Remission of Proteinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by Sodium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship?
Abstract
1. Introduction
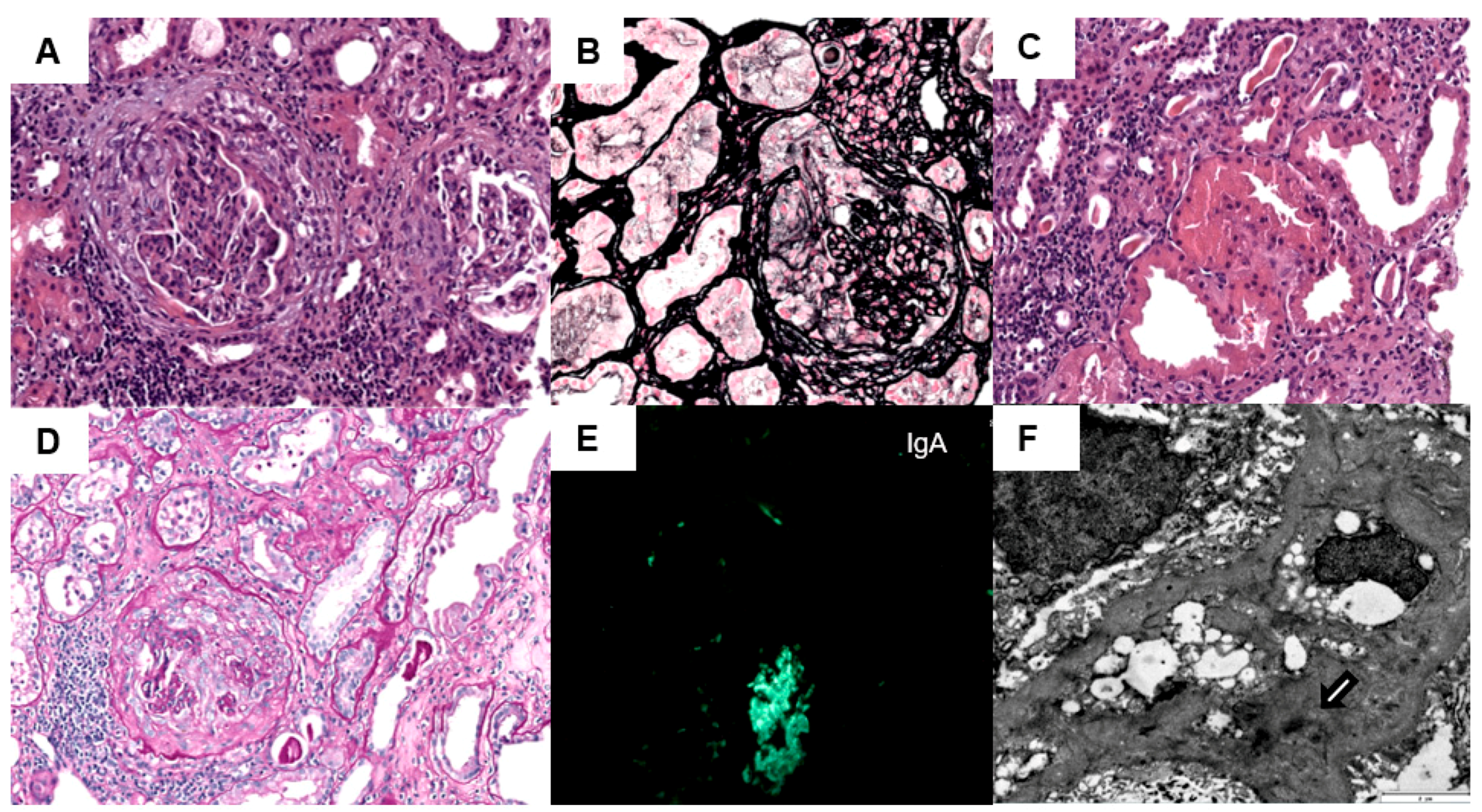
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murugapandian, S.; Mansour, I.; Hudeeb, M.; Hamed, K.; Hammode, E.; Bijin, B.; Daheshpour, S.; Thajudeen, B.; Kadambi, P. Epidemiology of Glomerular Disease in Southern Arizona: Review of 10-Year Renal Biopsy Data. Medicine 2016, 95, e3633. [Google Scholar] [CrossRef]
- Chembo, C.L.; Marshall, M.R.; Williams, L.C.; Walker, R.J.; Lynn, K.L.; Irvine, J.; Pilmore, H.L. Long-term outcomes for primary glomerulonephritis: New Zealand Glomerulonephritis Study. Nephrology 2015, 20, 899–907. [Google Scholar] [CrossRef]
- Nakai, S.; Hanafusa, N.; Masakane, I.; Taniguchi, M.; Hamano, T.; Shoji, T.; Hasegawa, T.; Itami, N.; Yamagata, K.; Shinoda, T.; et al. An overview of regular dialysis treatment in Japan (as of 31 December 2012). Ther. Apher. Dial. 2014, 18, 535–602. [Google Scholar] [CrossRef]
- Haas, M.; Verhave, J.C.; Liu, Z.H.; Alpers, C.E.; Barratt, J.; Becker, J.U.; Cattran, D.; Cook, H.T.; Coppo, R.; Feehally, J.; et al. A Multicenter Study of the Predictive Value of Crescents in IgA Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 691–701. [Google Scholar] [CrossRef]
- Reich, H.N.; Floege, J. How I Treat IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2022, 17, 1243–1246. [Google Scholar] [CrossRef]
- Lv, J.; Yang, Y.; Zhang, H.; Chen, W.; Pan, X.; Guo, Z.; Wang, C.; Li, S.; Zhang, J.; Zhang, J.; et al. Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J. Am. Soc. Nephrol. 2013, 24, 2118–2125. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Roberts, I.S.D.; Cook, H.T.; Troyanov, S.; Alpers, C.E.; Amore, A.; Barratt, J.; Berthoux, F.; Bonsib, S.; Bruijn, J.A.; Cattran, D.C.; et al. The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int. 2009, 76, 546–556. [Google Scholar] [CrossRef]
- Barbour, S.J.; Coppo, R.; Zhang, H.; Liu, Z.H.; Suzuki, Y.; Matsuzaki, K.; Katafuchi, R.; Er, L.; Espino-Hernandez, G.; Kim, S.J.; et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern. Med. 2019, 179, 942–952. [Google Scholar] [CrossRef]
- Lai, K.N.; Tang, S.C.W.; Schena, F.P.; Novak, J.; Tomino, Y.; Fogo, A.B.; Glassock, R.J. IgA nephropathy. Nat. Rev. Dis. Prim. 2016, 2, 16001. [Google Scholar] [CrossRef]
