Physical Activity Behaviour in Solid Organ Transplant Recipients: Proposal of Theory-Driven Physical Activity Interventions
Abstract
:1. Introduction
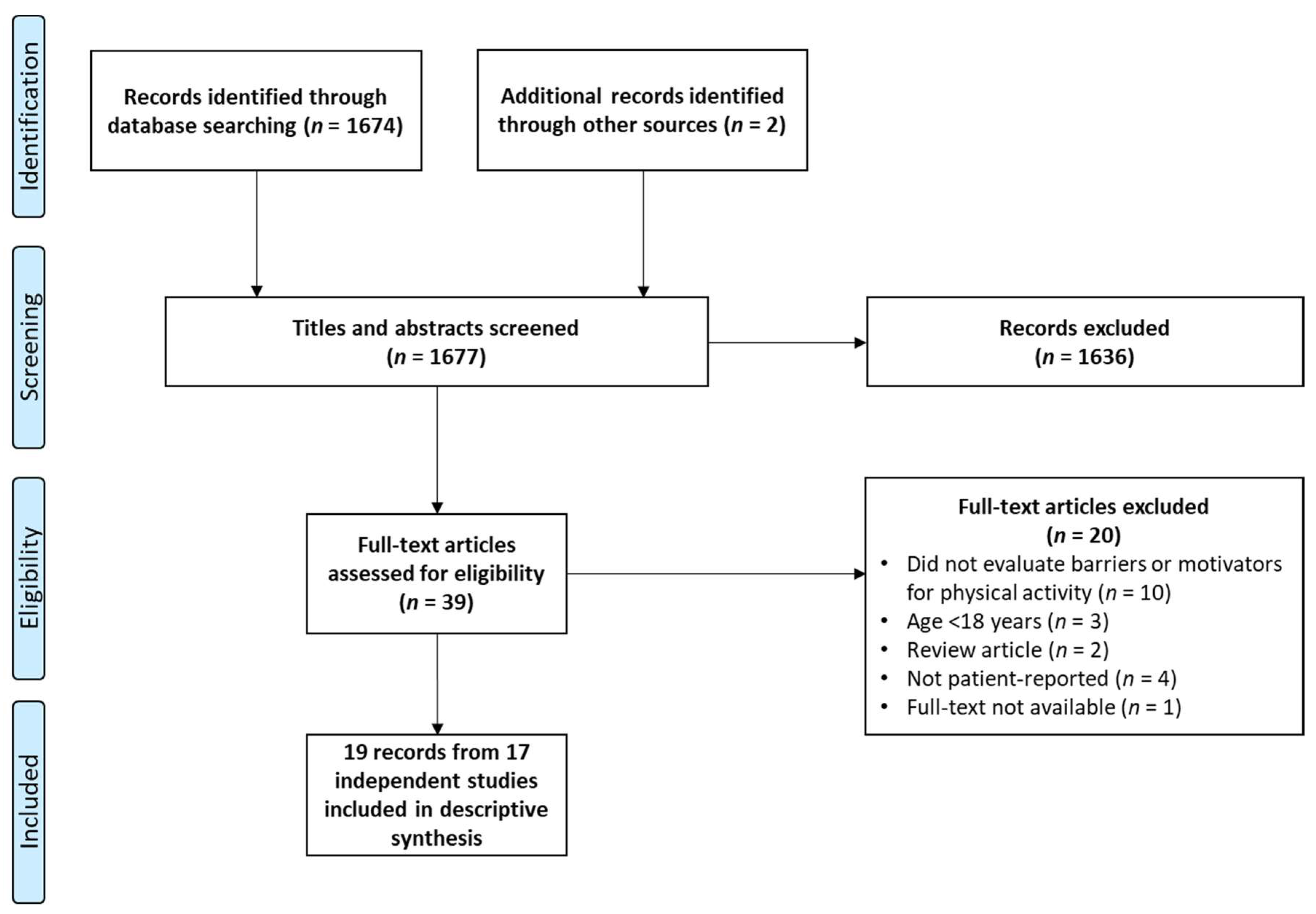
2. Methods
3. Results
3.1. Physical Inactivity after Organ Transplantation: Prevalence, Consequences, and Target Behaviour: BCW Steps 1–3
| Leading Question | Possible Answer |
|---|---|
| What is the problem/behaviour | The majority of transplant recipients do not meet physical activity guidelines and are less physically active compared to the general population [8,54]. Most studies evaluating adherence to physical activity recommendations evaluate participation in aerobic physical activity (150 min/week at moderate intensity, 75 min/week at vigorous intensity, or an equivalent of both). However, physical activity guidelines additionally include recommendations on muscle strengthening activities (≥2×/week) and multicomponent physical activities targeting postural balance during aerobic and/or muscle strengthening activities (≥3×/week), such as for instance dancing, yoga, gardening, and sports [13]. Nowadays, recommendations increasingly emphasize the need to replace sedentary behaviour with light physical activity [13,55,56,57]. Exercise and exercise-based physical rehabilitation are subsets of physical activity. For the sake of simplicity, low participation rates to posttransplant exercise-based rehabilitation are acknowledged, but in the present manuscript not discussed as the primary focus. |
| Where does it occur | Physical activity can be performed as incidental physical activity (e.g., housework, transportation-related physical activity), occupation-related physical activity, or as activities performed for enjoyment or to improve or maintain physical and mental well-being. Physical activity can be performed everywhere: indoors, outdoors, at home, at work, in sports centres, at rehabilitation centres, etc. |
| Who is involved? | Transplant recipients’ family members, friends, peers, health care providers (e.g., general practitioners, transplant physicians, transplant nurses, care assistants, physiotherapists, psychologists, social assistants, occupational therapists, dieticians) as well as patient organisations and policy makers (e.g., national policy makers, middle and top management of transplant centres) may modulate patient’s engagement in physical activity. |
| Possible Target Behaviour | Impact of Behaviour Change | Likelihood of Change | Spillover Effect | Measurement |
|---|---|---|---|---|
| Reduction of sedentary behaviour | Mortality: + [58] CV health: + [59,60] Physical fitness: + [61] HRQOL: + [62] | ++ | + | ++ (e.g., accelerometers such as ActivPal and Actigraph) |
| Aerobic activity: 150 min at moderate intensity, 75 min at vigorous intensity, or an equivalent combination of both | Mortality: ++ [63,64] CV health: ++ [63,65,66] Physical fitness (cardiorespiratory fitness): ++ [18,65] HRQOL: + [65] | + | ++ | ++ (e.g., Heart monitor, training diary) |
| Muscle strengthening activity: ≥2×/week | Mortality: + [63,64] CV health: + [18,63] Physical fitness (muscle fitness): ++ [18,67] HRQOL: + [68] | + | ++ | ++ (e.g., training diary) |
| Participation in WHO recommended volume and intensity of aerobic, muscle strengthening, and multicomponent physical activities | Mortality: ++ [64] CV health: ++ [63] Physical fitness (cardiorespiratory, muscle, and motor fitness): ++ [18] HRQOL: + [18,60] | + | ++ | ++ (e.g., accelerometers, training diary) |
| Target Behaviour | Physical Activity Participation |
|---|---|
| Who | Solid organ transplant recipients |
| What | Any bodily movement produced by the skeletal muscles that requires increased energy expenditure above resting requirements and involves household tasks, leisure time activity, and structured physical activity (including exercise and exercise-based rehabilitation) [13]. Physical activity dimensions include frequency, intensity, time/duration, and type. |
| When | After solid organ transplantation:
|
| Where | Engagement in physical activity can be performed everywhere: at home, at work, indoors, outdoors, in sports and health care centres, etc. |
| How | A plethora of physical activity types exists. E.g.,
|
| How often | Any reduction in sedentary behaviour and any increase in physical activity is believed beneficial for patients’ health, though specific physical activity goals have been reported as well (cf. Table 2.) |
| With whom | Physical activity can be performed with:
|
3.2. Contextual Factors Implicated in Posttransplant Participation in Physical Activity: BCW Step 4
3.2.1. Geographical Context
Environmental Context and Resources (TDF)
3.2.2. Epidemiological Context
Sex, Age, and Transplant Type
COVID-19 Pandemic
Physical Skills and Limitations (TDF)
3.2.3. Socio-Cultural Context
Ethnicity and Culture
Knowledge (TDF)
Social Influences; Social/Professional Role, and Identity (TDF)
Goals; Intentions; Emotional/Behavioural Regulation (TDF)
Emotions; Beliefs about Capabilities; Beliefs about Consequences (TDF)
3.2.4. Socio-Economic Context
Education Level of Transplant Recipients
Environmental Context and Resources (TDF)
3.2.5. Ethical Context
3.2.6. Political Context
3.2.7. Legal Context
3.3. Physical Activity Intervention Development: BCW Steps 5–7
3.3.1. Geographical Context—Interventions
Environmental Context and Resources (TDF)
3.3.2. Epidemiological Context—Interventions
COVID-19 Pandemic
Physical Skills and Limitations (TDF)
3.3.3. Socio-Cultural Context—Interventions
Ethnicity and Culture
Knowledge (TDF)
Social Influences; Social—Professional Identity (TDF)
Goals; Intentions; Emotional/Behavioural Regulation (TDF)
Emotions; Beliefs about Capabilities; Beliefs about Consequences (TDF)
3.3.4. Socio-Economic Context—Interventions
Environmental Context and Recourses (TDF)
3.3.5. Ethical Context—Interventions
3.3.6. Political Context—Interventions
3.3.7. Legal Context—Interventions
4. Limitations
5. Conclusions and Take Home Messages
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Black, C.K.; Termanini, K.M.; Aguirre, O.; Hawksworth, J.S.; Sosin, M. Solid organ transplantation in the 21st century. Ann. Transl. Med. 2018, 6, 409. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Ackah, R.L.; Webb, G.J.; Halazun, K.J.; Vierling, J.M.; Liu, H.; Wu, M.F.; Yoeli, D.; Kueht, M.; Mindikoglu, A.L.; et al. No gains in long-term survival after liver transplantation over the past three decades. Ann. Surg. 2019, 269, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; van Stralen, M.M.; West, R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. 2011, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfadenhauer, L.M.; Gerhardus, A.; Mozygemba, K.; Lysdahl, K.B.; Booth, A.; Hofmann, B.; Wahlster, P.; Polus, S.; Burns, J.; Brereton, L.; et al. Making sense of complexity in context and implementation: The Context and Implementation of Complex Interventions (CICI) framework. Implement. Sci. 2017, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- West, R.; Michie, S. A brief introduction to the COM-B Model of behaviour and the PRIME Theory of motivation. Qeios 2020, WW04E6. [Google Scholar] [CrossRef]
- Atkins, L.; Francis, J.; Islam, R.; O’Connor, D.; Patey, A.; Ivers, N.; Foy, R.; Duncan, E.; Colquhoun, H.; Grimshaw, J.; et al. A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems. Implement. Sci. 2017, 12, 77. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Clarke, A.L.; Nixon, D.G.D.; Hull, K.L.; Song, Y.; Burton, J.O.; Yates, T.; Smith, A. Prevalence and correlates of physical activity across kidney disease stages: An observational multicentre study. Nephrol. Dial. Transplant. 2021, 36, 641–649. [Google Scholar] [CrossRef]
