Combining Diffusion, Convection and Absorption: A Pilot Study of Polymethylmethacrylate versus Polysulfone Membranes in the Removal of P-Cresyl Sulfate by Postdilution On-Line Hemodiafiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Hemodialysis Procedures
2.3. Study Outcomes and Measurements
2.4. Blood Sampling
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Dialysis Features
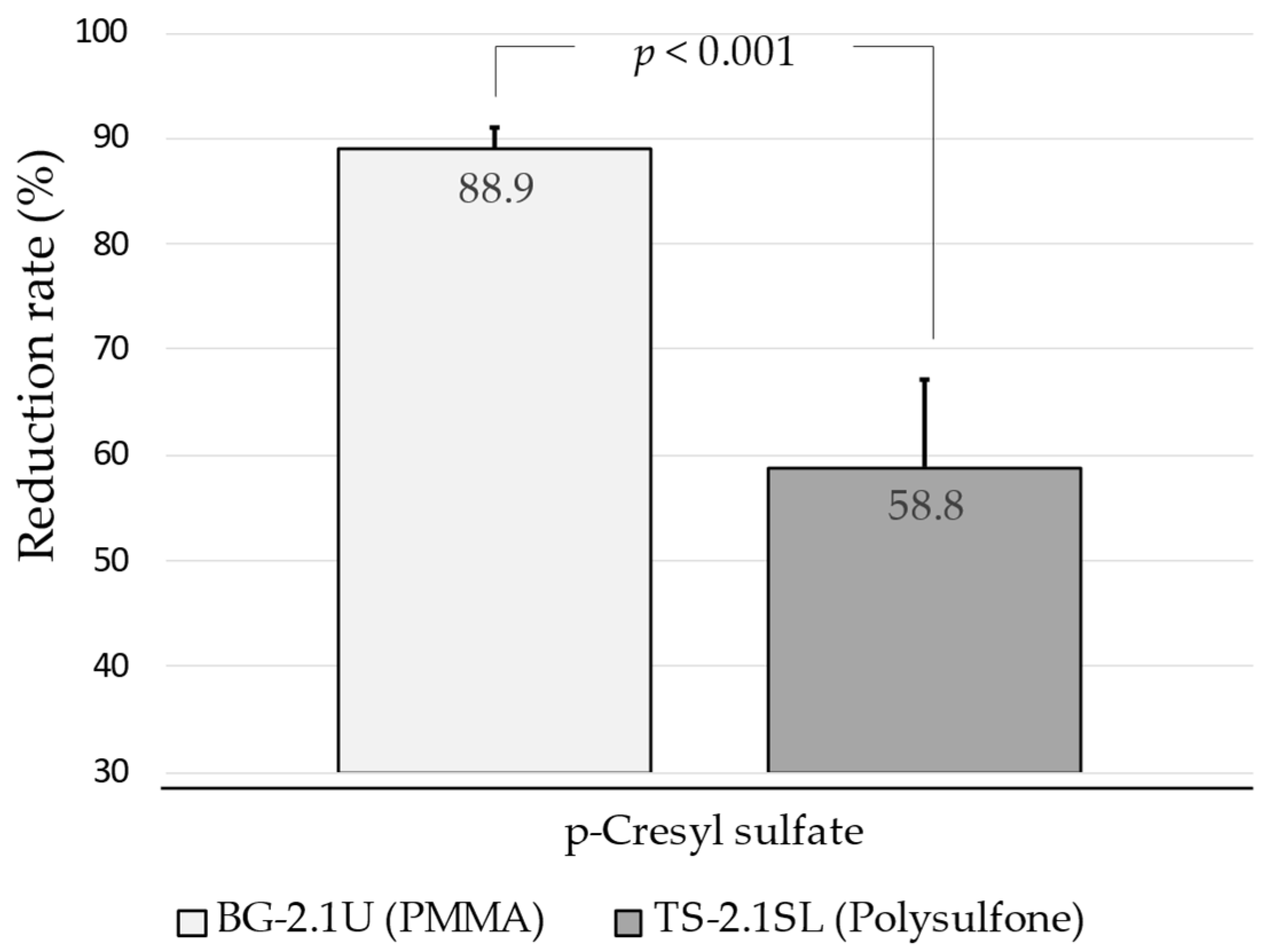
3.3. Solute Reduction Ratios and Dialysis Dose
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusion and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Study Investigators