- Berthoux, F.C.; Mohey, H.; Afiani, A. Natural history of primary IgA nephropathy. Semin. Nephrol. 2008, 28, 4–9. [Google Scholar] [CrossRef]
- Trimarchi, H.; Haas, M.; Coppo, R. Crescents and IgA Nephropathy: A Delicate Marriage. J. Clin. Med. 2022, 11, 3569. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Oshima, M.; Neuen, B.L.; Li, J.W.; Perkovic, V.; Charytan, D.M.; de Zeeuw, D.; Edwards, R.; Greene, T.; Levin, A.; Mahaffey, K.W.; et al. Early Change in Albuminuria with Canagliflozin Predicts Kidney and Cardiovascular Outcomes: A Post Hoc Analysis from the CREDENCE Trial. J. Am. Soc. Nephrol. 2020, 31, 2925–2936. [Google Scholar] [CrossRef]
- McQuarrie, E.P.; Gillis, K.A.; Mark, P.B. Seven suggestions for successful SGLT2i use in glomerular disease—A standalone CKD therapy? Curr. Opin. Nephrol. Hypertens. 2022, 31, 272–277. [Google Scholar] [CrossRef]
- Wheeler, D.C.; Toto, R.D.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Pecoits-Filho, R.; Correa-Rotter, R.; et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021, 100, 215–224. [Google Scholar] [CrossRef]
- Anders, H.-J.; Peired, A.J.; Romagnani, P. SGLT2 inhibition requires reconsideration of fundamental paradigms in chronic kidney disease, ‘diabetic nephropathy’, IgA nephropathy and podocytopathies with FSGS lesions. Nephrol. Dial. Transplant. 2022, 37, 1609–1615. [Google Scholar] [CrossRef]
- Natale, P.; Palmer, S.C.; Ruospo, M.; Saglimbene, V.M.; Craig, J.C.; Vecchio, M.; Samuels, J.A.; Molony, D.A.; Schena, F.P.; Strippoli, G.F.M. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst. Rev. 2020, 3, CD003965. [Google Scholar] [CrossRef]
- Miyata, K.N.; Zhang, S.-L.; Chan, J.S.D. The Rationale and Evidence for SGLT2 Inhibitors as a Treatment for Nondiabetic Glomerular Disease. Glomerular Dis. 2021, 1, 21–33. [Google Scholar] [CrossRef]
| Reference Range/Unit | ||
|---|---|---|
| WBC | 7800 | U/L |
| Hemoglobin (Hb) | 10.6 | 12–16 g/dL |
| Platelet count (PLt) | 188 | 103/μL |
| Reticulocytes count | 2.77 | 2–4% |
| Erythrocyte count | 3.43 | 4.2–5.8 × 106/μL |
| Lactate dehydrogenase (LDH) | 258 | 135–214 IU/L |
| Coombs Test | Negative | NA |
| Total Bilirubin | 0.42 | 0.1–1 mg/dL |
| Total protein | 6.1 | 6.4–8.7 g/dL |
| Serum Albumin (Alb) | 2.52 | 3–5.5 g/dL |
| GOT | 21 | 5–32 IU/L |
| GPT | 12 | 5–33 IU/L |
| Triglycerides | 161 | 30–150 mg/dL |
| Total cholesterol | 192 | 110–200 mg/dL |