- Van Adrichem, E.J.; Dekker, R.; Krijnen, W.P.; Verschuuren, E.A.M.; Dijkstra, P.U.; van der Schans, C.P. Physical activity, sedentary time, and associated factors in recipients of solid-organ transplantation. Phys. Ther. 2018, 98, 646–657. [Google Scholar] [CrossRef]
- Masiero, L.; Puoti, F.; Bellis, L.; Lombardini, L.; Totti, V.; Angelini, M.L.; Spazzoli, A.; Nanni, C.A.; Cardillo, M.; Sella, G.; et al. Physical activity and renal function in the Italian kidney transplant population. Ren. Fail. 2020, 42, 1192–1204. [Google Scholar] [CrossRef]
- Kallwitz, E.R.; Loy, V.; Mettu, P.; van Roenn, N.; Berkes, J.; Cotler, S.J. Physical activity and metabolic syndrome in liver transplant recipients. Liver Transplant. 2013, 19, 1125–1131. [Google Scholar] [CrossRef]
- Van Adrichem, E.J.; van de Zande, S.C.; Dekker, R.; Verschuuren, E.A.M.; Dijkstra, P.U.; van der Schans, C.P. Perceived barriers to and facilitators of physical activity in recipients of solid organ transplantation, a qualitative study. PLoS ONE 2016, 11, e0162725. [Google Scholar] [CrossRef] [Green Version]
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.; Altenburg, T.; Chinapaw, M.; Aminian, S.; et al. Sedentary Behavior Research Network (SBRN)—Terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [Green Version]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behavior; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Burra, P.; Burroughs, A.; Graziadei, I.; Pirenne, J.; Valdecasas, J.C.; Muiesan, P.; Samuel, D.; Forns, X. EASL Clinical Practice Guidelines: Liver transplantation. J. Hepatol. 2016, 64, 433–485. [Google Scholar]
- Costanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef]
- Wickerson, L.; Rozenberg, D.; Janaudis-Ferreira, T.; Deliva, R.; Lo, V.; Beauchamp, G.; Helm, D.; Gottesman, C.; Mendes, P.; Vieira, L.; et al. Physical rehabilitation for lung transplant candidates and recipients: An evidence-informed clinical approach. World J. Transplant. 2016, 6, 517. [Google Scholar] [CrossRef]
- Yamagata, K.; Hoshino, J.; Sugiyama, H.; Hanafusa, N.; Shibagaki, Y.; Komatsu, Y.; Konta, T.; Fujii, N.; Kanda, E.; Sofue, T.; et al. Clinical practice guideline for renal rehabilitation: Systematic reviews and recommendations of exercise therapies in patients with kidney diseases. Ren. Replace. Ther. 2019, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Janaudis-Ferreira, T.; Mathur, S.; Deliva, R.; Howes, N.; Patterson, C.; Räkel, A.; So, S.; Wickerson, L.; White, M.; Avitzur, Y.; et al. Exercise for solid organ transplant candidates and recipients: A joint position statement of the Canadian society of transplantation and CAN-RESTORE. Transplantation 2019, 103, e220–e238. [Google Scholar] [CrossRef]
- Berben, L.; Engberg, S.J.; Rossmeissl, A.; Gordon, E.J.; Kugler, C.; Schmidt-Trucksäss, A.; Klem, M.; Sereika, S.; De Simone, P.; Dobbels, F.; et al. Correlates and outcomes of low physical activity posttransplant: A systematic review and meta-analysis. Transplantation 2019, 103, 679–688. [Google Scholar] [CrossRef]
- Takahashi, A.; Hu, S.L.; Bostom, A. Physical activity in kidney transplant recipients: A Review. Am. J. Kidney Dis. 2018, 72, 433–443. [Google Scholar] [CrossRef]
- Jakovljevic, D.G.; McDiarmid, A.; Hallsworth, K.; Seferovic, P.M.; Ninkovic, V.M.; Parry, G.; Schueler, S.; Trenell, M.; Macgowan, G. Effect of left ventricular assist device implantation and heart transplantation on habitual physical activity and quality of life. Am. J. Cardiol. 2014, 114, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Langer, D.; Gosselink, R.; Pitta, F.; Burtin, C.; Verleden, G.; Dupont, L.; Decramer, M.; Troosters, T. Physical activity in daily life 1 year after lung transplantation. J. Heart Lung Transplant. 2009, 28, 572–578. [Google Scholar] [CrossRef]
- Walsh, J.R.; Chambers, D.C.; Yerkovich, S.T.; Hopkins, P.M.A.; Morris, N.R. Low levels of physical activity predict worse survival to lung transplantation and poor early post-operative outcomes. J. Heart Lung Transplant. 2016, 35, 1041–1043. [Google Scholar] [CrossRef] [Green Version]
- Ney, M.; Haykowsky, M.J.; Vandermeer, B.; Shah, A.; Ow, M.; Tandon, P. Systematic review: Pre- and post-operative prognostic value of cardiopulmonary exercise testing in liver transplant candidates. Aliment. Pharmacol. Ther. 2016, 44, 796–806. [Google Scholar] [CrossRef]
- Kamo, N.; Kaido, T.; Hamaguchi, Y.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yao, S.; Yagi, S.; Uemoto, S. Impact of sarcopenic obesity on outcomes in patients undergoing living donor liver transplantation. Clin. Nutr. 2019, 38, 2202–2209. [Google Scholar] [CrossRef]
- Kaido, T.; Tamai, Y.; Hamaguchi, Y.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yagi, S.; Kamo, N.; Hammad, A.; Inagaki, N.; et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017, 33, 195–198. [Google Scholar] [CrossRef]
- Rosas, S.E.; Reese, P.P.; Huan, Y.; Doria, C.; Cochetti, P.T.; Doyle, A. Pretransplant physical activity predicts all-cause mortality in kidney transplant recipients. Am. J. Nephrol. 2012, 35, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Zelle, D.M.; Corpeleijn, E.; Stolk, R.P.; de Greef, M.H.G.; Gans, R.O.B.; van der Heide, J.J.H.; Navis, G.; Bakker, S. Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Jardine, A.G.; Gaston, R.S.; Fellstrom, B.C.; Holdaas, H. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 2011, 378, 1419–1427. [Google Scholar] [CrossRef]
- D’Avola, D.; Cuervas-Mons, V.; Martí, J.; Ortiz de Urbina, J.; Lladó, L.; Jimenez, C.; Otero, E.; Suarez, F.; Rodrigo, J.; Gómez, M.A.; et al. Cardiovascular morbidity and mortality after liver transplantation: The protective role of mycophenolate mofetil. Liver Transplant. 2017, 23, 498–509. [Google Scholar] [CrossRef]
- Fussner, L.A.; Heimbach, J.K.; Fan, C.; Dierkhising, R.; Coss, E.; Leise, M.D.; Watt, K. Cardiovascular disease after liver transplantation: When, what, and who is at risk. Liver Transplant. 2015, 21, 889–896. [Google Scholar] [CrossRef]
- Galindo, R.J.; Wallia, A. Hyperglycemia and Diabetes Mellitus Following Organ Transplantation. Curr. Diabetes Rep. 2016, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Byambasukh, O.; Osté, M.C.J.; Gomes-neto, A.W.; van den Berg, E.; Navis, G.; Bakker, S.J.L.; Corpeleijn, E. Physical activity and the development of post-transplant diabetes mellitus, and cardiovascular- and all-cause mortality in renal transplant recipients. J. Clin. Med. 2020, 9, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zelle, D.M.; Klaassen, G.; van Adrichem, E.; Bakker, S.J.L.; Corpeleijn, E.; Navis, G. Physical inactivity: A risk factor and target for intervention in renal care. Nat. Rev. Nephrol. 2017, 13, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar]
- Armstrong, K.; Rakhit, D.; Jeffriess, L.; Johnson, D.; Leano, R.; Prins, J.; Garske, L.; Marwick, T.; Isbel, N. Cardiorespiratory fitness is related to physical inactivity, metabolic risk factors, and atherosclerotic burden in glucose-intolerant renal transplant recipients. Clin. J. Am. Soc. Nephrol. 2006, 1, 1275–1283. [Google Scholar] [CrossRef]
- Engeseth, K.; Prestgaard, E.E.; Mariampillai, J.E.; Grundvold, I.; Liestol, K.; Kjeldsen, S.E.; Bodegard, J.; Erikssen, J.; Gjesdal, K.; Skretteberg, P. Physical fitness is a modifiable predictor of early cardiovascular death: A 35-year follow-up study of 2014 healthy middle-aged men. Eur. J. Prev. Cardiol. 2018, 25, 1655–1663. [Google Scholar] [CrossRef]
- Martinez-Gomez, D.; Lavie, C.J.; Hamer, M.; Cabanas-Sanchez, V.; Garcia-Esquinas, E.; Pareja-Galeano, H.; Struijk, E.; Sadarangani, K.; Ortega, F.; Rodriguez-Artalejo, F. Physical activity without weight loss reduces the development of cardiovascular disease risk factors—A prospective cohort study of more than one hundred thousand adults. Prog. Cardiovasc. Dis. 2019, 62, 522–530. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Park, M. Post-transplant cardiovascular disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 1878–1889. [Google Scholar] [CrossRef]
