Abstract
Acute kidney injury (AKI) in the setting of hypothyroidism has been documented in the literature. However, hypothyroidism is not generally considered a cause during investigation of an acute kidney injury. Most of the cases described have been reported in setting of rhabdomyolysis, while fewer cases describe AKI occurring in the absence of rhabdomyolysis. Only rarely have case reports been supplemented by renal biopsy findings to ensure other etiologies of acute kidney injury were ruled out, and none of these reports have documented changes in the kidney that could be associated with the hypothyroid state. We report a case of AKI in chronic kidney disease in the absence of rhabdomyolysis, occurring during severe hypothyroidism, that resolved completely after achievement of a euthyroid state. In addition, we provide renal biopsy findings likely associated with the hypothyroid state. We propose that evaluation of the thyroid function should be considered in any patient during evaluation of an acute kidney injury.
1. Introduction
Acute kidney injury (AKI) occurring in the setting of hypothyroidism is uncommon but has been reported. Most of the cases describe AKI occurring from rhabdomyolysis from hypothyroid myopathy. However, AKI from hypothyroidism can also occur in the absence of rhabdomyolysis. While some case reports describe this association of hypothyroidism and AKI in the absence of rhabdomyolysis, rarely have concomitant renal biopsies been carried out to conclusively rule out other causes of the AKI. We describe a patient with CKD who developed an AKI in setting of severe hypothyroidism that resolved completely after thyroid hormone replacement and achievement of a euthyroid state. In addition, we present histological changes in the kidney that could be attributed to the hypothyroid state. Our case emphasizes that evaluation of the thyroid function should be considered during workup of an unexplained AKI.
2. Case Report
A 52-year-old male was diagnosed with interstitial lung disease from a lung biopsy in January 2015 at an outside institute who was diagnosed with pauci-immune glomerulonephritis in July 2015. At that time, he had presented with worsening difficulty breathing, hemoptysis and hypoxia as well as acute kidney injury with microscopic hematuria and proteinuria. A bronchoscopy showed diffuse alveolar hemorrhage. The renal biopsy showed focal necrotizing glomerulonephritis (GN), pauci-immune. Anti-neutrophil cytoplasmic antibodies were negative. He was treated with high dose steroids and oral cyclophosphamide and achieved clinical remission. He received rituximab for maintenance therapy which he completed in July 2017; he was tapered off steroids completely. He had been in complete renal remission with serum creatinine in the range of 1.7 to 1.9, estimated glomerular filtration rate (eGFR) of 46–51 mL/min (eGFR 46–51 