Appendix B. Measurement of pCS Levels
Appendix B.1. Chemicals and Reagents for pCS Measurement
Appendix B.2. Preparation of pCS Standard Solutions
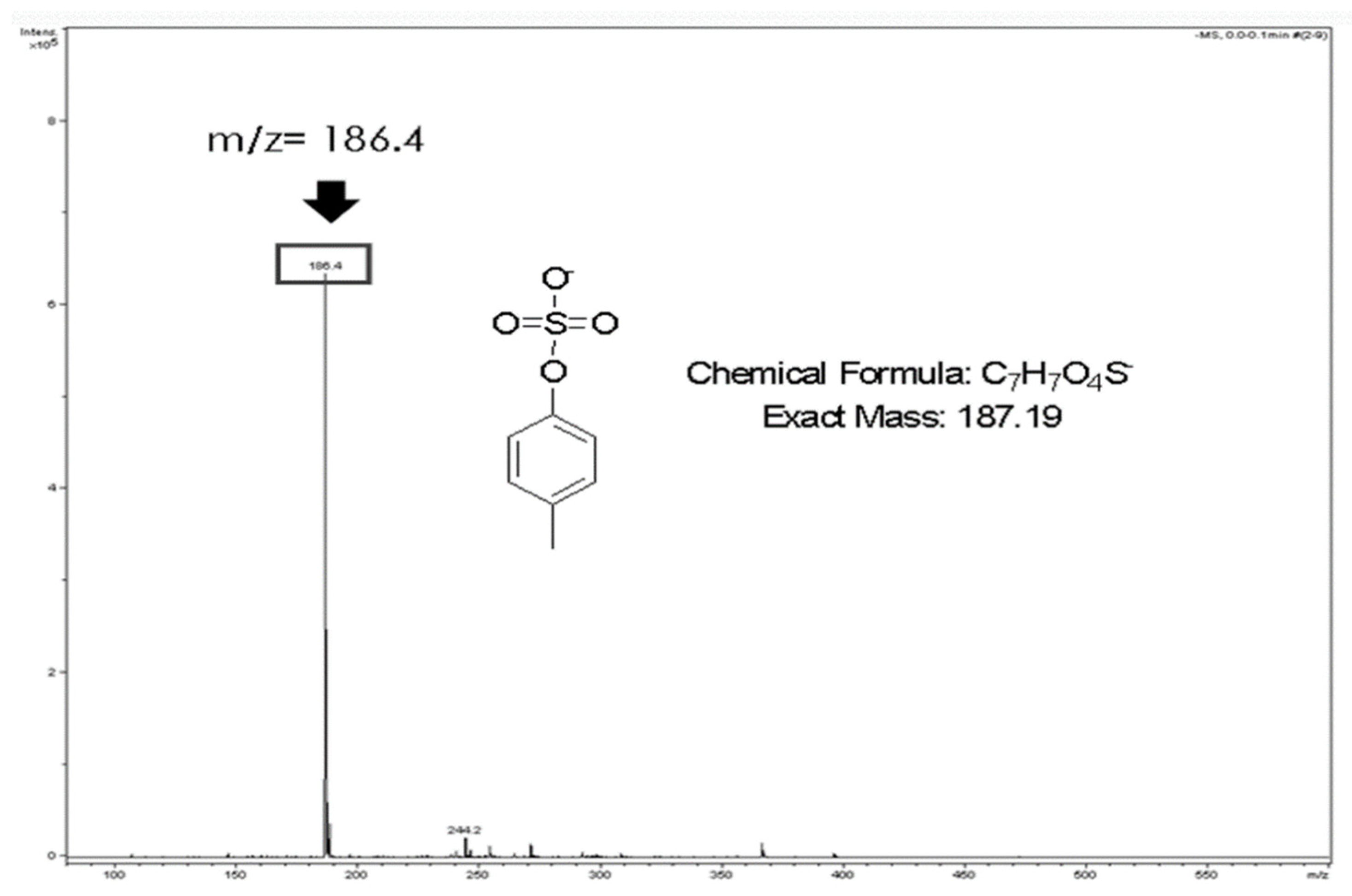

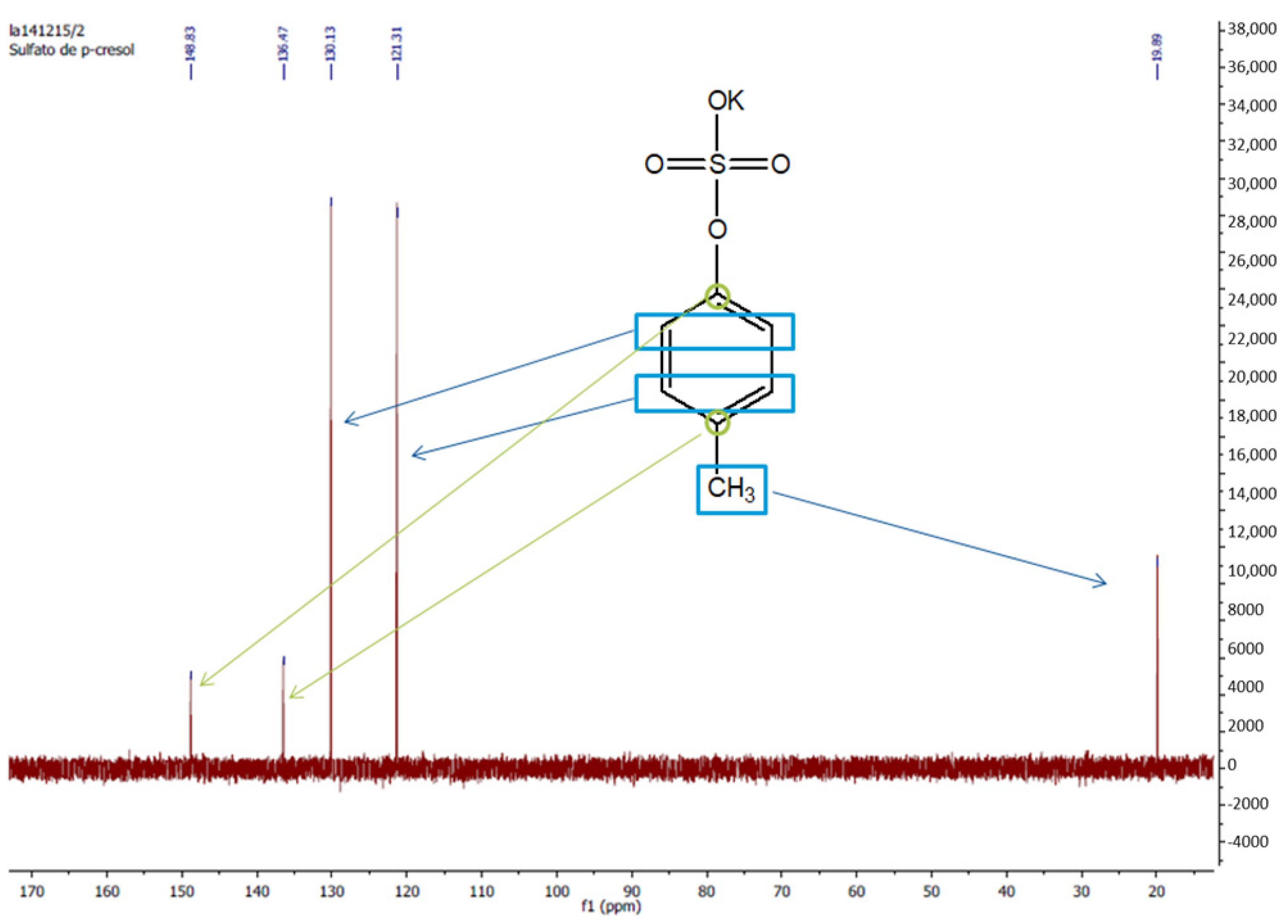
Appendix B.3. Serum Sample Preparation
Appendix B.4. HPLC Analytical Methodology
Appendix B.5. Chromatographic Method Validation
Appendix C. Chromatographic Method Validation
- -
- Linearity: r2 was 0.9999. The slope but not the intercept was statistically significant at 95% confidence level, the equation being y = 165.08·x.
- -
- Accuracy: The method was found to be accurate since percentage of recovery was between 98.9 and 100.6%.
- -
- Precision: The % RSD was ≤1.2% and ≤0.8% in all cases for repeatability and intermediate precision, respectively, which indicated that the method was precise.
- -
- Specificity: It was adequate since no interferences were observed at retention time of pCS.
- -
- LOD and LOQ: Under the experimental conditions used, LOD and LOQ were 0.009 mg/mL and 0.029 mg/mL, respectively.