- Silverborn, M.; Jeppsson, A.; Mårtensson, G.; Nilsson, F. New-onset cardiovascular risk factors in lung transplant recipients. J. Heart Lung Transplant. 2005, 24, 1536–1543. [Google Scholar] [CrossRef]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Yucel, R.; Conti, D.; Siminoff, L. Longitudinal analysis of physical activity, fluid intake, and graft function among kidney transplant recipients. Transpl. Int. 2009, 22, 990–998. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.; Cohen, D. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Penedo, F.J.; Dahn, J.R.; Williams, L. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 2005, 18, 189–193. [Google Scholar] [CrossRef]
- Netz, Y.; Wu, M.J.; Becker, B.J.; Tenenbaum, G. Physical activity and psychological well-being in advanced age: A meta-analysis of intervention studies. Psychol. Aging 2005, 20, 272–284. [Google Scholar] [CrossRef] [Green Version]
- Eddolls, W.T.B.; McNarry, M.A.; Lester, L.; Winn, C.O.N.; Stratton, G.; Mackintosh, K.A. The association between physical activity, fitness and body mass index on mental well-being and quality of life in adolescents. Qual. Life Res. 2018, 27, 2313–2320. [Google Scholar] [CrossRef]
- Van den Berg-Emons, R.J.G.; van Ginneken, B.T.J.; Nooijen, C.F.J.; Metselaar, H.J.; Tilanus, H.W.; Kazemier, G.; Stam, H. Fatigue after liver transplantation: Effects of a rehabilitation program including exercise training and physical activity counseling. Phys. Ther. 2014, 94, 857–865. [Google Scholar] [CrossRef]
- Neale, J.; Smith, A.C.; Bishop, N.C. Effects of exercise and sport in solid organ transplant recipients: A review. Am. J. Phys. Med. Rehabil. 2017, 96, 273–288. [Google Scholar] [CrossRef] [Green Version]
- Kastelz, A.; Fernhall, B.; Wang, E.; Tzvetanov, I.; Spaggiari, M.; Shetty, A.; Gallon, L.; Hachaj, G.; Kaplan, B.; Benedetti, E. Personalized physical rehabilitation program and employment in kidney transplant recipients: A randomized trial. Transpl. Int. 2021, 34, 1083–1092. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, Y.R.; Han, H.S.; Yoon, Y.S.; Cho, J.Y.; Kim, S.; Kim, K.; Hyun, I.; Shehta, A. Fatigue and weakness hinder patient social reintegration after liver transplantation. Clin. Mol. Hepatol. 2018, 24, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Aberg, F. From prolonging life to prolonging working life: Tackling unemployment among liver-transplant recipients. World J. Gastroenterol. 2016, 22, 3701–3711. [Google Scholar] [CrossRef]
- Ochman, M.; Latos, M.; Orzeł, G.; Pałka, P.; Urlik, M.; Nęcki, M.; Stacel, T.; Zembala, M. Employment after lung transplantation in Poland—A single center study. Int. J. Occup. Med. Environ. Health 2019, 32, 379–386. [Google Scholar] [CrossRef]
- Raiz, L.; Monroe, J. Employment post-transplant: A biopsychosocial analysis. Soc. Work Health Care 2007, 45, 19–37. [Google Scholar] [CrossRef]
- Wiltshire, G.; Clarke, N.J.; Phoenix, C.; Bescoby, C. Organ transplant recipients’ experiences of physical activity: Health, self-Care, and transliminality. Qual. Health Res. 2021, 31, 385–398. [Google Scholar] [CrossRef]
- Dogra, S.; Copeland, J.L.; Altenburg, T.M.; Heyland, D.K.; Owen, N.; Dunstan, D.W. Start with reducing sedentary behavior: A stepwise approach to physical activity counseling in clinical practice. Patient Educ. Couns. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Zhao, R.; Bu, W.; Chen, Y.; Chen, X. The dose-response associations of sedentary time with chronic diseases and the risk for all-cause mortality affected by different health status: A systematic review and meta-analysis. J. Nutr. Health Aging 2020, 24, 63–70. [Google Scholar] [CrossRef]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults a systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef]
- Tarp, J.; Fagerland, M.W.; Dalene, K.E.; Johannessen, J.S.; Hansen, B.H.; Jefferis, B.J.; Whincup, P.; Diaz, K.; Hooker, S.; Hooker, S.; et al. Device-measured physical activity, adiposity and mortality: A harmonised meta-analysis of eight prospective cohort studies. Br. J. Sports Med. 2021, 104827, Online ahead of print. [Google Scholar] [CrossRef]
- Bellizzi, V.; Cupisti, A.; Capitanini, A.; Calella, P.; D’Alessandro, C. Physical activity and renal transplantation. Kidney Blood Press. Res. 2014, 39, 212–219. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Lyden, K.; Boucher, R.; Wei, G.; Zhou, N.; Christensen, J.; Chertow, G.M.; Beddhu, S.; Greene, T. Targeting sedentary behavior in ckd a pilot and feasibility randomized controlled trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 717–726. [Google Scholar] [CrossRef]
- Raymond, J.; Johnson, S.T.; Diehl-Jones, W.; Vallance, J.K. Walking, sedentary time and health-related quality life among kidney transplant recipients: An exploratory study. Transplant. Proc. 2016, 48, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.L.; Rezende, L.F.M.; Lee, D.H. Muscle-strengthening activities and risk of cardiovascular disease, type 2 diabetes, cancer and mortality: A review of prospective cohort studies. J. Intern. Med. 2021, 290, 789–805. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, C.A.; Stommel, M. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am. J. Prev. Med. 2011, 40, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lv, A.; Wang, J.; Xu, N.; Ma, G.; Zhai, Z.; Zhang, B.; Gao, J.; Ni, C. Exercise training and outcomes in hemodialysis patients: Systematic review and meta-analysis. Am. J. Nephrol. 2019, 50, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitao, C.; Gouveia, M.; Figueiredo, D.; Alves, A.; Polonia, J.; Oliveira, J.; et al. Exercise training reduces arterial stiffness in adults with hypertension: A systematic reviewand meta-analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hotta, K.; Ota, E.; Mori, R.; Matsunaga, A. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: A meta-analysis. J. Cardiol. 2016, 68, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, C.; Karahalios, A.; Neil, C.; Allen, J.; Levinger, I. The effects of resistance training on muscle strength, quality of life and aerobic capacity in patients with chronic heart failure—A meta-analysis. Int. J. Cardiol. 2017, 227, 413–423. [Google Scholar] [CrossRef]
- Sánchez, Z.V.; Cashion, A.K.; Cowan, P.A.; Jacob, S.R.; Wicks, M.N.; Velasquez-Mieyer, P. Perceived barriers and facilitators to physical activity in kidney transplant recipients. Prog. Transplant. 2007, 17, 324–331. [Google Scholar] [CrossRef]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.P.; Sehgal, A.R.; Strogatz, D.; Conti, D.; Siminoff, L. Prevalence and determinants of physical activity and fluid intake in kidney transplant recipients. Clin. Transplant. 2010, 24, E69–E81. [Google Scholar] [CrossRef] [Green Version]
- Spillman, L.N.; Melville-Claxton, A.; Gatiss, G.A.; Fernandez, N.; Madden, A.M. Diet and physical activity after liver transplant: A qualitative study of barriers and facilitators to following advice. J. Hum. Nutr. Diet. 2021, 34, 910–919. [Google Scholar] [CrossRef]
- Gustaw, T.; Schoo, E.; Barbalinardo, C.; Rodrigues, N.; Zameni, Y.; Motta, V.N.; Mathur, S.; Janaudis-Ferreira, T. Physical activity in solid organ transplant recipients: Participation, predictors, barriers, and facilitators. Clin. Transplant. 2017, 31, e12929. [Google Scholar] [CrossRef]
- Wietlisbach, M.; Benden, C.; Koutsokera, A.; Jahn, K.; Soccal, P.M.; Radtke, T. Perceptions towards physical activity in adult lung transplant recipients with cystic fibrosis. PLoS ONE 2020, 15, e0229296. [Google Scholar] [CrossRef]