mL/min), though intermittently in the 1.6 to 2.1 mg/dL range. Urinalysis was without blood or protein. His breathing had significantly improved as well, and he did not require home oxygen on activity. He had been on lisinopril since his diagnosis of pauci-immune GN. In September 2019, he was diagnosed with angioedema after starting Augmentin and azithromycin prescribed by his local doctor for pneumonia. It was not clear if the angioedema was from the antibiotics or the lisinopril, but lisinopril was stopped. He was started on losartan 25 mg daily, which he had been tolerating.
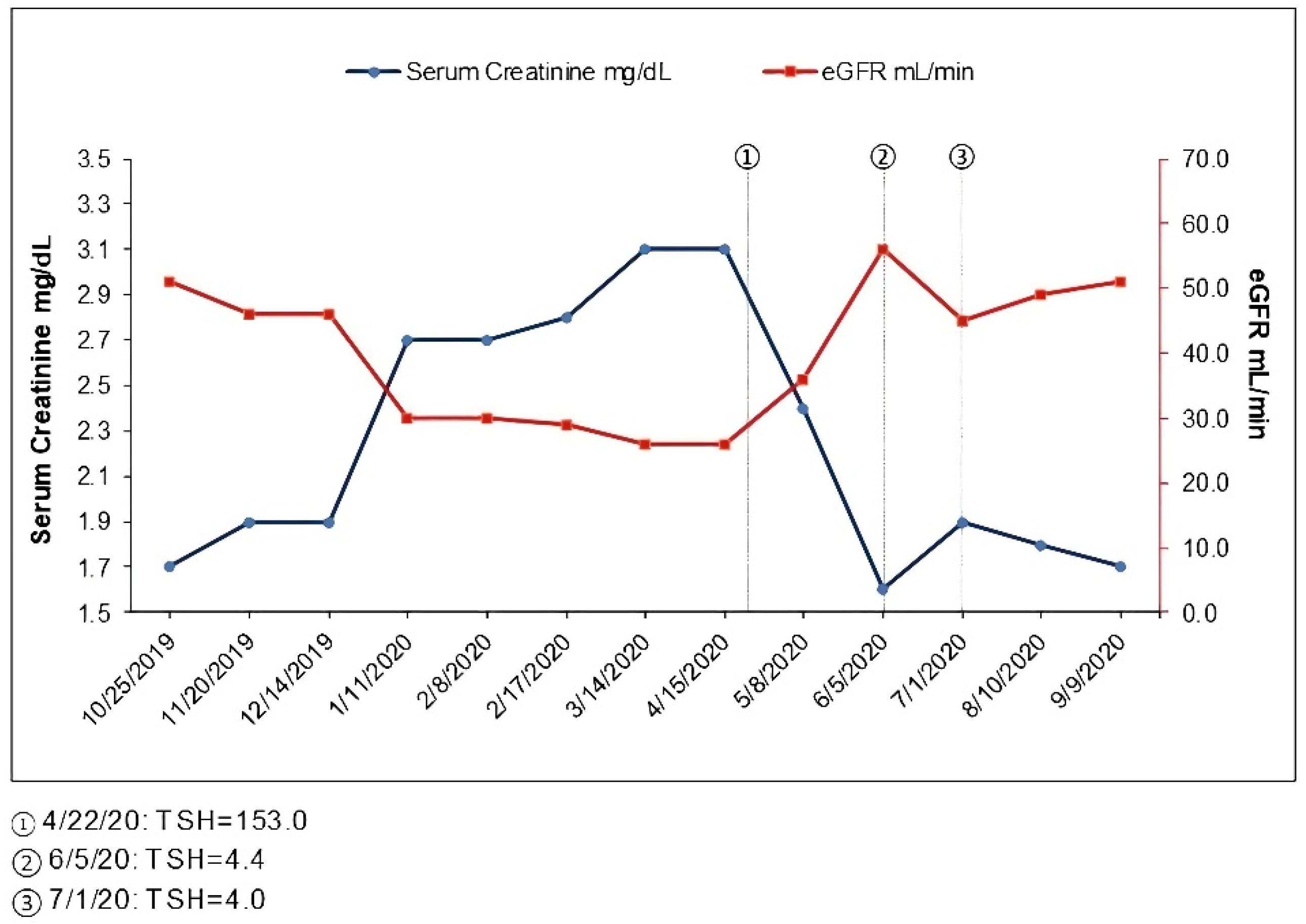
From routine labs in January 2020, his creatinine was noted to be elevated to 2.7 mg/dL. Urinalysis showed no blood or protein, and the random protein creatinine ratio was <0.1 g/g. These labs were repeated a few weeks later, and serum creatinine remained 2.7 mg/dL. He reported a good appetite, denied any new medications including NSAID use and denied use of over-the-counter supplements including creatine supplements. His home blood pressure had risen above 130/80 and losartan was increased to 50 mg daily. Upon clinical evaluation in February 2020, he reported a 10-pound weight gain which he attributed to weight gain during the holiday season that had persisted. His wife reported he had started snoring for about a month since he had gained weight. He reported a change in his voice and felt it was hoarse but only intermittently. He denied any difficulty breathing, wheezing or swallowing. He had no urinary complaints and denied any change in color of urine or amount of urine. Upon assessment, he did have a deeper voice compared to before, though his oropharyngeal exam was normal. Urinalysis was bland without red cells, pyuria or protein. Due to concern with angioedema with lisinopril previously, his losartan was held temporarily and switched to nifedipine XL until further evaluation was completed. He was referred to otolaryngology (ENT) and also asked to schedule a sleep study. He was evaluated by ENT in March 2020 and had no stridor, andno focal neck masses were noted. On laryngoscopy, no abnormalities of the glottis or vocal cords were noted, although there was some edema in the arytenoid and post cricoid area thought to be related to acid reflux. Use of a proton pump inhibitor was advised; however, given his worsening renal function and to avoid further confounders, this was not started. Repeat labs showed worsening creatinine to 3.1 in March 2020, and his urinalysis remained without blood or protein (Figure 1, Table 1). He had no new symptoms, and his weight remained stable. It was unclear if he had an interstitial or an infiltrative process, though the urinalysis was without pyuria. In the absence of clear cause and significant drop in GFR, he underwent a renal biopsy in March 2020. The renal ultrasound prior to the biopsy was unremarkable.
