Development of a Nanogold-Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Proteins and Antibodies
Abstract
1. Introduction
2. Materials and Methods
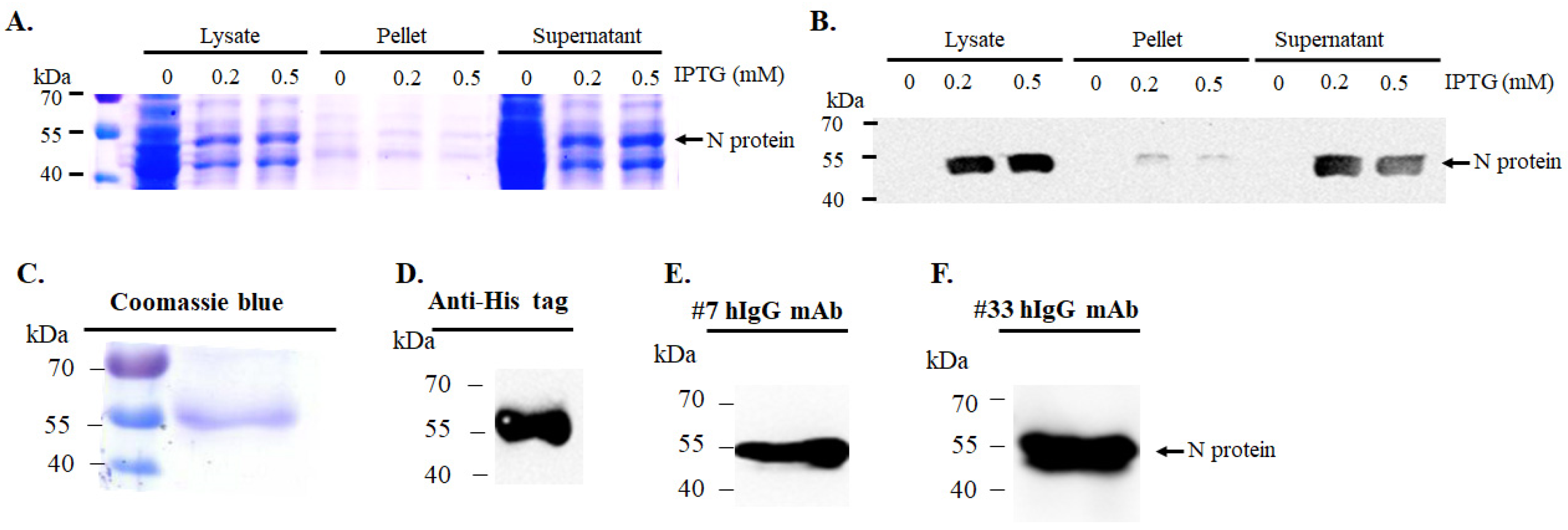
2.1. Expression and Purification of Recombinant SARS-CoV-2 N Protein
2.2. Immunoreagents and Chemicals
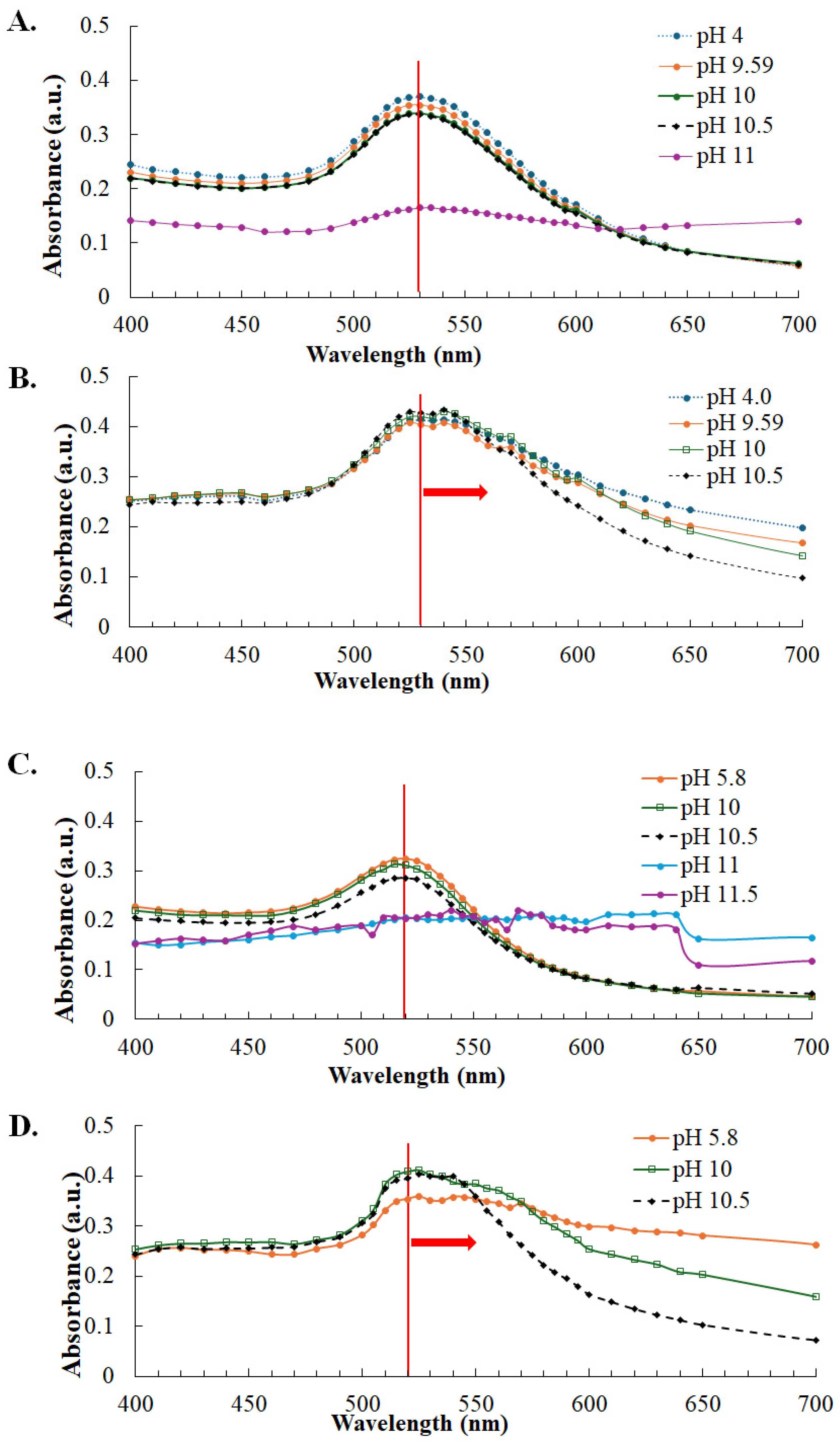
2.3. Preparation of Colloidal Gold Nanoparticles
2.4. Optimization of Colloidal Gold–N Antigen Conjugation
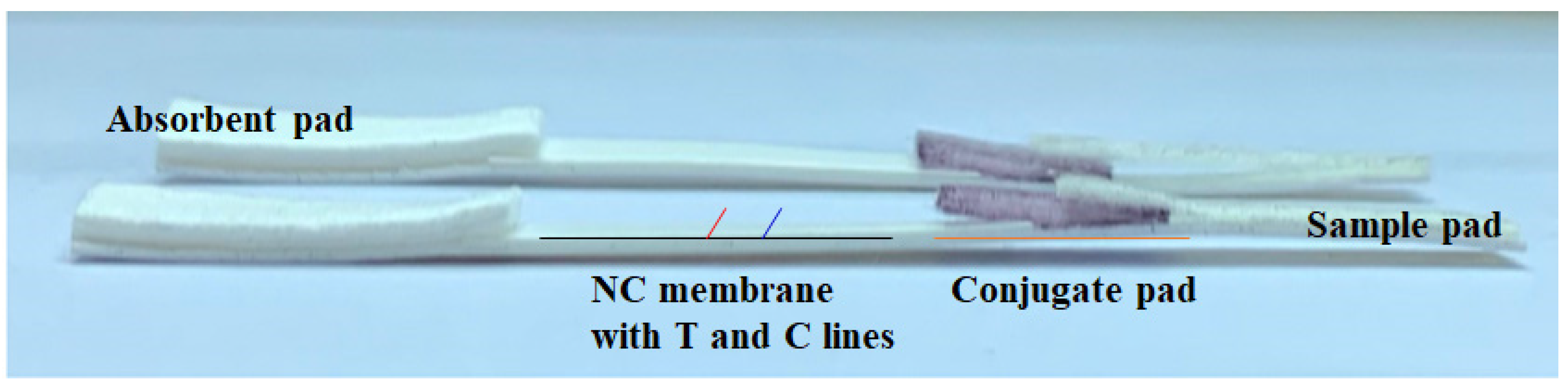
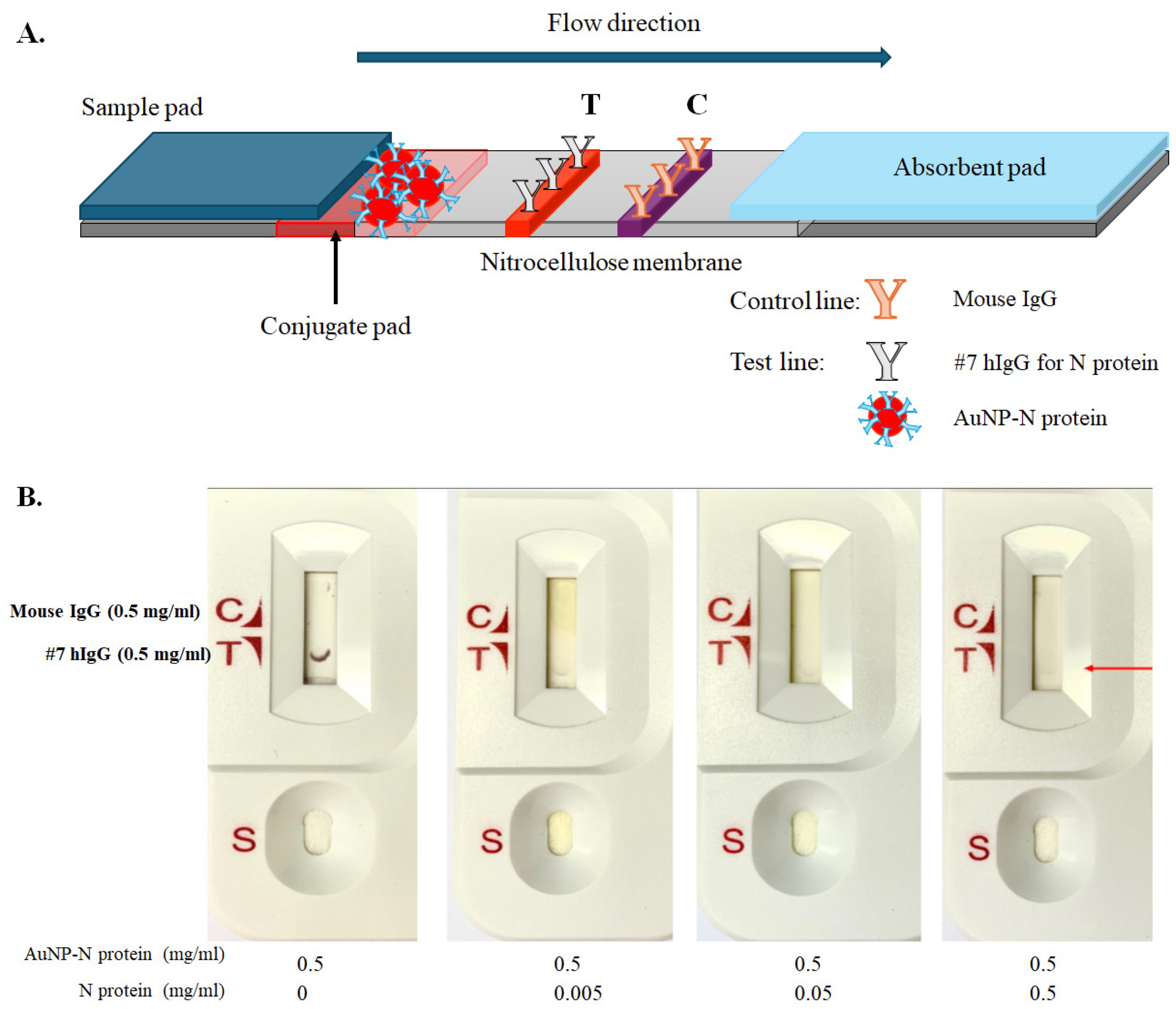
2.5. Preparation of LFIA Strips
3. Results
3.1. Expression and Purification of Recombinant SARS-CoV-2 N Protein
3.2. Stability of Gold Nanoparticles and Optimization of SARS-CoV-2 N Protein Conjugation
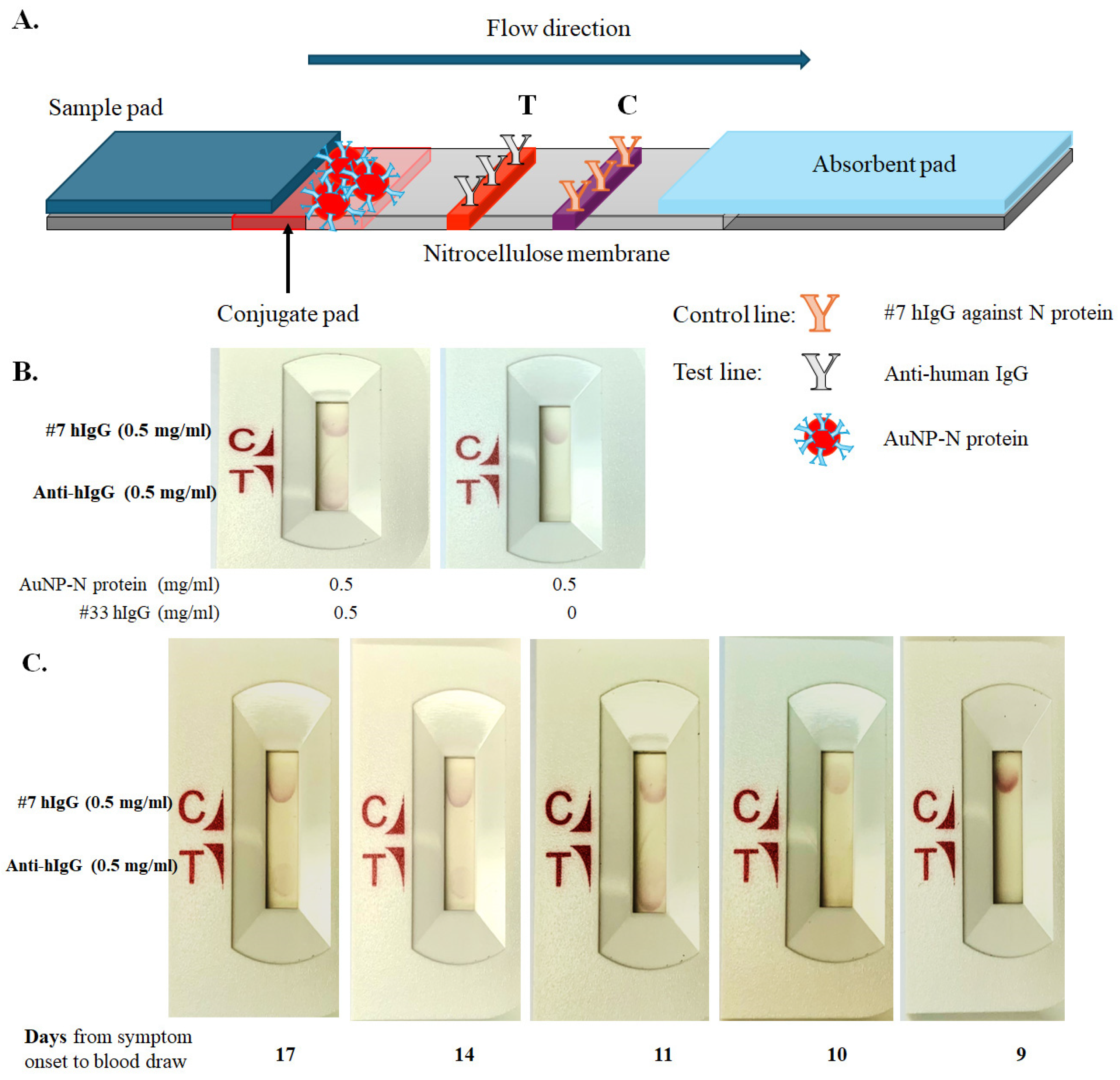
3.3. Assembly of Lateral Flow Immunoassay (LFIA) Strips for SARS-CoV-2 N Protein and Antibody Detection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Novel Coronavirus 2019—Situation Report; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 1 August 2020).