- Billany, R.E.; Smith, A.C.; Stevinson, C.; Clarke, A.L.; Graham-Brown, M.P.M.; Bishop, N.C. Perceived barriers and facilitators to exercise in kidney transplant recipients: A qualitative study. Health Expect. 2022, 25, 764–774. [Google Scholar] [CrossRef]
- Zelle, D.M.; Corpeleijn, E.; Klaassen, G.; Schutte, E.; Navis, G.; Bakker, S.J.L. Fear of movement and low self-efficacy are important barriers in physical activity after renal transplantation. PLoS ONE 2016, 11, e0147609. [Google Scholar] [CrossRef]
- Wesolowska-Gorniak, K.; Wojtowicz, M.; Gierus, J.; Czarkowska-Paczek, B. The correlation of patients’ anxiety after a liver or kidney transplantation with functional and self-reported work ability. Medicine 2020, 99, e20108. [Google Scholar] [CrossRef]
- Mathur, S.; Janaudis-Ferreira, T.; Hemphill, J.; Cafazzo, J.A.; Hart, D.; Holdsworth, S.; Lovas, M.; Wickerson, L. User-centered design features for digital health applications to support physical activity behaviors in solid organ transplant recipients: A qualitative study. Clin. Transplant. 2021, 35, e14472. [Google Scholar] [CrossRef]
- Segatto, B.L.; Sabiston, C.M.; Harvey, W.J.; Bloom, G.A. Exploring relationships among distress, psychological growth, motivation, and physical activity among transplant recipients. Disabil. Rehabil. 2013, 35, 2097–2103. [Google Scholar] [CrossRef]
- Gordon, E.J.; Prohaska, T.R.; Gallant, M.; Siminoff, L.A. Self-care strategies and barriers among kidney transplant recipients: A qualitative study. Chronic Illn. 2009, 5, 75–91. [Google Scholar] [CrossRef] [Green Version]
- Schoo, E.; Gustaw, T.; Barbalinardo, C.; Rodrigues, N.; Zameni, Y.; Mathur, S.; Janaudis-Ferreira, T. Solid organ transplant recipients’ opinions of pre- and post-transplant supervised exercise programmes: A brief report. Physiother. Can. 2017, 69, 178–183. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, C.; Tansey, C.M.; Fontaine, S.; Baker, S.; Laberge, É.; Mathur, S.; Lambert, H.; Janaudis-Ferreira, T. Community-based exercise program for solid organ transplant recipients: Views of exercise professionals and patients. McGill J. Med. 2021, 19. [Google Scholar] [CrossRef]
- Tığlı, A.; Soy, E.H.A.; Aytar, A.; Moray, G.; Haberal, M. Relationship between exercise perception with physical activity level, body awareness, and illness cognition in renal transplant patients: A pilot study. Exp. Clin. Transplant. 2019, 17, 270–276. [Google Scholar] [CrossRef]
- Jeng, C.; Rn, D.; Chu, F.-L. Empowering: The experiences of exercise among heart transplantation patients in Taiwan. J. Adv. Nurs. 2002, 40, 560–567. [Google Scholar] [CrossRef]
- D’Ambrosio, A.; Toulouse, C.; Bélanger-Marceau, S.; Savary, S.; Mathur, S.; Segatto, B.; Hartell, D.; Janaudis-Ferreira, T. Characteristics and motivation of solid organ transplant recipients attending the canadian transplant games. Transplant. Proc. 2021, 53, 581–589. [Google Scholar] [CrossRef]
- Suaya, J.A.; Shepard, D.S.; Normand, S.L.T.; Ades, P.A.; Prottas, J.; Stason, W.B. Use of cardiac rehabilitation by medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation 2007, 116, 1653–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, H.; Ramos, C.; Grantham, S.C. Drivers of racial and ethnic disparities in cardiac rehabilitation use: Patient and provider perspectives. Med. Care Res. Rev. 2016, 73, 251–282. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasegaram, S.; Oh, P.; Reid, R.D.; McCumber, T.; Grace, S.L. Cardiac rehabilitation barriers by rurality and socioeconomic status: A cross-sectional study. Int. J. Equity Health 2013, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathews, L.; Brewer, L.C. A review of disparities in cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2021, 41, 375–382. [Google Scholar] [CrossRef]
- Trojetto, T.; Elliott, R.J.; Rashid, S.; Wong, S.; Dlugosz, K.; Helm, D.; Wickerson, L.; Brooks, D. Availability, characteristics, and barriers of rehabilitation programs in organ transplant populations across Canada. Clin. Transplant. 2011, 25, 571–578. [Google Scholar] [CrossRef]
- Gebel, K.; Bauman, A.E.; Petticrew, M. The physical environment and physical activity. A critical appraisal of review articles. Am. J. Prev. Med. 2007, 32, 361–369. [Google Scholar] [CrossRef]
- Sallis, J.F.; Cerin, E.; Conway, T.L.; Adams, M.A.; Frank, L.D.; Pratt, M.; Salvo, D.; Schipperijn, J.; Smith, G.; Cain, K.; et al. Physical activity in relation to urban environments in 14 cities worldwide: A cross-sectional study. Lancet 2016, 387, 2207–2217. [Google Scholar] [CrossRef] [Green Version]
- Koohsari, M.J.; Sugiyama, T.; Sahlqvist, S.; Mavoa, S.; Hadgraft, N.; Owen, N. Neighborhood environmental attributes and adults’ sedentary behaviors: Review and research agenda. Prev. Med. 2015, 77, 141–149. [Google Scholar] [CrossRef]
- Puoti, F.; Ricci, A.; Nanni-Costa, A.; Ricciardi, W.; Malorni, W.; Ortona, E. Organ transplantation and gender differences: A paradigmatic example of intertwining between biological and sociocultural determinants. Biol. Sex Differ. 2016, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Bennie, J.A.; de Cocker, K.; Tittlbach, S. The epidemiology of muscle-strengthening and aerobic physical activity guideline adherence among 24, 016 German adults. Scand. J. Med. Sci. Sports 2021, 31, 1096–1104. [Google Scholar] [CrossRef]
- Bennie, J.A.; Teychenne, M.J.; de Cocker, K.; Biddle, S.J.H. Associations between aerobic and muscle-strengthening exercise with depressive symptom severity among 17,839 U.S. adults. Prev. Med. 2019, 121, 121–127. [Google Scholar] [CrossRef]
- Stockwell, S.; Trott, M.; Tully, M.; Shin, J.; Barnett, Y.; Butler, L.; McDermott, D.; Schuch, F.; Smith, L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: A systematic review. BMJ Open Sport Exerc. Med. 2021, 7, e000960. [Google Scholar] [CrossRef]
- Farah, B.Q.; do Prado, W.L.; Malik, N.; Lofrano-Prado, M.C.; de Melo, P.H.; Botero, J.P.; Cucato, G.; de Almeida Correia, M.; Ritti-Dias, R. Barriers to physical activity during the COVID-19 pandemic in adults: A cross-sectional study. Sport Sci. Health 2021, 17, 441–447. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O. COVID-19 vaccination in kidney transplant recipients. Nat. Rev. Nephrol. 2021, 17, 785–787. [Google Scholar] [CrossRef]
- Weber, S.; Rek, S.; Eser-Valeri, D.; Padberg, F.; Reiter, F.P.; de Toni, E.; Hohenester, S.; Zimny, S.; Rehm, M.; Guba, M.; et al. The psychosocial burden on liver transplant recipients during the COVID-19 pandemic. Visc. Med. 2021, 37, 542–549. [Google Scholar] [CrossRef]
- Mayes, J.; Castle, E.M.; Greenwood, J.; Ormandy, P.; Howe, P.D.; Greenwood, S.A. Cultural influences on physical activity and exercise beliefs in patients with chronic kidney disease: ‘The Culture-CKD Study’—A qualitative study. BMJ Open 2022, 12, e046950. [Google Scholar] [CrossRef]
- Mabry, R.M.; Al-Busaidi, Z.Q.; Reeves, M.M.; Owen, N.; Eakin, E.G. Addressing physical inactivity in Omani adults: Perceptions of public health managers. Public Health Nutr. 2014, 17, 674–681. [Google Scholar] [CrossRef] [Green Version]
- Aljayyousi, G.F.; Abu Munshar, M.; Al-Salim, F.; Osman, E.R. Addressing context to understand physical activity among Muslim university students: The role of gender, family, and culture. BMC Public Health 2019, 19, 1452. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, I.N. Socio-cultural barriers to attaining recommended levels of physical activity among gemales: A review of literature. Quest 2014, 66, 448–467. [Google Scholar] [CrossRef]
- NHS Blood and Transplant. Organ Donation and Transplantation Data for Black, Asian and Minority Ethnic (BamE) Communities: Report for 2018/2019 (1 April 2013–31 March 2018). 2019. Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/17496/organ-donation-and-transplantation-bame-activity-report-2018-2019.pdf (accessed on 20 March 2022).