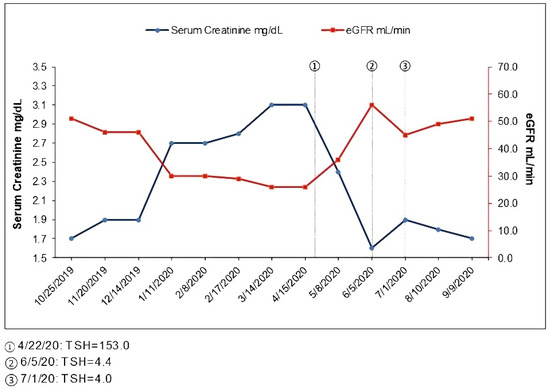
Figure 1.
Time course of serum creatinine and thyroid function.

Table 1.
Time course of renal and thyroid function.
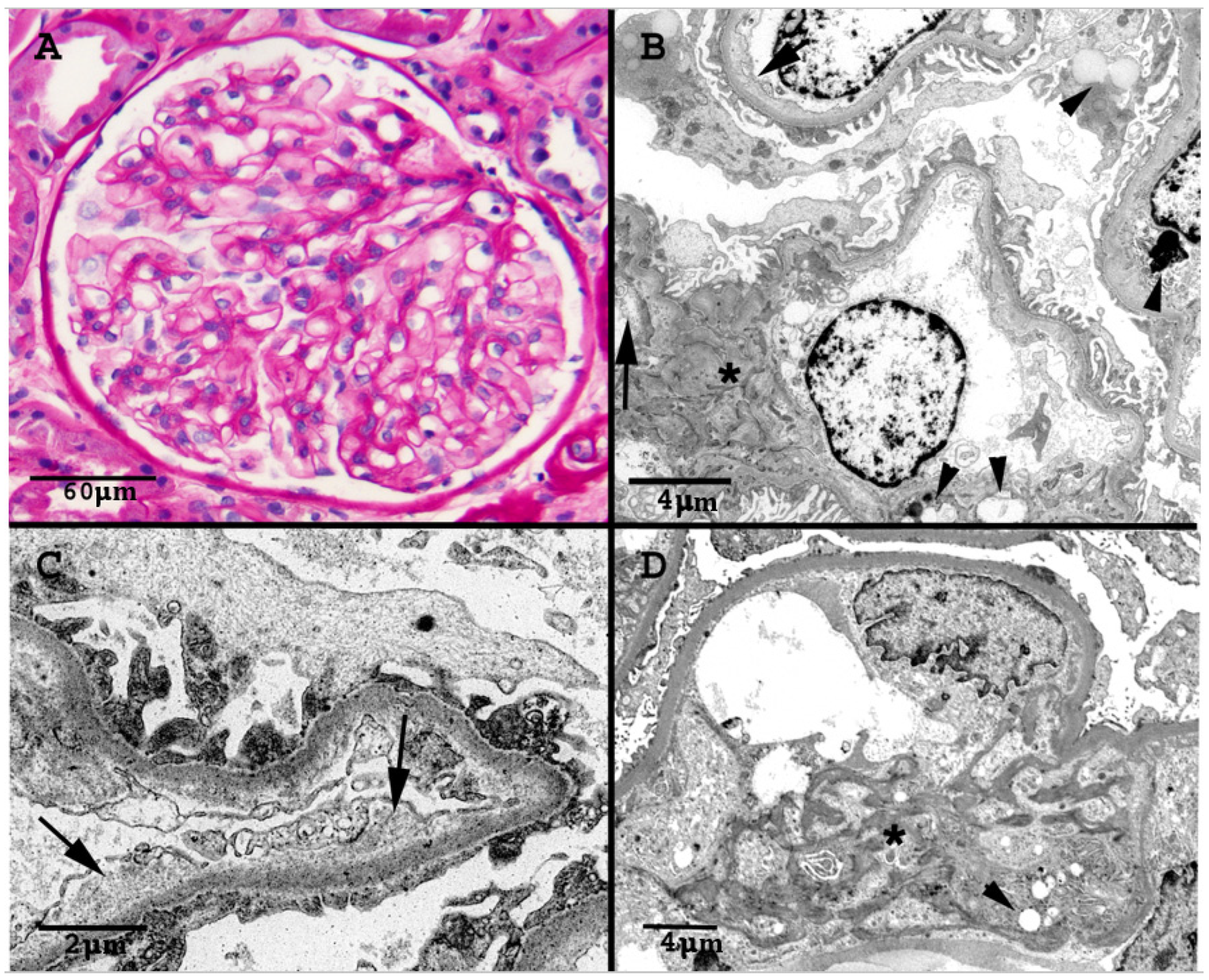
The renal biopsy showed 17 glomeruli, 8 globally sclerosed (Figure 2). No proliferative, necrotizing or thrombotic lesions were present. The viable glomeruli had a mild increase in the mesangial matrix and overall patent capillary lumina. There was one bland segmental area of sclerosis, consistent with the focal necrotizing glomerulonephritis seen in the previous biopsy. Interlobular and preglomerular arterial branches were within normal limits and interstitial fibrosis and tubular atrophy were mild (15%). Immunofluorescence staining was negative. Electron microscopic (EM) examination confirmed the expanded mesangium, that appeared heterogeneous and mottled with increased electron-dense strands admixed with electro-lucent areas. The peripheral capillary loops were patent without any electron-dense immune deposits. There were no amyloid deposits, fibrils or tubuloreticular inclusions. There was multifocal widening of the subendothelial space/lamina rara interna due to accumulation of granular or loosely fibrillary material. Lipid inclusion was present in epithelial, endothelial and mesangial cells. The tubules often showed irregular multilayering of their basement membranes. The ultrastructural changes seen in this biopsy were not present in the previous biopsy. The clinical significance of these EM findings was not clear at that time. The EM findings were not consistent with diabetic nephropathy, thrombotic microangiopathy or IgA nephropathy. Clinically, the patient had no proteinuria and no findings suggestive of thrombotic microangiopathy.
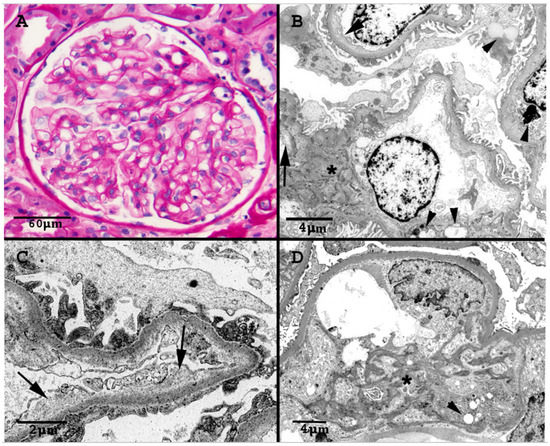
Figure 2.