- Jalandra, R.; Yadav, A.K.; Verma, D.; Dalal, N.; Sharma, M.; Singh, R.; Kumar, A.; Solanki, P.R. Strategies and perspectives to develop SARS-CoV-2 detection methods and diagnostics. Biomed. Pharmacother. 2020, 129, 110446. [Google Scholar] [CrossRef] [PubMed]
- Keshav, V.; Scott, L.; David, A.; Noble, L.; Mayne, E.; Stevens, W. Antigen-based point of care testing (POCT) for diagnosing SARS-CoV-2: Assessing performance. In SARS-CoV-2; Chu, J.J.H., Ahidjo, B.A., Mok, C.K., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2452, pp. 45–62. [Google Scholar]
- Kakkar, S.; Gupta, P.; Yadav, S.P.S.; Raj, D.; Singh, G.; Chauhan, S.; Mishra, M.K.; Martín-Ortega, E.; Chiussi, S.; Kant, K. Lateral flow assays: Progress and evolution of recent trends in point-of-care applications. Mater Today Bio. 2024, 25, 100817. [Google Scholar] [CrossRef]
- Ang, G.Y.; Chan, K.G.; Yean, C.Y.; Yu, C.Y. Lateral flow immunoassays for SARS-CoV-2. Diagnostics 2022, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Mirica, A.C.; Stan, D.; Chelcea, I.C.; Mihailescu, C.M.; Ofiteru, A.; Bocancia-Mateescu, L.A. Latest trends in lateral flow immunoassay (LFIA) detection labels and conjugation process. Front. Bioeng. Biotechnol. 