| Urea | 185 | 17–60 mg/dL |
| Creatinine | 6.07 | 0.6–1.2 mg/dL |
| Na | 138 | 135–145 mmol/L |
| K | 4.2 | 3.5–5.5 mmol/L |
| Cl | 99 | 95–110 mmol/L |
| P | 7.5 | 2.5–4.5 mg/dL |
| Albumin-corrected calcium | 8.78 | 8–10.4 mg/dL |
| CRP | 1.02 | 0.1–0.5 mg/dL |
| Hbs-Ag | Negative | NA |
| HCV-Ab | Negative | NA |
| HIV | Negative | NA |
| Cryoglobulins | Negative | NA |
| CFH | 250 | 225–760 μg/mL |
| Autoantibodies CFH | Negative | <18 AU/L |
| C3 nephritic factor (C3NF) | Negative | Ratio > 1.022 |
| C3 | 110 | 90–180 mg/dL |
| C4 | 25.1 | 10–40 mg/dL |
| RF | Negative | <15 IU/ml |
| ANA, Antids-DNA, ANCA and cryoglobulin | Negative | NA |
| Anti-GBM | Negative | <1 AI |
| Anti-PLA2R Ab (ELISA) | Negative | NA |
| Beta 2 microglobulin | 1.09 | <0–20 mg/dL |
| IgG | 885 | 800–1600 mg/dL |
| IgA | 517 | 70–400 mg/dL |
| IgM | 98 | 90–180 mg/dL |
| UPCR | 6350 | <20 mg/g |
| UACR | 5655 | <30 mg/g |
| Urine red blood cells | 50–100 | /HPF |
| 24-h urine total protein excretion | 6.5 | <0.15 g/24-h |
| SPEP M-protein concentration | No monoclonal band | NA |
| Urine immunofixation-electrophoresis: | No monoclonal band | NA mg/dL |
| Baseline | At 1 Month | At 2 Month | At 3 Month | At 4 Month | At 5 Month | At 8 Month | |
|---|---|---|---|---|---|---|---|
| Hb (mg/dL) | 10.6 | 11.3 | 13 | 12.4 | 13 | 12 | 12.3 |
| Ferritin (ng/mL) | 233 | 224 | 599 | 468 | 556 | 372 | 437 |
| Tsat (%) | 30 | 21 | 32 | 38 | 35 | 33 | 41 |
| Serum creatinine (mg/dL) | 6.07 | 4.14 | 5.14 | 2.35 | 2.66 | 2.69 | 2.66 |
| eGFR (CKD-EPI) (mL/min) | 8.8 | 13.9 | 10.7 | 27.6 | 23.8 | 23.4 | 23.8 |
| Serum urea (mg/dL) | 185 | 123 | 103 | 76 | 99 | 89 | 88 |
| Kru (mL/min) | 2.66 | 3.44 | - | 10.96 | 6.9 | 7.4 | 7.33 |
| Urine volume (mL/24 h) | 800 | 1900 | 1600 | 1900 | 2400 | 1900 | 1600 |
| CrCl (mL/min) | 9.9 | 13 | - | 31.8 | 29.6 | 28.7 | 29.6 |
| UACR (mg/g) | 5655 | 6769 | 5599 | 3200 | 1271 | 443 | 200 |
| Urine red blood cells/HPF | 50–100 | 50–100 | - | 25–35 | - | 15–25 | 10–15 |
| Urine glucose (mg/dL) | Negative | 5 | Negative | 100 | 300 | 288 | 310 |
| Albumin-corrected calcium (mg/dL) | 8.78 | 9.1 | 9.1 | 8.5 | 9.3 | 8.9 | 8.8 |
| Phos (mg/dL) | 7.5 | 5.6 | 4.7 | 4.5 | 4.6 | 3.3 | 2.9 |
| Serum Albumin (mg/dL) | 2.52 | 2.84 | 2.97 | 3.34 | 3.11 | 3.43 | 3.69 |
| PTH (pg/mL) | 154 | 202 | 232 | - | 101 | - | 79 |
| Calcitriol (ng/mL) | 6.31 | 33.5 | 46.9 | 21 | 25 | 20.6 | 27.8 |