- Persson, G.; Brorsson, A.; Ekvall Hansson, E.; Troein, M.; Strandberg, E.L. Physical activity on prescription (PAP) from the general practitioner’s perspective—A qualitative study. BMC Fam. Pract. 2013, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Wee, C.C.; McCarthy, E.P.; Davis, R.B.; Phillips, R.S. Physician counseling about exercise. J. Am. Med. Assoc. 1999, 282, 1583–1588. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, M.F.; Meeuwisse, W.H. Exercise counselling by family physicians in Canada. Prev. Med. 2003, 37, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Graham, R.C.; Dugdill, L.; Cable, N.T. Health professionals’ perspectives in exercise referral: Implications for the referral process. Ergonomics 2005, 48, 1411–1422. [Google Scholar] [CrossRef]
- Albert, F.A.; Crowe, M.J.; Malau-Aduli, A.E.O.; Malau-Aduli, B.S. Physical activity promotion: A systematic review of the perceptions of healthcare professionals. Int. J. Environ. Res. Public Health 2020, 17, 4358. [Google Scholar] [CrossRef]
- Regolisti, G.; Maggiore, U.; Sabatino, A.; Gandolfini, I.; Pioli, S.; Torino, C.; Aucella, F.; Cupisti, A.; Pistolesi, V.; Capitanini, A.; et al. Interaction of healthcare staff’s attitude with barriers to physical activity in hemodialysis patients: A quantitative assessment. PLoS ONE 2018, 13, e0196313. [Google Scholar]
- Delgado, C.; Johansen, K.L. Deficient counseling on physical activity among nephrologists. Nephron Clin. Pract. 2010, 116, c330–c336. [Google Scholar] [CrossRef]
- Elwell, L.; Povey, R.; Grogan, S.; Allen, C.; Prestwich, A. Patients’ and practitioners’ views on health behaviour change: A qualitative study. Psychol. Health 2013, 28, 653–674. [Google Scholar] [CrossRef]
- Pang, A.; Lingham, S.; Zhao, W.; Leduc, S.; Räkel, A.; Sapir-Pichhadze, R.; Mathur, S.; Janaudis-Ferreira, T. Physician practice patterns and barriers to counselling on physical activity in solid organ transplant recipients. Ann. Transplant. 2018, 23, 345–359. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, G.M.; Donaghue, N.; Walker, I.; Wood, C.A. Deservingness and gratitude in the context of heart transplantation. Qual. Health Res. 2014, 24, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, G.; Kennedy, A.; Puggina, A.; Aleksovska, K.; Buck, C.; Burns, C.; Cardon, G.; Carlin, A.; Ciarapica, D.; Colotto, M.; et al. Socio-economic determinants of physical activity across the life course: A “DEterminants of DIet and Physical ACtivity” (DEDIPAC) umbrella literature review. PLoS ONE 2018, 13, e0190737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Mei, S.; van Sonderen, E.; van Son, W.; de Jong, P.; Groothoff, J.W.; van den Heuvel, W.J.A. Social participation after successful kidney transplantation. Disabil. Rehabil. 2007, 29, 473–483. [Google Scholar] [CrossRef] [Green Version]
- De Baere, C.; Delva, D.; Kloeck, A.; Remans, K.; Vanrenterghem, Y.; Verleden, G.; Vanhaecke, J.; Nevens, F.; Dobbels, F. Return to work and social participation: Does type of organ transplantation matter? Transplantation 2010, 89, 1009–1015. [Google Scholar] [CrossRef]
- Danuser, B.; Simcox, A.; Studer, R.; Koller, M.; Wild, P. Employment 12 months after kidney transplantation: An in-depth bio-psycho-social analysis of the Swiss Transplant Cohort. PLoS ONE 2017, 12, e0175161. [Google Scholar] [CrossRef]
- Siminoff, L.A.; Chillag, K. The fallacy of the “gift of life.” The Hastings Center Report. JSTOR 1999, 29, 34. [Google Scholar]
- Lauritzen, P.; McClure, M.; Smith, M.L.; Trew, A. The gift of life and the common good: The need for a communal approach to organ procurement. Hastings Cent. Rep. 2001, 31, 29–35. [Google Scholar] [CrossRef]
- Rütten, A.; Abu-Omar, K.; Gelius, P.; Schow, D. Physical inactivity as a policy problem: Applying a concept from policy analysis to a public health issue. Health Res. Policy Syst. 2013, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.F.; Winsett, R.P.; Gaber, A.O.; Hathaway, D.K. Cost-effectiveness of post-transplantation quality of life intervention among kidney recipients. Clin. Transplant. 2004, 18, 407–414. [Google Scholar] [CrossRef]
- Briffa, T.G.; Eckermann, S.D.; Griffiths, A.D.; Keech, A.C.; Harris, P.J.; Heath, M.R.; Freedman, S.; Donaldson, L.; Briffa, N. Cost-effectiveness of rehabilitation after an acute coronary event: A randomised controlled trial. Med. J. Aust. 2005, 183, 450–455. [Google Scholar] [CrossRef]
- Tam, A.; Mac, S.; Isaranuwatchai, W.; Bayley, M. Cost-effectiveness of a high-intensity rapid access outpatient stroke rehabilitation program. Int. J. Rehabil. Res. 2019, 42, 56–62. [Google Scholar] [CrossRef]
- IJsbrandy, C.; van Harten, W.H.; Gerritsen, W.R.; Hermens, R.P.M.G.; Ottevanger, P.B. Healthcare professionals’ perspectives of barriers and facilitators in implementing physical activity programmes delivered to cancer survivors in a shared-care model: A qualitative study. Supportive Care Cancer 2020, 28, 3429–3440. [Google Scholar] [CrossRef] [Green Version]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.; Cane, J.; Wood, C. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Howlett, N.; Trivedi, D.; Troop, N.A.; Chater, A.M. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl. Behav. Med. 2019, 9, 147–157. [Google Scholar] [CrossRef]
- Samdal, G.B.; Eide, G.E.; Barth, T.; Williams, G.; Meland, E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.M.; Brennan, S.F.; French, D.P.; Patterson, C.C.; Kee, F.; Hunter, R.F. Effectiveness of physical activity interventions in achieving behaviour change maintenance in young and middle aged adults: A systematic review and meta-analysis. Soc. Sci. Med. 2017, 192, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Kwasnicka, D.; Dombrowski, S.U.; White, M.; Sniehotta, F. Theoretical explanations for maintenance of behaviour change: A systematic review of behaviour theories. Health Psychol. Rev. 2016, 10, 277–296. [Google Scholar] [CrossRef]
- Richardson, C.R.; Franklin, B.; Moy, M.L.; Jackson, E.A. Advances in rehabilitation for chronic diseases: Improving health outcomes and function. BMJ 2019, 365, 12191. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.J.; Scharloo, M.; Abbink, J.J.; Thijs-Van Nies, A.; Rudolphus, A.; Snoei, L.; Weinman, J.; Kaptein, A. Participation and drop-out in pulmonary rehabilitation: A qualitative analysis of the patient’s perspective. Clin. Rehabil. 2007, 21, 212–221. [Google Scholar] [CrossRef]
- Beatty, A.L.; Fukuoka, Y.; Whooley, M.A. Using mobile technology for cardiac rehabilitation: A review and framework for development and evaluation. J. Am. Heart Assoc. 2013, 2, e000568. [Google Scholar] [CrossRef]
- Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2018.
