.P.; Toka, H.R.; Hariri, A.; Walsh, S.B.; Bockenhauer, D. Mitochondrial DNA mutations in renal disease: An overview. Pediatr. Nephrol. 2021, 36, 9–17. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Emma, F.; Bertini, E.; Salviati, L.; Montini, G. Renal involvement in mitochondrial cytopathies. Pediatr. Nephrol. 2012, 27, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Niaudet, P.; Rotig, A. The kidney in mitochondrial cytopathies. Kidney Int. 1997, 51, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Ogier, H.; Lombes, A.; Scholte, H.R.; Poll-The, B.T.; Fardeau, M.; Alcardi, J.; Vignes, B.; Niaudet, P.; Saudubray, J.M. de Toni-Fanconi-Debré syndrome with Leigh syndrome revealing severe muscle cytochrome c oxidase deficiency. J. Pediatr. 1988, 112, 734–739. [Google Scholar] [CrossRef]
- Mochizuki, H.; Joh, K.; Kawame, H.; Imadachi, A.; Nozaki, H.; Ohashi, T.; Usui, N.; Eto, Y.; Kanetsuna, Y.; Aizawa, S. Mitochondrial encephalomyopathies preceded by de-Toni-Debré-Fanconi syndrome or focal segmental glomerulosclerosis. Clin. Nephrol. 1996, 46, 347–352. [Google Scholar]
- Mori, K.; Narahara, K.; Ninomiya, S.; Goto, Y.I.; Nonaka, I. Renal and skin involvement in a patient with complete Kearns–Sayre syndrome. Am. J. Med. Genet. 1991, 38, 583–587. [Google Scholar] [CrossRef]
- Topaloglu, R.; Lebre, A.S.; Demirkaya, E.; Kuskonmaz, B.; Coskun, T.; Derman, O.; Gurgey, A.; Gumruk, F. Two new cases with Pearson syndrome and review of Hacettepe experience. Turk. J. Pediatr. 2008, 50, 572–576. [Google Scholar] [PubMed]
- Lee, Y.S.; Yap, H.K.; Barshop, B.A.; Lee, Y.S.; Rajalingam, S.; Loke, K.Y. Mitochondrial tubulopathy: The many faces of mitochondrial disorders. Pediatr. Nephrol. 2001, 16, 710–712. [Google Scholar] [CrossRef]
- Martín-Hernández, E.; García-Silva, M.T.; Vara, J.; Campos, Y.; Cabello, A.; Muley, R.; Del Hoyo, P.; Martín, M.A.; Arenas, J. Renal pathology in children with mitochondrial diseases. Pediatr. Nephrol. 2005, 20, 1299–1305. [Google Scholar] [CrossRef]
- Emma, F.; Pizzini, C.; Tessa, A.; Di Giandomenico, S.; Onetti-Muda, A.; Santorelli, F.M.; Bertini, E.; Rizzoni, G. ‘Bartter-like’ phenotype in Kearns–Sayre syndrome. Pediatr. Nephrol. 2006, 21, 355–360. [Google Scholar] [CrossRef]
- Goto, Y.I.; Itami, N.; Kajii, N.; Tochimaru, H.; Endo, M.; Horai, S. Renal tubular involvement mimicking Bartter syndrome in a patient with Kearns–Sayre syndrome. J. Pediatr. 1990, 116, 904–910. [Google Scholar] [CrossRef]
- Graham, B.H. Diagnostic challenges of mitochondrial disorders: Complexities of two genomes. Mitochondrial Disord. Biochem. Mol. Anal. 2012, 837, 35–46. [Google Scholar]
- Hallberg, B.M.; Larsson, N.G. Making proteins in the powerhouse. Cell Metab. 2014, 20, 226–240. [Google Scholar] [CrossRef]
- Ling, J.; Reynolds, N.; Ibba, M. Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 2009, 63, 61–78. [Google Scholar] [CrossRef]
- Antonellis, A.; Green, E.D. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu. Rev. Genom. Hum. Genet. 2008, 9, 87–107. [Google Scholar] [CrossRef] [PubMed]
- Wellner, K.; Betat, H.; Morl, M. A tRNA’s fate is decided at its 3’ end: Collaborative actions of CCA-adding enzyme and RNases involved in tRNA processing and degradation. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2018, 1861, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Scheper, G.C.; van der Klok, T.; van Andel, R.J.; van Berkel, C.G.; Sissler, M.; Smet, J.; Muravina, T.I.; Serkov, S.V.; Uziel, G.; Bugiani, M.; et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat. Genet. 2007, 39, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Serrano, L.E.; Chihade, J.W.; Sissler, M. When a common biological role does not imply common disease outcomes: Disparate pathology linked to human mitochondrial aminoacyl-tRNA synthetases. J. Biol. Chem. 2019, 294, 5309–5320. [Google Scholar] [CrossRef] [PubMed]