2022, 10, 922772. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, S.; Zhou, J.; Jiang, W.; Yin, L.; Su, L.; Zhang, X.; Chen, Q.; Li, X. Rapid detection of SARS-CoV-2: The gradual boom of lateral flow immunoassay. Front. Bioeng. Biotechnol. 2023, 10, 1090281. [Google Scholar] [CrossRef] [PubMed]
- Ince, B.; Sezgintürk, M.K. Lateral flow assays for viruses diagnosis: Up-to-date technology and future prospects. TrAC Trends Anal. Chem. 2022, 157, 116725. [Google Scholar] [CrossRef] [PubMed]
- Fagúndez, P.; Botasini, S.; Tosar, J.P.; Méndez, E. Systematic process evaluation of the conjugation of proteins to gold nanoparticles. Heliyon 2021, 7, e07392. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, T.; Javed, B.; Sharma, V.; Tian, F. pH and NaCl optimisation to improve the stability of gold and silver nanoparticles’ anti-zearalenone antibody conjugates for immunochromatographic assay. Methods Protoc. 2023, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, H.; Lin, W.; Lin, L.; Gao, Y.; Yang, F.; Du, M.; Fang, W.; Huang, J.; Sun, D.; et al. Quantitative nucleation and growth kinetics of gold nanoparticles via model-assisted dynamic spectroscopic approach. J. Colloid Interface Sci. 2013, 407, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Haage, V.C.; Bleicker, T.; Schmidt, M.L.; Mühlemann, B.; Zuchowski, M.; Jo, W.K.; Tscheak, P.; Möncke-Buchner, E.; Müller, M.A.; et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e311–e319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.; Banerjee, M.; Bajpayee, A.; Mandal, S.; Mitra, P.; Sharma, P.; Misra, S.; Bhardwaj, P. SARS-CoV-2 IgG Antibody and its Clinical Correlates in Convalescent Plasma Donors: An Indian Experience. Indian J. Clin. Biochem. 2022, 37, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asympto-matic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Liu, C.; Yu, X.; Gao, C.; Zhang, L.; Zhai, H.; Hu, Y.; Liu, E.; Wang, Q.; Gao, Y.; Wei, D.; et al. Characterization of antibody responses to SARS-CoV-2 in convalescent COVID-19 patients. J. Med. Virol. 2020, 93, 2227–2233. [Google Scholar] [CrossRef]