- Wickerson, L.; Helm, D.; Gottesman, C.; Rozenberg, D.; Singer, L.G.; Keshavjee, S.; Sidhu, A. Telerehabilitation for lung transplant candidates and recipients during the COVID-19 pandemic: Program evaluation. JMIR Mhealth Uhealth 2021, 9, e28708. [Google Scholar] [CrossRef]
- Thorsen, I.K.; Kayser, L.; Teglgaard Lyk–Jensen, H.; Rossen, S.; Ried-Larsen, M.; Midtgaard, J. “I tried forcing myself to do It, but then it becomes a boring chore”: Understanding (dis)engagement in physical activity among individuals with type 2 diabetes using a practice theory approach. Qual. Health Res. 2021, 32, 520–530. [Google Scholar] [CrossRef]
- Costello, E.; Kafchinski, M.; Vrazel, J.; Sullivan, P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J. Geriatr. Phys. Ther. 2011, 34, 138–147. [Google Scholar] [CrossRef]
- Holley, U.A. Social isolation: A practical guide for nurses assisting clients with chronic illness. Rehabil. Nurs. 2007, 32, 51–56. [Google Scholar] [CrossRef]
- Cappelle, M.; Masschelein, E.; de Smet, S.; Vos, R.; Vanbekbergen, J.; Gryp, S.; Van Craenenbroeck, A.; Cornelissen, V.; Verreydt, J.; Van Belleghem, Y.; et al. Transplantoux. Beyond the successful climb of Mont Ventoux. Beyond road to sustained physical activity in organ transplantation. Transplantation 2021, 105, 471–473. [Google Scholar] [CrossRef]
- Stamatakis, E.; Johnson, N.A.; Powell, L.; Hamer, M.; Rangul, V.; Holtermann, A. Short and sporadic bouts in the 2018 US physical activity guidelines: Is high-intensity incidental physical activity the new HIIT? Br. J. Sports Med. 2019, 53, 1137–1139. [Google Scholar] [CrossRef] [Green Version]
- Sanders, J.P.; Biddle, S.J.H.; Gokal, K.; Sherar, L.B.; Skrybant, M.; Parretti, H.M.; Ives, N.; Yates, T.; Mutrie, N.; Daley, A. ‘SnacktivityTM’ to increase physical activity: Time to try something different? Prev. Med. 2021, 153, 106851. [Google Scholar] [CrossRef]
- Jenkins, E.M.; Nairn, L.N.; Skelly, L.E.; Little, J.P.; Gibala, M.J. Do stair climbing exercise “snacks” improve cardiorespiratory fitness? Appl. Physiol. Nutr. Metab. 2019, 44, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Bodenheimer, T.; Handley, M.A. Goal-setting for behavior change in primary care: An exploration and status report. Patient Educ. Couns. 2009, 76, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Swann, C.; Jackman, P.C.; Lawrence, A.; Hawkins, R.M.; Goddard, S.G.; Williamson, O.; Schweickle, M.; Vella, S.; Rosenbaum, S.; Ekkebkakis, P. The (over)use of SMART goals for physical activity promotion: A narrative review and critique. Health Psychol. Rev. 2022, 1–16, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.A.; Latham, G.P. New Developments in Goal Setting and Task Performance; Routledge: Hove, UK; Taylor Fr.: New York, NY, USA, 2013. [Google Scholar]
- Greenwood, S.A.; Koufaki, P.; Mercer, T.H.; Rush, R.; O’Connor, E.; Tuffnell, R.; Lindup, H.; Haggis, L.; Dew, T.; Abdulnassir, L.; et al. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: A 12-week pilot randomized controlled trial (the Exercise in Renal Transplant [ExeRT] Trial). Am. J. Kidney Dis. 2015, 66, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Painter, P.L.; Hector, L.; Ray, K.; Lynes, L.; Paul, S.M.; Dodd, M.; Tomlanovich, S.; Ascher, N. Effects of exercise training on coronary heart disease risk factors in renal transplant recipients. Am. J. Kidney Dis. 2003, 42, 362–369. [Google Scholar] [CrossRef]
- Painter, P.L.; Hector, L.; Ray, K.; Lynes, L.; Dibble, S.; Paul, S.M.; Tomlanovich, S.; Ascher, N. A randomized trial of exercise training after renal transplantation. Transplantation 2002, 74, 42–48. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.; Corrado, D.; Drezner, J.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Armstrong, M.; Paternostro-Bayles, M.; Conroy, M.B.; Franklin, B.A.; Richardson, C.; Kriska, A. Preparticipation screening prior to physical activity in community lifestyle interventions. Transl. J. Am. Coll. Sports Med. 2018, 3, 176–180. [Google Scholar]
- Serper, M.; Barankay, I.; Chadha, S.; Shults, J.; Jones, L.S.; Olthoff, K.M.; Reese, P. A randomized, controlled, behavioral intervention to promote walking after abdominal organ transplantation: Results from the LIFT study. Transpl. Int. 2020, 33, 632–643. [Google Scholar] [CrossRef]
- Abu-Omar, K.; Rütten, A.; Burlacu, I.; Schätzlein, V.; Messing, S.; Suhrcke, M. The cost-effectiveness of physical activity interventions: A systematic review of reviews. Prev. Med. Rep. 2017, 8, 72–78. [Google Scholar] [CrossRef]
- Werbrouck, A.; Schmidt, M.; Putman, K.; Seghers, J.; Simoens, S.; Verhaeghe, N.; Annemans, L. Cost-effectiveness of exercise referral schemes: A systematic review of health economic studies. Eur. J. Public Health 2022, 32, 87–94. [Google Scholar] [CrossRef]
- Edwards, K.; Jones, N.; Newton, J.; Foster, C.; Judge, A.; Jackson, K.; Arden, N.; Pinedo-Villanueva, R. The cost-effectiveness of exercise-based cardiac rehabilitation: A systematic review of the characteristics and methodological quality of published literature. Health Econ. Rev. 2017, 7, 37. [Google Scholar] [CrossRef]
- De Smet, S.; van Craenenbroeck, A.H. Exercise training in patients after kidney transplantation. Clin. Kidney J. 2021, 14, ii15–ii24. [Google Scholar] [CrossRef]
- Clarke, A.L.; Jhamb, M.; Bennett, P.N. Barriers and facilitators for engagement and implementation of exercise in end-stage kidney disease: Future theory-based interventions using the Behavior Change Wheel. Semin. Dial. 2019, 32, 308–319. [Google Scholar] [CrossRef]
- Leppla, L.; Mielke, J.; Kunze, M.; Mauthner, O.; Teynor, A.; Valenta, S.; Vanhoof, J.; Dobbels, F.; Berben, L.; Zeiser, R.; et al. Clinicians and patients perspectives on follow-up care and eHealth support after allogeneic hematopoietic stem cell transplantation: A mixed-methods contextual analysis as part of the SMILe study. Eur. J. Oncol. Nurs. 2020, 45, 101723. [Google Scholar] [CrossRef]
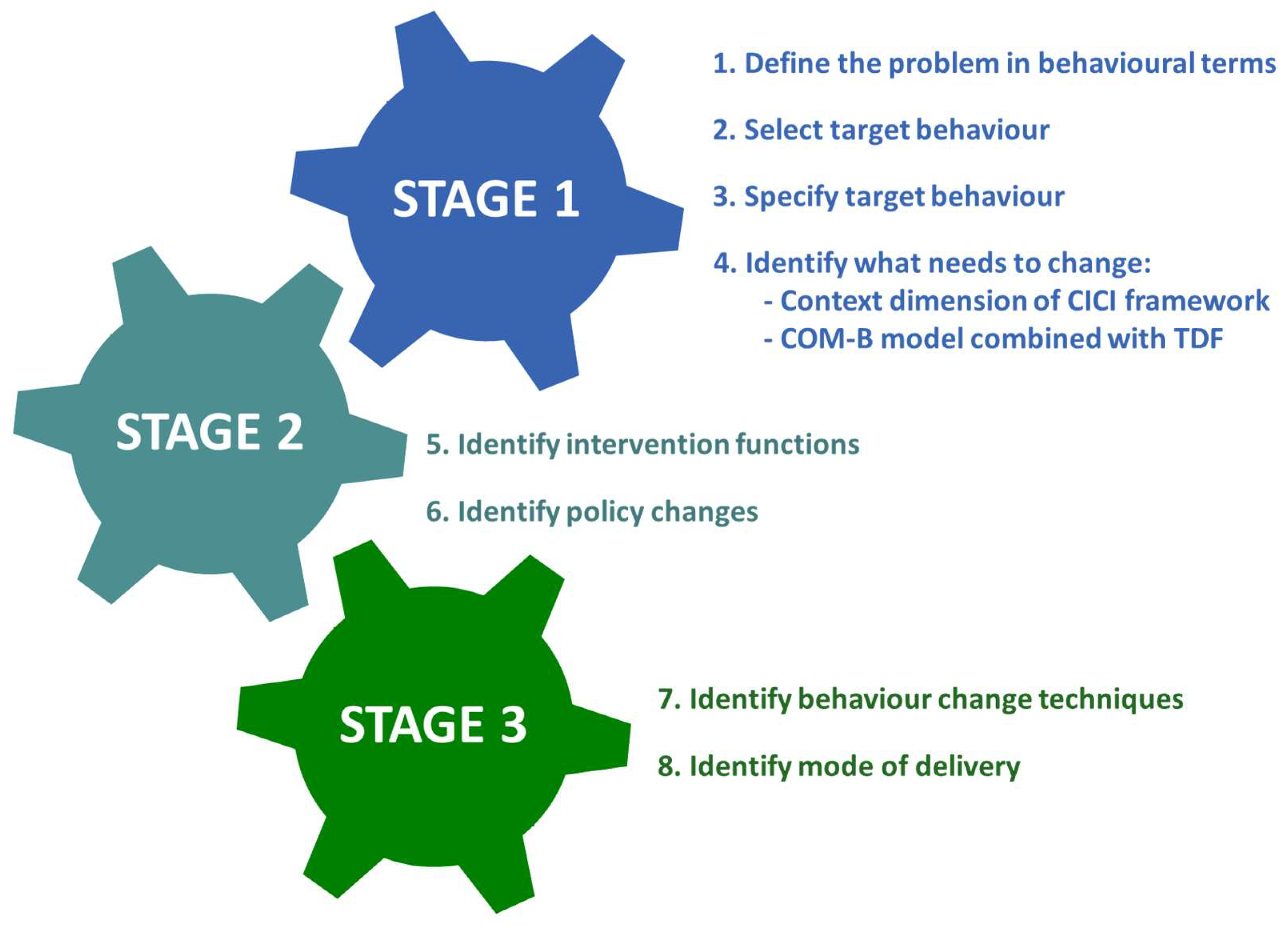
| COM-B | TDF | What Needs to Happen for Target Behaviour To Occur? | Barriers | Motivators |
|---|---|---|---|---|
| Physical Capability | Physical skills | Being physically able to be physically active | Evaluated in 12/19 records General health and symptoms [69,71,80] (K/Li/M) Kidney disease [69] (K) Comorbidities that interfere with physical activity [11,80,82] (K/M) Physical limitations in relation to transplantation [11,82] or slow recovery after transplantation [71] (K/Li/M) Feeling too fatigued or low energy levels [11,69,73,82,83] (K/Lu/H/M) Feeling too sick to exercise [79] (K) Physical pain [70,83] (K/H) Having open incision [79] (K) Shortness of breath [69,83] (K/H) Inadequate strength to perform activities [11,72] (M) Side effects of immunosuppressant’s [11,72] or medication [81] (M) Being overweight [11] (M) Avoiding direct sunlight due to higher risk of skin cancer when taking immunosuppressant’s [79] (K) Restrictions towards exercise (lifting and contact sports) [74] (K) | Evaluated in 11/19 records Perceiving feelings of health and benefits from physical activity [72,81,82] Feel healthier and generally better [71,74,81,83] (K/Li/H/M) Reduce specific health risks [74] (K) Increase energy [69,83] (K/H) Decrease pain [69] (K) Increase mobility [74] and muscle strength [11,69] (K/M) Manage weight [11,69,81] (K/M) Improve endurance [73] (Lu) Consequences of inactivity [11,82] (K/M) Longevity of the transplanted kidney [74] (K) Improvements in body and transplant conditions and feeling the transplant becoming stronger [83] (H) Recognition that PA is essential for prolonging their lives and maintain the quality of their health [77] (M) |