(A): Normocellular glomerulus with open capillary lumina and mildly expanded mesangium due to increase in matrix (PAS stain). (B): Electron micrograph of several capillary lumina. There is expansion of the lamina rara interna (arrows), and multiple lipid inclusions are noted (arrowheads). The mesangium is minimally accentuated (asterisk). (C): Electron micrograph demonstrating areas of prominent expansion of the subendothelial space with accumulation of electro-lucent granular material. (D): Electron micrograph highlights heterogeneous mesangial matrix (asterisk) with increased electron-dense mottled strands, admixed with loose more clear areas. Arrowhead marks lipid inclusions.
During subsequent weeks, he noted some mild leg swelling but also had been started on nifedipine XL; otherwise, he had no new symptoms. Upon further evaluation, in April 2020, he was noted to have some thyroid fullness, and a thyroid stimulating hormone (TSH) test was performed, with results showing elevated levels at 153 mcIU/mL (reference range 0.47–4.68 mcIU/mL), while free T4 was <0.07 ng/dL (reference 0.6–2.5 ng/dL) consistent with hypothyroidism. The patient had no prior history of any thyroid disorder. A thyroid peroxidase antibody was elevated at 477 IU/mL (reference < 9 IU/mL). He was started on 100 mcg levothyroxine. He felt symptomatically better after the start of the thyroid supplement and felt his voice normalize; he lost the weight he had gained over the preceding months. Serum creatinine was 3.1 as of April 2020 but was 2.4 mg/dL in May 2020 and 1.6 in June 2020, at which time his repeat TSH was normal (Figure 1, Table 1). The patient had acute worsening of his renal function in the setting of severe hypothyroidism that completely resolved with achievement of the euthyroid state. Renal function since remained at his baseline with a serum creatinine in the 1.7 to 1.9 mg/dL range (Figure 1, Table 1).