References
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; on behalf of the European Uremic Toxin Work Group. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. Protein-Bound Toxins-Update. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- De Loor, H.; Meijers, B.K.; Meyer, T.W.; Bammens, B.; Verbeke, K.; Dehaen, W.; Evenepoel, P. Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J. Chromatogr. A 2009, 1216, 4684–4688. [Google Scholar] [CrossRef]
- Al Za’Abi, M.; Ali, B.; Al Toubi, M. HPLC-Fluorescence Method for Measurement of the Uremic Toxin Indoxyl Sulfate in Plasma. J. Chromatogr. Sci. 2012, 51, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, H.; Nakahashi, H. Determination of 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid, a major endogenous ligand substance in uremic serum, by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Biomed. Sci. Appl. 1987, 415, 110–117. [Google Scholar] [CrossRef]
- Meijers, B.; de Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl Sulfate and Indoxyl Sulfate in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.-Y. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N. Eng. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Depner, T.; Himmelfarb, J. Uremic Retention Solutes: The Free and the Bound. J. Am. Soc. Nephrol. 2007, 18, 675–676. [Google Scholar] [CrossRef] [Green Version]
- Schulman, G.; Agarwal, R.; Acharya, M.; Berl, T.; Blumenthal, S.; Kopyt, N. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging Study of AST-120 (Kremezin) in Patients with Moderate to Severe CKD. Am. J. Kidney Dis. 2006, 47, 565–577. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Omori, K.; Matsuo, K.; Takahashi, Y.; Kawamura, K.; Matsuto, T.; Watanabe, H.; Maruyama, T.; Narita, I. Continuous Reduction of Protein-Bound Uraemic Toxins with Improved Oxidative Stress by Using the Oral Charcoal Adsorbent AST-120 in Haemodialysis Patients. Sci. Rep. 2015, 5, 14381. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Glorieux, G. The intestine and the kidneys: A bad marriage can be hazardous. Clin. Kidney J. 2015, 8, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Bammens, B.; Evenepoel, P.; Verbeke, K.; Vanrenterghem, Y. Removal of the protein-bound solute p-cresol by convective transport: A randomized crossover study. Am. J. Kidney Dis. 2004, 44, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Peattie, J.W.; Miller, J.D.; Dinh, D.C.; Recht, N.S.; Walther, J.L.; Hostetter, T.H. Increasing the Clearance of Protein-Bound Solutes by Addition of a Sorbent to the Dialysate. J. Am. Soc. Nephrol. 2007, 18, 868–874. [Google Scholar] [CrossRef]
- Meijers, B.K.; Weber, V.; Bammens, B.; Dehaen, W.; Verbeke, K.; Falkenhagen, D.; Evenepoel, P. Removal of the Uremic Retention Solute p-Cresol Using Fractionated Plasma Separation and Adsorption. Artif. Organs 2008, 32, 214–219. [Google Scholar] [CrossRef]
- Pham, N.M.; Recht, N.S.; Hostetter, T.H.; Meyer, T.W. Removal of the Protein-Bound Solutes Indican and P-Cresol Sulfate by Peritoneal Dialysis. Clin. J. Am. Soc. Nephrol. 2007, 3, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirich, T.L.; Luo, F.J.-G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Selectively increasing the clearance of protein-bound uremic solutes. Nephrol. Dial. Transplant. 2012, 27, 1574–1579. [Google Scholar] [CrossRef]
- Campistol, J.; Torregrosa, J.; Ponz, E.; Fenollosa, B. β2-Microglobulin Removal by Hemodialysis with Polymethylmethacrylate Membranes. Polymethylmethacrylate 1998, 125, 76–85. [Google Scholar] [CrossRef]
- Santoro, A.; Guadagni, G. Dialysis membrane: From convection to adsorption. Clin. Kidney J. 2010, 3, i36–i39. [Google Scholar] [CrossRef] [Green Version]