- Rivera, H.; Martín-Hernández, E.; Delmiro, A.; García-Silva, M.T.; Quijada-Fraile, P.; Muley, R.; Arenas, J.; Martín, M.A.; Martínez-Azorín, F. A new mutation in the gene encoding mitochondrial seryl-tRNA synthetase as a cause of HUPRA syndrome. BMC Nephrol. 2013, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Linnankivi, T.; Neupane, N.; Richter, U.; Isohanni, P.; Tyynismaa, H. Splicing Defect in Mitochondrial Seryl-tRNA Synthetase Gene Causes Progressive Spastic Paresis Instead of HUPRA Syndrome. Hum. Mutat. 2016, 37, 884–888. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, C.; Yang, Q.; Zhang, G.; Yang, H.; Li, Q.; Wang, M. Novel SARS2 variants identified in a Chinese girl with HUPRA syndrome. Mol. Genet. Genom. Med. 2021, 9, 1650. [Google Scholar] [CrossRef]
- Göknar, N.; Keleşoğlu, E.; Kasap, N.; Üçkardeş, D.; Candan, C. A case of chronic kidney disease with pulmonary hypertension, hyperuricemia, immunodeficiency and other extrarenal findings: Questions. Pediatr. Nephrol. 2022, 37, 2615–2616. [Google Scholar] [CrossRef]
- Blethen, S.L.; Van Wyk, J.J.; Lorentz, W.B.; Jennette, J.C. Reversal of Bartter’s syndrome by renal transplantation in a child with focal segmental glomerular sclerosis. Am. J. Med. Sci. 1985, 289, 31–36. [Google Scholar] [CrossRef]
- Su, I.H.; Frank, R.; Gauthier, B.G.; Valderrama, E.; Simon, D.B.; Lifton, R.P.; Trachtman, H. Bartter syndrome and focal segmental glomerulosclerosis: A possible link between two diseases. Pediatr. Nephrol. 2000, 14, 970–972. [Google Scholar] [CrossRef]
- Bulucu, F.; Vural, A.; Yenicesu, M.; Caglar, K. Association of Gitelman’s syndrome and focal glomerulosclerosis. Nephron 1998, 79, 244. [Google Scholar] [CrossRef]
- Bettinelli, A.; Borsa, N.; Syren, M.L.; Mattiello, C.; Coviello, D.; Edefonti, A.; Giani, M.; Travi, M.; Tedeschi, S. Simultaneous mutations in the CLCNKB and SLC12A3 genes in two siblings with phenotypic heterogeneity in classic Bartter syndrome. Pediatr. Res. 2005, 58, 1269–1273. [Google Scholar] [CrossRef]
- Bendayan, D.; Shitrit, D.; Ygla, M.; Huerta, M.; Fink, G.; Kramer, M.R. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir. Med. 2003, 97, 130–133. [Google Scholar] [CrossRef] [PubMed]
| Analysis | At Presentation 1 | Reference Value |
|---|---|---|
| Blood Hemoglobin, g/L | 100 | 110–140 |
| Red blood cell, × 1012/L | 4.5 | 3.9–4.5 |
| Platelet, ×109/L | 106 | 180–400 |
| White blood cell, ×109/L | 2.13 | 5.5–12.5 |
| Creatinine, µmol/L | 90.4 | 17–44 |
| Urea, mmol/L | 11 | 2.5–7.1 |
| Uric acid, µmol/L | 978 | 120–320 |
| LDH, U/L | 498 | <320 |
| ALT, U/L | 13 | <36 |
| AST, U/L | 120 | <48 |
| PTH, pg/mL | 233 | 25–65 |
| Sodium, mmol/L | 124 | 135–145 |
| Potassium, mmol/L | 3.1 | 3.6–5.6 |
| Chloride, mmol/L | 79 | 96–111 |
| Magnesium, mmol/L | 0.67 | 0.71–0.95 |
| Lactic acid (mmol/lL) | 3.0 | 0.27–2.2 |
| Coagulation test APPT, s | 47 | 24–34 |
| D-dimers, ng/mL | 218 | <243 |
| Blood gases (venous) | ||
| pH | 7.49 | 7.35–7.45 |
| HCO3−, mmol/L | 27.6 | 22–26 |
| Urine | ||
| Protein, g/L | abs | 0–0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrosyan, E.; Molchanova, M.; Kushnir, B.; Povilaitite, P.; Tsygankova, P.; Zakharova, E.; Proskura, M. Genetic Variability of HUPRA Syndrome—A Case Report. Kidney Dial. 2023, 3, 196-203. https://doi.org/10.3390/kidneydial3020018
Petrosyan E, Molchanova M, Kushnir B, Povilaitite P, Tsygankova P, Zakharova E, Proskura M. Genetic Variability of HUPRA Syndrome—A Case Report. Kidney and Dialysis. 2023; 3(2):196-203. https://doi.org/10.3390/kidneydial3020018
Chicago/Turabian StylePetrosyan, Edita, Maria Molchanova, Berta Kushnir, Patritsia Povilaitite, Polina Tsygankova, Ekaterina Zakharova, and Maria Proskura. 2023. "Genetic Variability of HUPRA Syndrome—A Case Report" Kidney and Dialysis 3, no. 2: 196-203. https://doi.org/10.3390/kidneydial3020018
APA StylePetrosyan, E., Molchanova, M., Kushnir, B., Povilaitite, P., Tsygankova, P., Zakharova, E., & Proskura, M. (2023). Genetic Variability of HUPRA Syndrome—A Case Report. Kidney and Dialysis, 3(2), 196-203. https://doi.org/10.3390/kidneydial3020018