| Psychological Capability | Knowledge | Patients (and health care providers) require the knowledge about why, how, when, how often, and with who to be physically activity in a safe way | Evaluated in 9/19 records Lack of knowledge about the benefits of physical activity [11,69] (K/M) Lack of knowledge about appropriate exercise [74] or unsure how to exercise safely [81] (K/M) Health care providers’ lack of expertise, lack of medical clearance, lack of specific advice, and conflicting or vague advice [11,71,74,77,84] (K/Li/M) Health care providers not recommending or advising against physical activity [79,80] (K/M) Health care providers not providing answers on questions about exercise limitations [74] (K) Desire for (currently lacking) exercise guidelines [74] (K)Desire to three different types of guidance: (i) standardized guidance, (ii) prescriptive (individualized) guidance, and (iii) supervised guidance/sessions both individual and in group [74] (K) Exercise advice and guidance not priority of the National Health Service (UK) [74] (K) | Evaluated in 7/19 records Knowing the value and benefits of increased PA [69,79] (K) Having knowledge about PA [72] (M) Receiving information on how to exercise [69] (K) Expertise of personnel [11] (M) Physician recommendations to PA [72,80] (M) Individualized timely advice consistent across the multidisciplinary team [71] (Li) Accessible and comprehensive rehabilitation as a potential source for guidelines around proper exercise and transplant appropriate milestones [77] (M) |
| Emotion and behavioural regulation | Apply the knowledge about correct physical activity frequency, intensity, type, and duration. Development of coping strategies for barriers | Evaluated in 2/19 records A previous routine without physical activity [11,82] (K/M) Post-transplantation life events [11] (M) | Evaluated in 5/19 records Coping [11,82] (K/M) Physical activity as routine habit [11,79,82] (K/M) Exploration of new capabilities and refine their understanding of their trans liminal, transplanted body-self [54] (N) Self-determination [71] (Li) | |
| Memory, attention & decision processing | Notice and remember to be physically active during daily life | Evaluated in 1/19 records Not remembering to be physically active [79] (K) | Evaluated in 0/19 records | |
| Physical Opportunity | Environmental context and resources | Availability and accessibility of physical activity facilities and opportunities. Financial resources and insurances to be physically active | Evaluated in 8/19 records Lack of access to (safe) physical activity facilities [69,74,77,79,81] and a lack of opportunities to participate in a physical activity program [80] (K/M) Costs of physical activity [74,81] costs of fitness/rehabilitation centres [72,79,80], and limited financial resources [11,69] (K/M) Bad weather [11,69,74,79,81] (K/M) No private insurance [70,72] (K/M) No transportation to a gym [79] (K) Far distance from rehabilitation centre [80] (M) No place to sit down while exercising outside [69] (K) Poor sidewalks [69] (K) | Evaluated in 6/19 records Having financial resources [69] and having private insurance [70] (K) Proximity to an exercise facility [72] and environmental opportunity to be physically active [71] (Li/M) Outdoor activities (views and fresh air) and walking (preferred activity as it could be easily fitted into daily life) [74] (K) Exercise classes (structured and motivational) and individual exercise preferences (especially influencing continued exercise behaviour) [74] (K) Workable and constructive exercise program [83] Taking precautions when training outdoors and adjusting the way of exercise to fit themselves [83] (H) |
| Social Opportunity | Social influence | An encouraging and supporting social network | Evaluated in 4/19 records Lack of general encouragement, lack of support from family and friends [69], and lack of support from physicians [81] (K/M) Low expectations from family, friends, and health-care providers [69] (K) Negative social influence [71] (Li) Not knowing other kidney transplant recipients who are physically active [74] (K) Expectations of others that kidney transplant recipients should not exercise [74] (K) | Evaluated in 9/19 records Having support and encouragement from family, friends, peers (peer modeling), and others [11,69,71,72,79,81,83] (K/Li/H/M) Physical activities with others [69,79], friends/family [74], and in group [11,81] (K/M) Making new friends by physical activity [74] (K) Encouragement, support, and empathy from healthcare providers [69,71,83] (K/Li/H) High expectations from family, friends and healthcare providers [69] (K) Having a supportive exercise leader [69] (K) Exercising on the job [74,79] (K) Not wanting to let people down if they had planned to exercise together [74] (K) |
| Automatic motivation | Emotion | Positive emotions related to physical activity | Evaluated in 11/19 records General anxiety [73,82], anxiety about physical activity [69], and fear of movement [75] (K/Lu) Fear of damaging the transplanted organ [11,81,83], increasing pain or injury [69,81]; negative effects [69,81]; infection [81]; rejection [73]; making health worse [69], and falling [69] (K/Lu/H/M) General fear of activities outdoors [83], or fear of outdoor activities due to fear for crime [69] or fear of being affected by a certain disease [83] (K/H) Depression [69] and low health-related quality of life [72] (K/M) Heightened sense of self-awareness during exercise and heightened awareness of normal exercise effects (i.e., increased blood pressure, heart rate) [74], insecurity with the body and body signals [11,77], and unpleasant sensations associated with exercise [69] (K/M) Greatened awareness of normal exercise effects, such as dehydration [74]; concerns exercise will make you too thirsty [69] (K) Being cautious about doing too much, feeling fatigued and not wanting to become more fatigued [69,74] (K) Self-consciousness about appearance [69] (K) Emotional trauma, most often as a direct result of transplant experience, including illness, the transplant procedure itself, and post-transplant recovery [77] (M) | Evaluated in 10/19 records Wanting to decrease depression and anxiety [69] (K) Perceived health related quality of life, well-being and benefits [77,81,82,83] (K/H/M) Encourage a return to leisurely and meaningful activities [81] (M) Sense of duty to enact health, self-care, and donor-directed gratitude [11,54,74,77] and moral imperative to move [77] (K/N/M) Physical activity as a way of connecting to their donor as means of keeping part of their donor ‘alive’ for the sake of their respective donor families [77] (M) Transplant specific distress relates to feelings of ‘needing to’ participate in physical activity in order to avoid negative feelings such as guilt or shame [78] (M) Feeling better and giving ‘mental clarity’ [74] (K) Becoming more optimistic and outspoken by physical activity [83] (H) Stress relief and ‘take their mind off their transplant and related worries’ [74] (K) Frustration, stress, and guilt for missing exercise sessions [74] (K) Feeling favourable towards exercise [79] (K) Positive psychological growth (also correlates with autonomous self-regulation) [78] (M) Enjoying new physical experiences and sensations [77] (M) Managing emotional and physical trauma [77] (M) |
| Reinforcement | Strategies for possible problems | Evaluated in 0/19 records | Evaluated in 0/19 records | |
| Reflective motivation | Intentions | Have willingness to be physically activeHave a plan on physical activity | Evaluated in 6/19 records Lack of motivation [54,69,71,74,79,81] (K/Li/N/M) Lack of interest in physical activity [69] (K) Dislike exercise [79] (K) Being lazy [79] (K) | Evaluated in 4/19 records Being motivated to be physically active [11,82] or internal need to exercise [74] (K/M) High level of motivation or a desire to stay healthy [72] (M) |
| Beliefs about consequence | Correct beliefs of resulting consequences of physical inactivity | Evaluated in 3/19 records Not believing the advice that is given [71] (Li) Perceiving only few health benefits by physical activity [11,80] (M) | Evaluated in 2/19 records The belief that implementing advice would be beneficial [71] (Li) | |
| Beliefs about capabilities | Correct beliefs about capabilities to be physical activity | Evaluated in 9/19 records Low exercise self-efficacy [8,11,70,75] (K/M) Low expectations by self [8,69] (K/M) Low self-confidence [8,69,71,74,79,80] (K/Li/M) | Evaluated in 8/19 records Beliefs in one’s ability to be physically active [69] (K) Having confidence about physical activity [72,80] and becoming more confident by physical activity [83] (H/M)High self-efficacy [8,11,75] (K) Self-management [74] (K) | |
| Goals | Correct beliefs of own responsibility for outcome and priority setting | Evaluated in 6/19 records Having other priorities [11,69] and other commitments [73] (K/Lu/M) Lack of time [69,73,79,81] or time commitment [80] (K/Lu/M) | Evaluated in 4/19 records Setting and wanting to achieve goals, goal progress, and priorities [11,82] (K/M) Structured approach [74] (K) Building toward more challenging and ambitious physical activity while realizing new capabilities [54] (N) | |