- Aucella, F.; Gesuete, A.; Vigilante, M.; Prencipe, M. Adsorption Dialysis: From Physical Principles to Clinical Applications. Blood Purif. 2013, 35, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Birk, H.-W.; Kistner, A.; Wizemann, V.; Schütterle, G. Protein Adsorption by Artificial Membrane Materials Under Filtration Conditions. Artif. Organs 1995, 19, 411–415. [Google Scholar] [CrossRef]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Hermida-Lama, E.; Rodas, L.M.; Gómez, M.; Arias-Guillén, M.; Fontseré, N.; Vera, M.; Rico, N. Evaluation and comparison of polysulfone TS-UL and PMMA NF-U dialyzers versus expanded hemodialysis and postdilution hemodiafiltration. Artif. Organs 2021, 45, E317–E323. [Google Scholar] [CrossRef]
- Cavalier, E.; Torres, P.U.; Dubois, B.E.; Smelten, N.; Pottel, H.; Krzesinski, J.-M.; Delanaye, P. Impact of the Type of Dialysis Membranes on the Circulating Concentration of Markers of Vitamin D Metabolism. Int. J. Artif. Organs 2017, 40, 43–47. [Google Scholar] [CrossRef]
- Maduell, F.; Arias, M.; Garro, J.; Vera, M.; Fontseré, M.; Barros, X.; Massó, E.; Martina, M.N.; Sentis, A.; Durán, C.; et al. Pauta de infusión manual automatizada: Una forma práctica de prescribir la hemodiafiltración on-line posdilucional [Guidelines for automated manual infusion: A practical way of prescribing postdilution on-line hemodiafiltration]. Nefrología 2010, 30, 349–353. [Google Scholar] [CrossRef]
- Masakane, I.; Esashi, S.; Yoshida, A.; Chida, T.; Fujieda, H.; Ueno, Y.; Sugaya, H. A new polymethylmetacrylate membrane improves the membrane adhesion of blood components and clinical efficacy. Ren. Replace. Ther. 2017, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.; Bañon-Maneus, E.; Arias-Guillén, M.; Maduell, F. Assessment of removal and adsorption enhancement of high-flux hemodialyzers in convective therapies by a novel in vitro uremic matrix. Sci. Rep. 2020, 10, 17403. [Google Scholar] [CrossRef] [PubMed]
- Maduell, F.; Arias-Guillen, M.; Fontsere, N.; Ojeda, R.; Rico, N.; Vera, M.; Elena, M.; Bedini, J.; Wieneke, P.; Campistol, J. Elimination of Large Uremic Toxins by a Dialyzer Specifically Designed for High-Volume Convective Therapies. Blood Purif. 2014, 37, 125–130. [Google Scholar] [CrossRef]
- Goldau, R.; Kuhlmann, U.; Samadi, N.; Gross, M.; Graf, T.; Orlandini, G.; Marcelli, D.; Lange, H. Ionic dialysance measurement is urea distribution volume dependent: A new approach to better results. Artif. Organs 2002, 26, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.; Wehle, B. No change in corrected β2-microglobulin concentration after cuprophane haemodialysis. Lancet 1987, 329, 628–629. [Google Scholar] [CrossRef]
- Meert, N.; Eloot, S.; Waterloos, M.-A.; Van Landschoot, M.; Dhondt, A.; Glorieux, G.; Ledebo, I.; Vanholder, R. Effective removal of protein-bound uraemic solutes by different convective strategies: A prospective trial. Nephrol. Dial. Transplant. 2008, 24, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.-C.; Wang, C.-Y.; Hsu, C.-Y.; Wu, C.-H.; Kuo, C.-C.; Wang, K.-C.; Yang, C.-C.; Wu, M.-T.; Chuang, F.-R.; Lee, C.-T. Freep-Cresol Sulfate Is Associated with Survival and Function of Vascular Access in Chronic Hemodialysis Patients. Kidney Blood Press. Res. 2012, 35, 583–588. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Yen, C.-H.; Wu, I.-W.; Hsu, K.-H.; Chen, C.-K.; Sun, C.-Y.; Chou, C.-C.; Chen, C.-Y.; Tsai, C.-J.; Wu, M.-S.; et al. The Association of Uremic Toxins and Inflammation in Hemodialysis Patients. PLoS ONE 2014, 9, e102691. [Google Scholar] [CrossRef]
- Lin, C.-J.; Pan, C.-F.; Liu, H.-L.; Chuang, C.-K.; Jayakumar, T.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 2012, 225, 173–179. [Google Scholar] [CrossRef]
- Wu, I.-W.; Hsu, K.-H.; Hsu, H.-J.; Lee, S.W.; Sun, C.-Y.; Tsai, C.-J.; Wu, M.-S. Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients--a prospective cohort study. Nephrol. Dial. Transplant. 2012, 27, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Lesaffer, G.; De Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R. Intradialytic removal of protein-bound uraemic toxins: Role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Meert, N.; Beerenhout, C.; Schepers, E.; Glorieux, G.; Kooman, J.; Vanholder, R. Evolution of protein-bound urae-mic solutes during predilution haemofiltration. J. Nephrol. 2009, 22, 352–357. [Google Scholar]