3. Discussion
There are multiple reports of renal dysfunction attributed to rhabdomyolysis in the setting of hypothyroid associated myopathy; in some of these reports, levels of creatine kinase (CK) were significantly elevated [1,2,3,4,5,6,7,8]. In other reports, CK was only moderately elevated, though most but not all the patients had some muscle weakness, pain or stiffness [9,10,11,12,13,14,15,16]. However, renal dysfunction from hypothyroidism can also occur in the absence of rhabdomyolysis. In these cases, the AKI reversed with thyroid hormone replacement and reestablishment of euthyroid state [17,18,19,20,21,22,23,24,25,26,27]; renal biopsies were carried out in only two of these reports [18,21] and were reported as unremarkable. We present a patient with AKI in CKD resulting from severe hypothyroidism, in the absence of myopathy, and with the renal biopsy suggesting findings seen in hypothyroid states.
The thyroid hormone has multiple effects on the cardiovascular system including increased cardiac contractility, increased heart rate, decreased peripheral vascular resistance and increased cardiac output. Hypothyroidism results in decreased cardiac output and increased peripheral resistance [28]. In the kidney, hypothyroidism causes multiple effects including decreased renal plasma flow, intrarenal vasoconstriction, decreased GFR, decreased renin angiotensinogen aldosterone system (RAAS) activity, inability to excrete free water, decreased urinary concentrating ability and decreased sodium absorption [29,30,31,32]. Hypothyroid patients have decreased GFR, decreased proximal tubular sodium absorption, decreased maximal urinary flow rate and free water clearance similar to patients with chronic renal failure, thought to be likely related to the reduced eGFR [33].
In 41 patients with primary hypothyroidism, the creatinine clearance was lower than 85 mL/min in all cases, and mean creatinine clearance was 62 (±4) mL/min. Repeat readings two months after starting thyroid hormone replacement showed normal creatinine clearance in all but three elderly patients whose creatinine clearance remained below 80 mL/min; the mean creatinine clearance improved to 90 (±3) mL/min after thyroid replacement [34].
In another study, serum creatinine was compared before and after a period of iatrogenic hypothyroidism, induced prior to radioactive iodine scanning for thyroid carcinoma monitoring. In the 29 episodes of hypothyroidism in 15 patients, the hypothyroid creatinine values were greater in 89.7% and equal in 10.3% cases compared with the creatinine during the prior euthyroid state. In addition, in 36 episodes of hypothyroidism in 20 patients, the creatinine during the hypothyroid state was greater in 91.7% of cases compared with creatinine values when levothyroxine was reinstituted and euthyroid state achieved again. Among the 29 episodes of hypothyroidism in the 15 patients in whom all three creatinine values were available, there was no difference in creatinine obtained during euthyroid states before and after the hypothyroid state [35].
The elevation in the creatinine with hypothyroidism is thought to be due to reduction in the GFR and not due to increased production from myopathy or reduced renal tubular secretion. Effective renal plasma blood flow asmeasured by 131 I-Hippuran clearance and GFR measured by 51Cr-EDTA was found to be low in patients with overt hypothyroidism and increased after therapy with thyroid hormone replacement [36]. Similarly, in a study of 27 patients after total thyroidectomy for thyroid carcinoma, the GFR as measured by 51Cr-EDTA clearance was significantly lower and serum creatinine higher in hypothyroid state than after two months of treatment with thyroid hormone replacement [37].
Our patient had no symptoms of myalgia or muscle weakness, though these symptoms may not always be present even with myopathy. Muscle creatinine kinase was not measured; however, he reported no change in color of the urine, and the urinalysis showed no blood or heme pigment. While a normal urinalysis may not rule out mild cases of rhabdomyolysis, his renal biopsy showed no acute tubular injury or pigment casts to suggest this as the cause of the AKI. Thyroid disorders can be associated with glomerular pathologies [31,32]; however, the urinalysis was without red blood cells or protein, and the renal biopsy showed no glomerulonephritis. The patient had no evidence of volume depletion from either history or physical examination. His blood pressure had been higher than his baseline; this was partly attributed to his weight gain. Subsequently, he was normotensive with adjustment of his antihypertensive. Even though the etiology of the decrease in GFR with hypothyroidism is due to decreased renal plasma flow, BPs were reported to be normal to mildly hypertensive in most cases of hypothyroidism associated AKI in the absence of rhabdomyolysis. No urine sodium or urine urea was measured in this patient who developed persistent renal impairment over months, since there was no clinical suspicion for volume depletion, and he was non oliguric.
