Cystatin C-Based eGFR Predicts Post-Treatment Kidney Prognosis in Patients with Severe Obstructive Nephropathy
Abstract
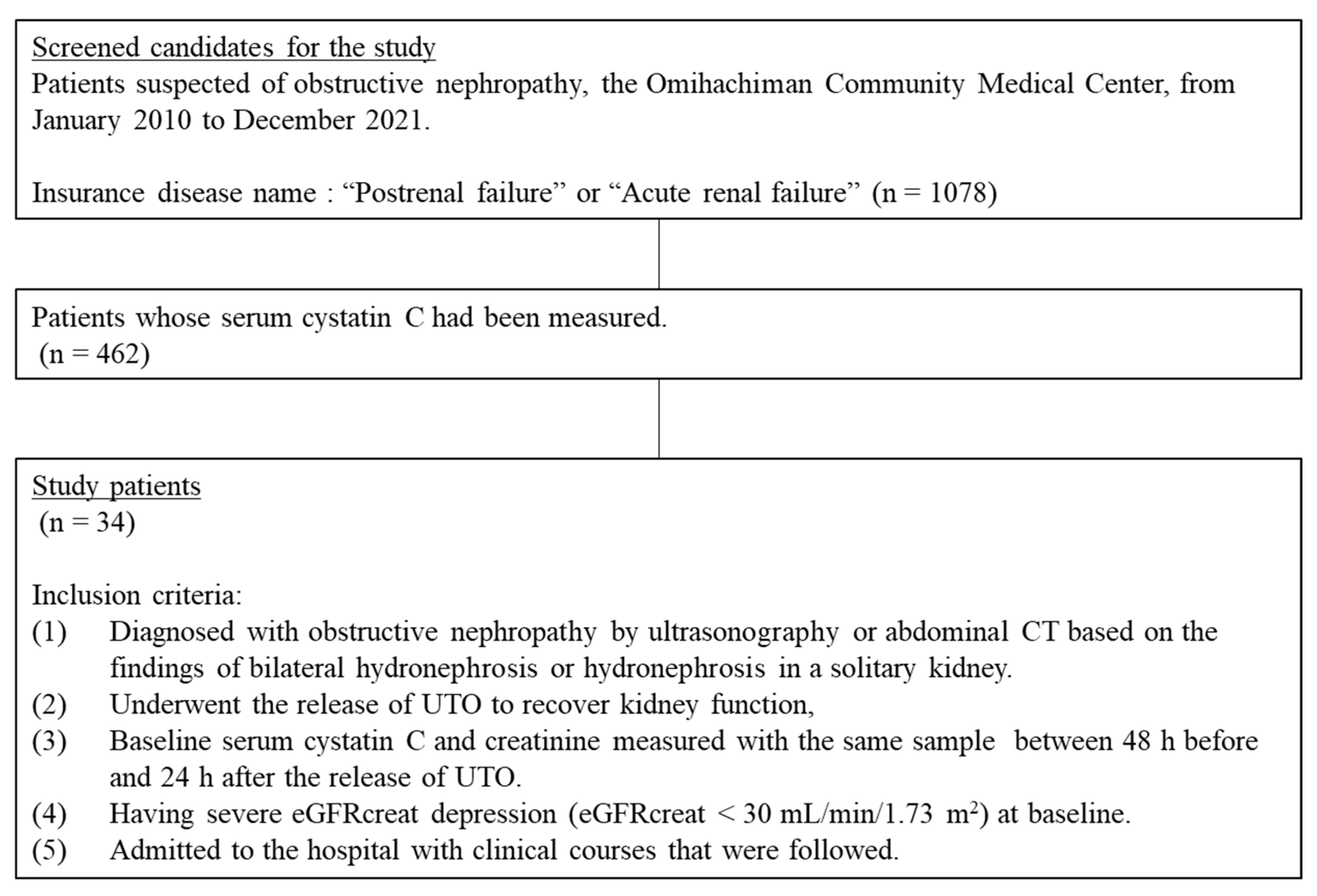
:1. Introduction
2. Materials and Methods
2.1. Patients and Measurements
2.2. Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Baseline eGFR and Recovery from Severe eGFRcreat Depression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of acute kidney injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Obstructive nephropathy. Intern. Med. 2000, 39, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Okuda, Y.; Namba, S.; Nagata, M.; Hara, H.; Morita, T. Plasma creatinine and cystatin C ratio is useful for discriminate diagnosis of postrenal renal failure. Rinsho Byori. Jpn. J. Clin. Pathol. 2008, 56, 101–107. [Google Scholar]
- Fujisawa, N.; Yashiro, M.; Segawa, H.; Kadoya, Y.; Kamata, T. Discrepancy between cystatin C and creatinine points leading to a diagnosis of postrenal acute kidney injury and its reversibility: Three case reports. Clin. Exp. Nephrol. 2010, 14, 608–613. [Google Scholar] [CrossRef]
- İnal, S.; Altuntaş, A.; Kidir, V.; Özorak, A.; İlgin, Y.; Sezer, M.T. Utility of serum creatinine/cystatin C ratio in diagnosis of postrenal acute kidney injury. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 1086–1089. [Google Scholar]
- Matsuki, M.; Tanaka, T.; Maehana, T.; Kyoda, Y.; Ichihara, K.; Hashimoto, K.; Yanase, M.; Matsukawa, M.; Adachi, H.; Takahashi, S.; et al. The discrepancy between serum creatinine and cystatin C can predict renal function after treatment for postrenal acute kidney injury: Multicenter study and pooled data analysis. Clin. Exp. Nephrol. 2017, 21, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Dharnidharka, V.R.; Kwon, C.; Stevens, G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am. J. Kidney Dis. 2002, 40, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Perkins, B.A.; Nelson, R.G.; Ostrander, B.E.; Blouch, K.L.; Krolewski, A.S.; Myers, B.D.; Warram, J.H. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J. Am. Soc. Nephrol. JASN 2005, 16, 1404–1412. [Google Scholar] [CrossRef]
- Peralta, C.A.; Katz, R.; Sarnak, M.J.; Ix, J.; Fried, L.F.; De Boer, I.; Palmas, W.; Siscovick, D.; Levey, A.S.; Shlipak, M.G. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J. Am. Soc. Nephrol. JASN 2011, 22, 147–155. [Google Scholar] [CrossRef]
- Horio, M.; Imai, E.; Yasuda, Y.; Watanabe, T.; Matsuo, S. GFR estimation using standardized serum cystatin C in Japan. Am. J. Kidney Dis 2013, 61, 197–203. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, A.; Hajage, D.; Van Glabeke, E.; Belenfant, X.; Vincent, F.; Gonzalez, F.; Ciroldi, M.; Obadia, E.; Chelha, R.; Pallot, J.L.; et al. Severe post-renal acute kidney injury, post-obstructive diuresis and renal recovery. BJU Int. 2012, 110, E1027–E1034. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Isaka, Y.; Takahara, S.; Horio, M. Discrepancy between serum levels of low molecular weight proteins in acute kidney injury model rats with bilateral ureteral obstruction and bilateral nephrectomy. Clin. Exp. Nephrol. 