- Abad, S.; Vega, A.; Quiroga, B.; Arroyo, D.; Panizo, N.; Reque, J.E.; López-Gómez, J.M. Toxinas unidas a proteínas: Valor añadido en su eliminación con altos volúmenes convectivos. Nefrología 2016, 36, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Krieter, D.H.; Kerwagen, S.; Rüth, M.; Lemke, H.-D.; Wanner, C. Differences in Dialysis Efficacy Have Limited Effects on Protein-Bound Uremic Toxins Plasma Levels over Time. Toxins 2019, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, E.; Cataldi, M.; Minco, M.; Argentino, G.; Russo, R.; Brancaccio, S.; Memoli, A.; Grumetto, L.; Postiglione, L.; Guida, B.; et al. Evidence That p-Cresol and IL-6 Are Adsorbed by the HFR Cartridge: Towards a New Strategy to Decrease Systemic Inflammation in Dialyzed Patients? PLoS ONE 2014, 9, e95811. [Google Scholar] [CrossRef] [Green Version]
- Esquivias-Motta, E.; Martín-Malo, A.; Buendia, P.; Álvarez-Lara, M.A.; Soriano, S.; Crespo, R.; Carracedo, J.; Ramírez, R.; Aljama, P. Hemodiafiltration With Endogenous Reinfusion Improved Microinflammation and Endothelial Damage Compared With Online-Hemodiafiltration: A Hypothesis Generating Study. Artif. Organs 2016, 41, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Removal of Protein-Bound Uraemic Toxins by Haemodialysis. Blood Purif. 2013, 35, 20–25. [Google Scholar] [CrossRef]
- Aucella, F.; Vigilante, M.; Gesuete, A. Review: The effect of polymethylmethacrylate dialysis membranes on uraemic pruritus. Clin. Kidney J. 2010, 3, i8–i11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoike, I. Clinical significance of protein adsorbable membranes—Long-term clinical effects and analysis using a proteomic technique. Nephrol. Dial. Transplant. 2007, 22, v13–v19. [Google Scholar] [CrossRef] [Green Version]
- Niwa, T.; Asada, H.; Tsutsui, S.; Miyazaki, T. Efficient Removal of Albumin-Bound Furancarboxylic Acid by Protein-Leaking Hemodialysis. Am. J. Nephrol. 1995, 15, 463–467. [Google Scholar] [CrossRef]
- Galli, F.; Benedetti, S.; Buoncristiani, U.; Piroddi, M.; Conte, C.; Canestrari, F.; Buoncristiani, E.; Floridi, A. The effect of PMMA-based protein-leaking dialyzers on plasma homocysteine levels. Kidney Int. 2003, 64, 748–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galli, F.; Benedetti, S.; Floridi, A.; Canestrari, F.; Piroddi, M.; Buoncristiani, E.; Buoncristiani, U. Glycoxidation and inflammatory markers in patients on treatment with PMMA-based protein-leaking dialyzers. Kidney Int. 2005, 67, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Oshihara, W.; Fujieda, H.; Ueno, Y. A New Poly(Methyl Methacrylate) Membrane Dialyzer, NF, with Adsorptive and Antithrombotic Properties. Contrib. Nephrol. 2017, 189, 230–236. [Google Scholar] [CrossRef]
- Liabeuf, S.; Villain, C.; Massy, Z.A. Protein-bound toxins: Has the Cinderella of uraemic toxins turned into a princess? Clin. Sci. 2016, 130, 2209–2216. [Google Scholar] [CrossRef]
- Florens, N.; Guebre-Egziabher, F.; Juillard, L. Reconsidering adsorption in hemodialysis: Is it just an epiphenomenon? A narrative review. J. Nephrol. 2021, 1–9. [Google Scholar] [CrossRef]
- Abe, M.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Renal Data Registry Committee, Japanese Society for Di-alysis Therapy, Effect of dialyzer membrane materials on survival in chronic hemodialysis patients: Results from the annual survey of the Japanese Nationwide Dialysis Registry. PLoS ONE 2017, 12, e0184424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and mortality in end-stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transplant. 2015, 31, 978–984. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonized Tripartite Guideline Q2 (R1) Validation of Analytical Procedures. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 21 April 2021).
- Reviewer Guidance. Validation of Chromatographic Methods. Centre for Drug Evaluation and Research. The Food and Drug Administration. FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reviewer-guidance-validation-chromatographic-methods (accessed on 29 October 2021).