In the case reports published thus far describing AKI in the setting of hypothyroidism in the absence of rhabdomyolysis, there are only two reports which documented renal biopsies [18,21]. Both these reports describe no significant abnormalities that were found in the biopsy. However, ours is the first case report which describes renal pathology findings similar to those reported in prior biopsy series in patients with hypothyroidism [38,39]. Solomon et al. describe a case series of renal biopsies in seven patients with clinical hypothyroidism; there was enlarged mesangium noted with some thickening of the tubular basement membrane. In electron microscopy, there was thickening of the basement membranes, increased mesangial matrix which appeared loose or “mottled” and lipid containing inclusions noted in epithelial, endothelial and mesangial cells. There was also thickening of the tubular basement membranes with inclusions that either contained lipids or were lysosomes, and some cells had hyaline droplets, which were probably protein [38]. Repeat biopsies carried out in two patients during euthyroid state showed marked decrease in intracellular inclusions as well decrease in the capillary basement membrane thickness [38].
In our patient, the light microscopic analysis showed mild mesangial expansion, and the EM findings were in agreement with the features described by Salomon et al. While hemoglobin A1c was not checked at the time, the biopsy findings were not suggestive of diabetic nephropathy, and even with diabetic nephropathy, such early changes would not account for the severe decrease in the renal function.
There is increased prevalence of hypothyroidism in patients with CKD, with increasing prevalence noted with a greater degree of renal dysfunction. In addition, CKD is associated with various alterations in thyroid hormone levels [31,40,41]. Thyroid hormone alterations in CKD can include reduced triiodothyronine (T3) levels; reduced total thyroxine (T4) levels due to reduced protein states in advanced CKD and alterations in thyrotropin (TSH) including decreased clearance, decreased response to thyrotropin releasing hormone (TRH), decreased pulsatility, increased half-life and decreased bioactivity. However, the level of TSH is usually normal and is a more reliable indicator of thyroid function than serum T3 in patients with CKD [31,40,41]. The elevation in the TSH in our patient with CKD reflected true hypothyroidism, rather than an alteration of thyroid function due to his CKD. There was no alternative explanation for his AKI, and there was complete resolution of the AKI with normalization of the patient’s hypothyroid status after thyroid hormone supplementation. Moreover, the biopsy findings supported findings in the kidney due to the hypothyroid state.
Even though severe hypothyroidism can result in acute renal dysfunction, this is not routinely discussed as a cause of AKI in clinical practice. We present a case of an AKI in CKD in which the clinical picture and biopsy findings strongly suggest the etiology to be related to the severe hypothyroid state. We suggest that measurement of thyroid function should be performed in any patient with unexplained renal dysfunction.
Author Contributions
P.C. reviewed the literature and wrote the manuscript. A.H. and C.D. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written consent for publication to publish clinical information can be made available when requested.
Data Availability Statement
Not applicable.
Conflicts of Interest
All the authors declare that they have no competing interest.