| Social/professional role and identity | Compatible set of behaviours with professional identity | Evaluated in 3/19 records Feeling if they are required to fulfil a social role (i.e., caretaker in their family) [11] (M) Work and family responsibilities [80] (M) Physically demanding job [69] (K) | Evaluated in 6/19 records Doing house chores and moving around at home [79] (K) Walking with the dog/walking to the bakery [79] (K) Self-identity shifting from ill, abnormal, and deficient body to a healthy body capable of physical performance and feelings as they have a ‘new’ body [54,77] (N/M) Feeling of normality [74] or come back to real life again [83] (K/H) |
| COM-B | TDF | Intervention Function | Policy Category | Potential BCT |
|---|---|---|---|---|
| Target | ||||
| Physical Capability | Physical skills | Enablement: Assessment and follow-up of physical fitness, co-morbidities, weight and medication. Manage fatigue and pain to improve patients’ ability to be physically active. Training: Create a personalized physical activity program and demonstrate exercises that get around physical limitations. | Service regulation | 8.1 Behavioural rehearsal/practice 6.1 Demonstration of the behaviour 4.1 Instructions on how to perform the behaviour 1.2 Problem solving |
| Being physically able to engage in physical activity within a wide range of intensities, volumes, and types. | ||||
| Psychological Capability | Knowledge | Education: Provide education to transplant recipients and health care providers to increase knowledge and awareness of the why, how, when, and how often transplant recipients should be physically active. Patients’ social network should accordingly be educated on these topics. Environmental restructuring: Development of uniform transplant-specific physical activity guidelines and recommendations. Environmental restructuring: Provision of physical activity and exercise information booklets to share with transplant recipients’ social network. | Communication Guidelines | 5.1 Information about health consequences 5.3 Information about social and environmental consequences 2.2 Feedback on behaviour 4.1 Instructions on how to perform the behaviour |
| Increase knowledge of patients, health care providers, and patients’ social network about why, when, how, and how often transplant recipients should participate in physical activity. Development of transplant-specific physical activity recommendations and guidelines. | ||||
| Physical Opportunity | Environmental context and resources | Training: health care providers should provide training in problem-solving thinking to reduce patients’ environmental barriers. Environmental restructuring: Provision of home-based exercise programs and/or governmental action to create opportunities to be physically active in the community (e.g., sidewalks, mixed land use, transport, parks, etc.). Environmental restructuring: Provide financial solutions/support for financial vulnerable transplant recipients. Environmental restructuring: Restructuring social environment/network in transplant unit to build a “movement culture”. | Service Provision Environmental/social planning Fiscal | 1.2 Problem solving 12.1 Restructuring the physical environment 12.5 Adding objects to the environment |
| Creating access and opportunities to participate in physical activity and rehabilitation.Reduce costs of physical activity/rehabilitation. | ||||
| Social Opportunity | Social influences Social—professional role and identity | Environmental restructuring: Provision of physical activity and exercise information booklets to share with transplant recipients’ social environment/network. Environmental restructuring: Provision of in group physical activity and exercise programs. Persuasion: Creating a social environment in which family, friends, and health care providers actively encourage and support patients to engage in physical activity. Modelling: Using champions (individuals who act as “the face” of an implementation effort) and encouraging ‘social comparison’ to increase perceptions of feasibility, safety, and acceptability of physical activity. Environmental restructuring: Integrate incidental physical activities in patients’ social roles, e.g., active transport to work or the store, gardening, housework, playing with the (grand)kids. | Environmental/social planning Regulation Service provision | 2.2 Feedback on behaviour 2.1 Monitoring of behaviour by others 3.1–3.3 Social support (general–practical–emotional) 6.2 Social comparison 10.4 Social reward 12.2 Restructuring the social environment |
| Creation of an encouraging and supporting environment to participate in physical activity.Integrate physical activity in to social/professional role and identity. | ||||
| Automatic and reflective motivation | Emotions Beliefs about consequences Beliefs about capabilities | Persuasion: Verbal persuasion from trusted health care providers that transplant recipient is fit to exercise safely. Education: Provide education about the safety and benefits of physical activity and in this way increase self-efficacy. Education/training: Provide education about the normal physiological effects of physical activity and provide training to recognize and familiarize bodily signals. Modelling: Identify experienced physically active transplant recipients to act as champions and role models to help build self-efficacy among other transplant recipients through vicarious learning. Training: Graded physical activity program to increase transplant recipients’ feelings of self-efficacy through mastery experiences. Focus on small goals and past successes. | Communication Environmental/social planning Regulation Service provision | 5.6 Emotional consequences 11.2 Regulate negative emotions 11.3 Conserving mental resources 8.7 Graded tasks 2.6 Biofeedback 15.1 Verbal persuasion to boost self-efficacy 15.2 Mental rehearsal of successful performance 15.4 Self-talk 15.3 Focus on past success |
| Reduce anxiety towards physical activity, promoting self-efficacy and confidence. | ||||
| Intentions Goals Emotion and behavioural regulation | Enablement: Physical activity action planning according to SMART goal setting. Persuasion: Discuss with patients’ activities and exercise that may be enjoyable to them. Persuasion: Discuss with patients how to overcome barriers to engage in physical activity and exercise. Persuasion: Increase intrinsic motivation by means of motivation interviewing techniques. Incentivizing: Incentivize transplant recipients’ physical activity by self-monitoring of physical activity behaviour (reaching goal) or holding individual or team-based play and competitions. Coercion: Creating awareness of association between low physical activity and health care costs. Enablement: Habit formation. | Communication Environmental/social planning Regulation Service provision | 1.2 Goal setting 1.4 Action planning (including implementation intentions) 1.6 Discrepancy between current behaviour and goal 1.7 Review outcome goals 2.3 Self-monitoring of behaviour 10.9 Self-reward | |
| Changing priorities and time management towards physical activity. Increase intrinsic motivation.Goal setting in a specific, measurable, achievable, realistic, and timely way.Routine formation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leunis, S.; Vandecruys, M.; Cornelissen, V.; Van Craenenbroeck, A.H.; De Geest, S.; Monbaliu, D.; De Smet, S. Physical Activity Behaviour in Solid Organ Transplant Recipients: Proposal of Theory-Driven Physical Activity Interventions. Kidney Dial. 2022, 2, 298-329. https://doi.org/10.3390/kidneydial2020029
Leunis S, Vandecruys M, Cornelissen V, Van Craenenbroeck AH, De Geest S, Monbaliu D, De Smet S. Physical Activity Behaviour in Solid Organ Transplant Recipients: Proposal of Theory-Driven Physical Activity Interventions. Kidney and Dialysis. 2022; 2(2):298-329. https://doi.org/10.3390/kidneydial2020029
Chicago/Turabian StyleLeunis, Sofie, Marieke Vandecruys, Véronique Cornelissen, Amaryllis H. Van Craenenbroeck, Sabina De Geest, Diethard Monbaliu, and Stefan De Smet. 2022. "Physical Activity Behaviour in Solid Organ Transplant Recipients: Proposal of Theory-Driven Physical Activity Interventions" Kidney and Dialysis 2, no. 2: 298-329. https://doi.org/10.3390/kidneydial2020029