- Bonomini, M.; Fiederling, B.; Bucciarelli, T.; Manfrini, V.; Di Ilio, C.; Albertazzi, A. A new polymethylmethacry-late membrane for hemodialysis. Int. J. Artif. Organs. 1996, 19, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y. Polymethylmethacrylate Membrane with a Series of Serendipity. Recent Adv. Pediatric Nephrol. 2011, 173, 137–147. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ficociello, L.H.; Bazzanella, J.; Mullon, C.; Anger, M.S. Slipping Through the Pores: Hypoalbuminemia and Albumin Loss During Hemodialysis. Int. J. Nephrol. Renov. Dis. 2021, 14, 11–21. [Google Scholar] [CrossRef]
- Molina, P.; Vizcaíno, B.; Molina, M.D.; Beltrán, S.; González-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Ávila, A.I.; Górriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transplant. 2018, 33, 1223–1235. [Google Scholar] [CrossRef]
- Feigenbaum, J.; Neuberg, C.A. Simplified Method for the Preparation of Aromatic Sulfuric Acid Esters. J. Am. Chem. Soc. 1941, 63, 3529–3530. [Google Scholar] [CrossRef]
- Martinez, A.W.; Recht, N.S.; Hostetter, T.H.; Meyer, T.W. Removal of P-Cresol Sulfate by Hemodialysis. J. Am. Soc. Nephrol. 2005, 16, 3430–3436. [Google Scholar] [CrossRef] [Green Version]
- Shabir, G. A Practical Approach to Validation of HPLC Methods under Current Good Manufacturing Practices. Equipment and Instrumentation Qualification. Institute of Validation Technology. Available online: https://www.ivtnetwork.com/sites/default/files/A%20Practical%20Approach%20to%20Validation%20of%20HPLC%20Methods%20Under%20Current%20Good%20Manufacturing%20Practices_0.pdf (accessed on 29 October 2021).
- Calaf, R.; Cerini, C.; Génovésio, C.; Verhaeghe, P.; Jourde-Chiche, N.; Bergé-Lefranc, D.; Gondouin, B.; Dou, L.; Morange, S.; Argilés, A.; et al. Determination of uremic solutes in biological fluids of chronic kidney disease patients by HPLC assay. J. Chromatogr. B 2011, 879, 2281–2286. [Google Scholar] [CrossRef]
| Characteristic | BG-2.1U (Toray®) | TS-2.1SL (Toray®) |
|---|---|---|
| Surface area (m2) | 2.1 | 2.1 |
| Membrane structure | PMMA | PS |
| Sterilization | γ radiation | γ radiation |
| Membrane thickness (μm) | 30 | 40 |
| Internal diameter (μm) | 200 | 200 |
| Membrane frame | Symmetrical | Asymmetrical |
| Pore diameter (Å) | 70 | 25 |
| Negative charge (mEq/g) 1 | 110 | NA |
| KUF in vitro (mL/h) 2 | 4300 | 5500 |
| SC β2-microglobulin | NA | 0.93 |
| SC myoglobin | NA | 0.7 |
| SC albumin | <0.05 | <0.003 |
| Urea clearance (mL/min) 3 | 192 | 199 |
| Creatinine clearance (mL/min) 3 | 191 | 197 |
| Phosphate clearance (mL/min) 3 | 179 | 196 |
| Vitamin B12 clearance (mL/min) 3 | 133 | 171 |
| Inulin clearance (mL/min) 1 | 81 | 142 |
| Characteristic | n = 35 |
|---|---|
| Age (yr) | 61.3 ± 15.4 |
| Sex (female/male; %) | 13/22 (37%/63%) |
| CKD etiology (n, %) | |
| -Nephrosclerosis | 6 (17%) |
| -Diabetic nephropathy | 7 (20%) |
| -Glomerular | 11 (31%) |
| -Interstitial | 2 (6%) |
| -Other causes | 7 (20%) |
| -Unknown | 2 (6%) |
| Dialysis vintage (mo; median, IQR) | 44 (24–162) |
| Comorbidity history (n, %) | |
| -Diabetes mellitus | 6 (18%) |
| -Coronary artery disease | 4 (12%) |
| -Chronic heart failure | 7 (21%) |
| -Cerebrovascular disease | 2 (6%) |
| -Peripheral vascular disease | 6 (18%) |
| Vascular access (n, %) | |
| -Arteriovenous fistula | 27 (77%) |
| -Arteriovenous graft | 1 (3%) |