References
- Nelson, S.R.; Phillips, A.O.; Hendry, B.M. Hypothyroidism and rhabdomyolysis in a marathon runner. Nephrol. Dial. Transpl. 1993, 8, 375–376. [Google Scholar]
- Sekine, N.; Yamamoto, M.; Michikawa, M.; Enomoto, T.; Hayashi, M.; Ozawa, E.; Kobayashi, T. Rhabdomyolysis and Acute Renal Failure in a Patient with Hypothyroidism. Intern. Med. 1993, 32, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.D.M.D.M.; Bridi, R.A.; Balbi, A.; Ponce, D. Hypothyroidism and acute kidney injury: An unusual association. BMJ Case Rep. 2013, 2013, bcr2013200585. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Issa, M. Rhabdomyolysis and Acute Renal Impairment in a Patient with Hypothyroidism: A Case Report. Case Rep. Med. 2014, 2014, 139170. [Google Scholar] [CrossRef]
- Ghayur, A.; Elahi, Q.; Patel, C.; Raj, R. Rhabdomyolysis-induced acute kidney injury in a patient with non-compliance to levothyroxine therapy. Endocrinol. Diabetes Metab. Case Rep. 2021, 21-0034. [Google Scholar] [CrossRef]
- Baghi, M.A.; Sirajudeen, J.; Naushad, V.A.; Alarbi, K.S.; Benshaban, N. Severe hypothyroidism-induced rhabdomyolysis: A case report. Clin. Case Rep. 2021, 9, e05107. [Google Scholar] [CrossRef] [PubMed]
- Janjua, I.; Bashir, T.; Haq, M.Z.U.; Arshad, M.F.; Sharif, M. Severe Hypothyroidism Presenting with Rhabdomyolysis in a Young Patient. Cureus 2021, 13, e13993. [Google Scholar] [CrossRef]
- Alshamam, M.S.; Gurung, D.O.; Nso, N.; Saliaj, M.; Seitaj, A. Rhabdomyolysis Secondary to Hypothyroidism: Report of a Case. Cureus 2021, 13, e12746. [Google Scholar] [CrossRef]
- Mooraki, A.; Broumand, B.; Neekdoost, F.; Amimokri, P.; Bastani, B. Reversible acute renal failure associated with hypothy-roidism: Report of four cases with a brief review of literature. Nephrology 2003, 8, 57–60. [Google Scholar] [CrossRef]
- Altay, M.; Duranay, M.; Ceri, M. Rhabdomyolysis due to hypothyroidism. Nephrol. Dial. Transplant. 2005, 20, 847–848. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Jones, D.; Rochford, A.; Giblin, L. Hypothyroidism and associated acute renal failure. J. R. Soc. Med. 2009, 102, 199–200. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.; Ceri, M.; Unverdi, S.; Duranay, M. An unusual cause of acute renal failure: Hypothyroidism. Clin. Kidney J. 2010, 3, 386–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ardalan, M.R.; Ghabili, K.; Mirnour, R.; Shoja, M.M. Hypothyroidism-Induced Rhabdomyolysis and Renal Failure. Ren. Fail. 2011, 33, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tang, L. Rare Acute Kidney Injury Secondary to Hypothyroidism-Induced Rhabdomyolysis. Yonsei Med. J. 2013, 54, 172–176. [Google Scholar] [CrossRef]
- Ahmed, G.S.; Zaid, H.M.; Moloney, M. Hashimoto’s thyroiditis presenting as Hoffman’s syndrome, rhabdomyolysis and acute kidney injury. BMJ Case Rep. 2014, 2014, bcr2013203269. [Google Scholar] [CrossRef]
- Katipoglu, B.; Ates, I.; Acehan, F.; Meteris, A.; Yılmaz, N. Rhabdomyolysis case based on hypothyroidism. Endocrinol. Diabetes Metab. Case Rep. 2016, 16-0083. [Google Scholar] [CrossRef]
- Woodrow, G.; Brownjohn, A.M.; Turney, J.H. Acute on chronic renal failure and hyponatremia associated with severe hy-pothyroidism. Nephrol. Dial. Transpl. 1993, 8, 557–559. [Google Scholar] [CrossRef]
- Connor, A.; Taylor, J.E. Renal impairment resulting from hypothyroidism. NDT Plus 2008, 1, 440–441. [Google Scholar] [CrossRef][Green Version]
- Silva, G.C.P.L.; Carneiro, J.B.; Tardelli, C.C.; Risso, M.; Ventura, M.D.M. Kidney failure in the elderly due to hypothyroidism: A case report. Sao Paulo Med. J. 2008, 126, 291–293. [Google Scholar] [CrossRef]
- Liakopoulas, V.; Dovas, S.; Simopoulou, T.; Zarogiannis, S.; Giannopoulou, M.; Kourti, P.; Arampatzis, P.; Eleftheriadis, T.; Stefanidis, I. Acute renal failure: A rare presentation of hypothyroidism. Ren Fail. 2009, 31, 323–326. [Google Scholar] [CrossRef]