2009, 13, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Tenstad, O.; Roald, A.B.; Grubb, A.; Aukland, K. Renal handling of radiolabelled human cystatin C in the rat. Scand. J. Clin. Lab. Investig. 1996, 56, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, E.D., Jr.; Marion, D.; Poppas, D.P.; Felsen, D. Pathophysiology of unilateral ureteral obstruction: Studies from Charlottesville to New York. J. Urol. 2004, 172, 2563–2569. [Google Scholar] [CrossRef]
- Chevalier, R.L. Specific molecular targeting of renal injury in obstructive nephropathy. Kidney Int. 2006, 70, 1200–1201. [Google Scholar] [CrossRef]
- Klahr, S.; Pukerson, M.L. The pathophysiology of obstructive nephropathy: The role of vasoactive compounds in the hemodynamic and structural abnormalities of the obstructed kidney. Am. J. Kidney Dis 1994, 23, 219–223. [Google Scholar] [CrossRef]
- Chevalier, R.L.; Forbes, M.S.; Thornhill, B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009, 75, 1145–1152. [Google Scholar] [CrossRef]
- Nejat, M.; Pickering, J.W.; Devarajan, P.; Bonventre, J.V.; Edelstein, C.L.; Walker, R.J.; Endre, Z.H. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012, 81, 1254–1262. [Google Scholar] [CrossRef]
- Teo, S.H.; Endre, Z.H. Biomarkers in acute kidney injury (AKI). Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 331–344. [Google Scholar] [CrossRef]
- Leem, A.Y.; Park, M.S.; Park, B.H.; Jung, W.J.; Chung, K.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Kim, Y.S.; et al. Value of Serum Cystatin C Measurement in the Diagnosis of Sepsis-Induced Kidney Injury and Prediction of Renal Function Recovery. Yonsei Med. J. 2017, 58, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Better, O.S.; Arieff, A.I.; Massry, S.G.; Kleeman, C.R.; Maxwell, M.H. Studies on renal function after relief of complete unilateral ureteral obstruction of three months’ duration in man. Am. J. Med. 1973, 54, 234–240. [Google Scholar] [CrossRef]
- Green, J.; Vardy, Y.; Munichor, M.; Better, O.S. Extreme unilateral hydronephrosis with normal glomerular filtration rate: Physiological studies in a case of obstructive uropathy. J. Urol. 1986, 136, 361–365. [Google Scholar] [CrossRef]

| Sex (male) | 20 (59%) |
| Age (years) | 76 (67, 83) |
| Weight (kg) | 58.7 (48.1, 66.4) |
| Etiology of obstructive nephropathy | |
| Benign prostatic hyperplasia | 11 (32%) |
| Malignant tumor | 7 (21%) |
| Neurogenic bladder | 12 (35%) |
| Retroperitoneal fibrosis | 3 (9%) |
| Ureteral stone (solitary functional kidney) | 1 (3%) |
| Recovery * (n = 20) | Non-recovery (n = 14) | p | |
|---|---|---|---|
| Sex (male) | 12 (60%) | 8 (57%) | 1.000 |
| Age (years) | 80 (73, 85) | 69 (64, 78) | 0.069 |
| Weight (kg) | 61.0 (49.8, 68.7) | 52.3 (44.5, 61.1) | 0.336 |
| Etiology of obstructive nephropathy | 0.521 | ||
| Benign prostatic hyperplasia | 5 (25%) | 6 (43%) | |