| Na (mmol/L) | 138 | 137 | 141 | 138 | 140 | 138 | 139 |
| K (mmol/L) | 4.2 | 4.5 | 4.2 | 4.2 | 3.9 | 4.2 | 3.7 |
| Blood pressure (mmHg) | 180/100 | 151/87 | 137/82 | 128/79 | 121/74 | 133/81 | 125/78 |
| Cyclophosphamide | 0.5 g/m2 monthly | 0.5 g/m2 monthly | 0.5 g/m2 monthly | 0.5 g/m2 monthly | 0.5 g/m2 monthly | 0.5 g/m2 monthly | - |
| Corticosteroids | MPD 1 g for 3 days | PDN 60 mg/day | PDN 60 mg/day | PDN 50 mg/day | PDN 30 mg/day | PDN 10 mg/day | - |
| ARB-2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Dapagliflozin (SGLT-2i) | - | - | - | 10 mg/day | 10 mg/day | 10 mg/day | 10 mg/day |
| Weekly HD regimens | Thrice | once | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Flor Merino, J.C.; Apaza Chávez, J.; Valga Amado, F.; Díaz Crespo, F.; Justo Avila, P.; Marschall, A.; Cieza Terrones, M.; Núñez Ramos, P.; Ruiz Cicero, E. Remission of Proteinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by Sodium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship? Kidney Dial. 2022, 2, 545-552. https://doi.org/10.3390/kidneydial2040049
De La Flor Merino JC, Apaza Chávez J, Valga Amado F, Díaz Crespo F, Justo Avila P, Marschall A, Cieza Terrones M, Núñez Ramos P, Ruiz Cicero E. Remission of Proteinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by Sodium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship? Kidney and Dialysis. 2022; 2(4):545-552. https://doi.org/10.3390/kidneydial2040049
Chicago/Turabian StyleDe La Flor Merino, José C., Jacqueline Apaza Chávez, Francisco Valga Amado, Francisco Díaz Crespo, Pablo Justo Avila, Alexander Marschall, Michael Cieza Terrones, Patricia Núñez Ramos, and Elisa Ruiz Cicero. 2022. "Remission of Proteinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by Sodium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship?" Kidney and Dialysis 2, no. 4: 545-552. https://doi.org/10.3390/kidneydial2040049
APA StyleDe La Flor Merino, J. C., Apaza Chávez, J., Valga Amado, F., Díaz Crespo, F., Justo Avila, P., Marschall, A., Cieza Terrones, M., Núñez Ramos, P., & Ruiz Cicero, E. (2022). Remission of Proteinuria in a Patient Affected by Crescentic IgA Nephropathy with Rapidly Progressive Glomerulonephritis Treated by Sodium-Glucose Cotransporter-2 Inhibitors: Casual or Causal Relationship? Kidney and Dialysis, 2(4), 545-552. https://doi.org/10.3390/kidneydial2040049