- Patel, M.L.; Unival, R. Two unusual cases of hypothyroidism with renal dyfunction. BMJ Case Rep. 2011, 2011, bcr0120113707. [Google Scholar] [CrossRef]
- Vikrant, S.; Chander, S.; Kumar, S.; Gupta, D. Hypothyroidism presenting as reversible renal impairment: An interesting case report. Ren. Fail. 2013, 35, 1292–1294. [Google Scholar] [CrossRef] [PubMed]
- Ters, M.E.; Patel, S.M.; Norby, S.M. Hypothyroidism and reversible kidney dysfunction: An essential relation to recognize. Endocr. Pract. 2014, 20, 490–499. [Google Scholar] [CrossRef] [PubMed]
- McAninch, E.A.; Lagari, V.S. Acute-on-Chronic Kidney Injury in Thyroid Hormone Withdrawal: A Case with Possible Implications for Radioactive Iodine Planning. Case Rep. Endocrinol. 2015, 2015, 1–3. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, D.M.; Kruel-Poel, Y.H.; Den Heijer, M.; Simek, S. Subacute renal injury in hypothyroidism: A case report of an unusual phenomenon. Neth. J. Med. 2018, 76, 339–342. [Google Scholar]
- Tiong, M.; Wilson, S.; Walker, R. Hypothyroidism and renal impairment: An easily missed diagnosis. Intern. Med. J. 2019, 49, 276–278. [Google Scholar] [CrossRef]
- Shakoor, M.T.; Moahi, K.; Shemin, D. Hypothyroidism-induced acute kidney injury and hyponatremia. Rhode Isl. Med. J. 2020, 103, 61–64. [Google Scholar]
- Klein, I.; Ojamaa, K. Thyroid Hormone and the Cardiovascular System. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Vargas, F.; Moreno, J.M.; Rodríguez-Gómez, I.; Wangensteen, R.; Osuna, A.; Álvarez-Guerra, M.; Garcia-Estañ, J. Vascular and renal function in experimental thyroid disorders. Eur. J. Endocrinol. 2006, 154, 197–212. [Google Scholar] [CrossRef]
- Iglesias, P.; Díez, J.J. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef]
- Mariani, L.H.; Berns, J.S. The Renal Manifestations of Thyroid Disease. J. Am. Soc. Nephrol. 2011, 23, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Basu, G.; Mohapatra, A. Interactions between thyroid disorders and kidney disease. Indian J. Endocrinol. Metab. 2012, 16, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Harrow, A.; Pasque, C.B.; Rodriguez, M. Renal sodium and water handling in hypothyroid patients: The role of renal insufficiency. J. Am. Soc. Nephrol. 1990, 1, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, J.; Gonzalez, O.; Saracho, R.; Aguirre, R.; Gonzalez, O.; Martinez, I. Change in renal function in primary hypo-thyroidism. Am. J. Kid. Dis. 1996, 27, 195–198. [Google Scholar] [CrossRef]
- Kreisman, S.H.; Hennessey, J.V. Consistent Reversible Elevations of Serum Creatinine Levels in Severe Hypothyroidism. Arch. Intern. Med. 1999, 159, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Villabona, C.; Sahun, M.; Roca, M.; Mora, J.; Gómez, N.; Gómez, J.M.; Puchal, R.; Soler, J. Blood Volumes and Renal Function in Overt and Subclinical Primary Hypothyroidism. Am. J. Med. Sci. 1999, 318, 277–280. [Google Scholar] [CrossRef]
- Karanikas, G.; Schutz, M.; Szabo, M.; Becherer, A.; Wiesner, K.; Dudczak, R.; Kletter, K. Iostopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. Am. J. Nephrol. 2004, 24, 41–45. [Google Scholar] [CrossRef]
- Salomon, M.; Di Scala, V.; Grishman, E.; Brener, J.; Churg, J. Renal lesions in hypothyroidism: A study based on kidney biopsies. Metabolism 1967, 16, 846–852. [Google Scholar] [CrossRef]
- Cassano, C.; Fabbrini, A.; Andres, G.A.; Cinotti, G.A.; DeMartino, C.; Minio, Z. Functional, light and electron microscopic studies of the kidney in myxoedema. Eur. Rev. Endocrinol. 1964, 1, 1–10. [Google Scholar]
- Rhee, C.M.; Brent, G.A.; Kovesdy, C.P.; Soldin, O.P.; Nguyen, D.; Budoff, M.J.; Brunelli, S.M.; Kalanter-Zadeh, K. Thyroid functional disease: An under-recognised cardiovascular risk factor in kidney disease patients. Nephrol. Dial. Tranplant. 2015, 30, 724–737. [Google Scholar] [CrossRef]
- Rhee, C.M. The interaction between thyroid and kidney disease: An overview of the evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 407–415. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).