| -Tunneled catheter | 7 (20%) |
| BMI (kg/m2) | 25.4 ± 4.1 |
| nPNA (g/kg/d) | 1.26 ± 0.43 |
| Albumin (g/dL) | 3.6 ± 0.3 |
| Hemoglobin (g/dL) | 10.4 ± 1.2 |
| hs-CRP (mg/L) | 3.7 (1.0–8.2) |
| Total p-cresyl sulfate (mg/L) | 4.3 (1.4–7.6) |
| β2-microglobulin (mg/L) | 24.2 ± 11.5 |
| Creatinine (mg/dL) | 9.3 ± 2.6 |
| Uric acid (mg/dL) | 6.7 ± 1.2 |
| Phosphate (mg/dL) | 3.9 ± 1.0 |
| Calcium adjusted by albumin (mg/dL) | 9.0 ± 0.6 |
| iPTH (pg/mL) | 222 (144–340) |
| BG-2.1U (PMMA) | TS-2.1 (PS) | p | |
|---|---|---|---|
| Length session (min) | 247 ± 12 | 247 ± 12 | 0.9 |
| Qb (mL/min) | 380 ± 31 | 379 ± 30 | 0.9 |
| Initial weight (kg) | 72.2 ± 16.7 | 72.5 ± 16.2 | 0.1 |
| Final weight (kg) | 69.8 ± 16.4 | 70.0 ± 15.9 | 0.1 |
| Ultrafiltration (L) | 2.4 ± 1.1 | 2.5 ± 1.3 | 0.4 |
| Arterial pressure (mmHg) | −198 ± 34 | −187 ± 34 | 0.06 |
| Venous pressure (mmHg) | 157 ± 40 | 151 ± 38 | 0.07 |
| Blood processed (L) | 93.9 ± 9.7 | 93.8 ± 9.1 | 0.9 |
| Replacement volume (L) | 18.8 ± 2.8 | 30.3 ± 7.8 | <0.001 |
| BG-2.1U (PMMA) | TS-2.1SL (PS) | p | |
|---|---|---|---|
| Urea RR (60 Da) | 79.4 ± 5.5 | 81.1 ± 5.4 | 0.013 |
| Phosphate RR (95 Da) | 52.5 ± 10.8 | 54.6 ± 13.5 | 0.2 |
| Creatinine RR (113 Da) | 72.3 ± 6.1 | 74.6 ± 5.7 | <0.001 |
| Uric acid RR (168 Da) | 79.5 ± 5.4 | 82.1 ± 4.4 | <0.001 |
| β2-microglobulin RR (11,800 Da) | 67.5 ± 7.1 | 81.0 ± 5.0 | <0.001 |
| Kt (L) | 60.2 ± 8.7 | 65.5 ± 9.4 | 0.01 |
| Kt/V | 1.9 ± 0.4 | 2.0 ± 0.5 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, P.; Peiró, J.; Martínez-Gómez, M.A.; Vizcaíno, B.; Esteller, C.; González-Moya, M.; García-Valdelvira, M.; Molina, M.D.; Maduell, F.; on behalf of the Collaborators. Combining Diffusion, Convection and Absorption: A Pilot Study of Polymethylmethacrylate versus Polysulfone Membranes in the Removal of P-Cresyl Sulfate by Postdilution On-Line Hemodiafiltration. Kidney Dial. 2021, 1, 121-134. https://doi.org/10.3390/kidneydial1020015
Molina P, Peiró J, Martínez-Gómez MA, Vizcaíno B, Esteller C, González-Moya M, García-Valdelvira M, Molina MD, Maduell F, on behalf of the Collaborators. Combining Diffusion, Convection and Absorption: A Pilot Study of Polymethylmethacrylate versus Polysulfone Membranes in the Removal of P-Cresyl Sulfate by Postdilution On-Line Hemodiafiltration. Kidney and Dialysis. 2021; 1(2):121-134. https://doi.org/10.3390/kidneydial1020015
Chicago/Turabian StyleMolina, Pablo, Julio Peiró, María A. Martínez-Gómez, Belén Vizcaíno, Cristina Esteller, Mercedes González-Moya, María García-Valdelvira, Mariola D. Molina, Francisco Maduell, and on behalf of the Collaborators. 2021. "Combining Diffusion, Convection and Absorption: A Pilot Study of Polymethylmethacrylate versus Polysulfone Membranes in the Removal of P-Cresyl Sulfate by Postdilution On-Line Hemodiafiltration" Kidney and Dialysis 1, no. 2: 121-134. https://doi.org/10.3390/kidneydial1020015
APA StyleMolina, P., Peiró, J., Martínez-Gómez, M. A., Vizcaíno, B., Esteller, C., González-Moya, M., García-Valdelvira, M., Molina, M. D., Maduell, F., & on behalf of the Collaborators. (2021). Combining Diffusion, Convection and Absorption: A Pilot Study of Polymethylmethacrylate versus Polysulfone Membranes in the Removal of P-Cresyl Sulfate by Postdilution On-Line Hemodiafiltration. Kidney and Dialysis, 1(2), 121-134. https://doi.org/10.3390/kidneydial1020015