- Alhammadi, M.; Yoo, J.; Sonwal, S.; Park, S.Y.; Umapathi, R.; Oh, M.H.; Huh, Y.S. A highly sensitive lateral flow immuno-assay for the rapid and on-site detection of enrofloxacin in milk. Front Nutr. 2022, 9, 1036826. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Su, F.; Jayaraj, J.; Shah, M.I.; Huang, Y. Paper microfluidics-based fluorescent lateral flow immunoassay for point-of-care diagnostics of noncommunicable diseases. Analyst 2019, 144, 7026–7034. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Jiménez, N.S.; García-Pérez, B.E.; Reyes-Rodríguez, N.E.; Vega-Sánchez, V.; Martínez-Juárez, V.M.; Hernández-González, J.C. Challenges in the detection of SARS-CoV-2: Evolution of the lateral flow immunoassay as a valuable tool for viral diagnosis. Biosensors 2022, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Swadźba, J.; Bednarczyk, M.; Anyszek, T.; Martin, E. A comparison of 7 commercial anti-SARS-CoV-2 antibody immunoassays. Arch. Med. Sci. 2020, 19, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-J.; Chen, Y.; Hsu, J.-L.; Lin, H.-C.; Hsueh, P.-R.; Lin, C.-W. Development of a Nanogold-Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Proteins and Antibodies. COVID 2025, 5, 158. https://doi.org/10.3390/covid5090158
Tsai W-J, Chen Y, Hsu J-L, Lin H-C, Hsueh P-R, Lin C-W. Development of a Nanogold-Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Proteins and Antibodies. COVID. 2025; 5(9):158. https://doi.org/10.3390/covid5090158
Chicago/Turabian StyleTsai, Wei-Jie, Yeh Chen, Jye-Lin Hsu, Hsiao-Chuan Lin, Po-Ren Hsueh, and Cheng-Wen Lin. 2025. "Development of a Nanogold-Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Proteins and Antibodies" COVID 5, no. 9: 158. https://doi.org/10.3390/covid5090158
APA StyleTsai, W.-J., Chen, Y., Hsu, J.-L., Lin, H.-C., Hsueh, P.-R., & Lin, C.-W. (2025). Development of a Nanogold-Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 Nucleocapsid Proteins and Antibodies. COVID, 5(9), 158. https://doi.org/10.3390/covid5090158