| Malignant tumor | 3 (15%) | 4 (29%) | |
| Neurogenic bladder | 9 (45%) | 3 (21%) | |
| Retroperitoneal fibrosis | 2 (10%) | 1 (7%) | |
| Ureteral stone (solitary functional kidney) | 1 (5%) | 0 (0%) | |
| Maintenance hemodialysis | – | 3 (21%) | – |
| Laboratory data | |||
| Serum creatinine (mg/dL) | 5.89 (4.74, 8.14) | 6.09 (4.53, 10.97) | 1.000 |
| Serum cystatin C (mg/L) | 1.91 (1.45, 3.05) | 3.83 (3.19, 4.17) | <0.001 |
| eGFRcreat (mL/min/1.73 m2) | 7 (5, 10) | 6 (4, 9) | 0.803 |
| eGFRcys (mL/min/1.73 m2) | 31 (14, 40) | 13 (9, 14) | 0.002 |
| Blood urea nitrogen (mg/dL) | 110.1 (53.0, 138.5) | 78.8 (55.4, 120.1) | 0.877 |
| White blood cells (103/μL) | 9.1 (6.6, 10.6) | 7.6 (5.3, 9.3) | 0.345 |
| Hemoglobin (g/L) | 11.5 (9.4, 13.0) | 9.3 (8.3, 9.9) | 0.004 |
| Platelets (103/μL) | 221 (175, 341) | 167 (134, 215) | 0.083 |
| Serum albumin (g/dL) | 3.0 (2.8, 3.8) | 3.2 (2.8, 4.0) | 0.726 |
| Serum sodium (mmol/L) | 139 (136, 147) | 141 (135, 143) | 0.672 |
| Serum potassium (mmol/L) | 5.0 (4.4, 6.3) | 4.8 (3.4, 5.6) | 0.201 |
| Bicarbonate (mmol/L) | 17.2 (13.8, 18.4) | 17.9 (13.5, 22.3) | 0.551 |
| Serum calcium (mg/dL) | 8.7 (8.5, 9.0) | 8.2 (7.6, 9.0) | 0.072 |
| Serum phosphate (mg/dL) | 5.5 (3.9, 8.0) | 5.1 (3.9, 6.4) | 0.619 |
| Serum uric acid (mg/dL) | 9.8 (7.2, 14.1) | 8.7 (7.6, 9.6) | 0.241 |
| C-reactive protein (mg/dL) | 8.4 (3.4, 15.2) | 4.9 (1.6, 10.6) | 0.138 |
| OR (95% CI) | p | |
|---|---|---|
| eGFRcys (mL/min/1.73 m2) | 1.17 (1.04–1.31) | 0.010 |
| eGFRcreat (mL/min/1.73 m2) | 1.00 (0.87–1.15) | 0.987 |
| Recovery | Total | |||
|---|---|---|---|---|
| Yes | No | |||
| eGFRcys ≥ 25 | Yes | 14 | 0 | 14 |
| No | 6 | 14 | 20 | |
| Total | 20 | 14 | 34 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakai, K.; Segawa, H.; Yashiro, M.; Yoshii, K.; Kusaba, T.; Matoba, S.; Tamagaki, K.; Hatta, T.; Kado, H. Cystatin C-Based eGFR Predicts Post-Treatment Kidney Prognosis in Patients with Severe Obstructive Nephropathy. Kidney Dial. 2022, 2, 474-481. https://doi.org/10.3390/kidneydial2030043
Nakai K, Segawa H, Yashiro M, Yoshii K, Kusaba T, Matoba S, Tamagaki K, Hatta T, Kado H. Cystatin C-Based eGFR Predicts Post-Treatment Kidney Prognosis in Patients with Severe Obstructive Nephropathy. Kidney and Dialysis. 2022; 2(3):474-481. https://doi.org/10.3390/kidneydial2030043
Chicago/Turabian StyleNakai, Kunihiro, Hiroyoshi Segawa, Masatomo Yashiro, Kengo Yoshii, Tetsuro Kusaba, Satoaki Matoba, Keiichi Tamagaki, Tsuguru Hatta, and Hiroshi Kado. 2022. "Cystatin C-Based eGFR Predicts Post-Treatment Kidney Prognosis in Patients with Severe Obstructive Nephropathy" Kidney and Dialysis 2, no. 3: 474-481. https://doi.org/10.3390/kidneydial2030043
APA StyleNakai, K., Segawa, H., Yashiro, M., Yoshii, K., Kusaba, T., Matoba, S., Tamagaki, K., Hatta, T., & Kado, H. (2022). Cystatin C-Based eGFR Predicts Post-Treatment Kidney Prognosis in Patients with Severe Obstructive Nephropathy. Kidney and Dialysis, 2(3), 474-481. https://doi.org/10.3390/kidneydial2030043






