Breaking the Oxygen Dogma: How High FiO2 May Disrupt Pulmonary Physiology in COVID-19
Abstract
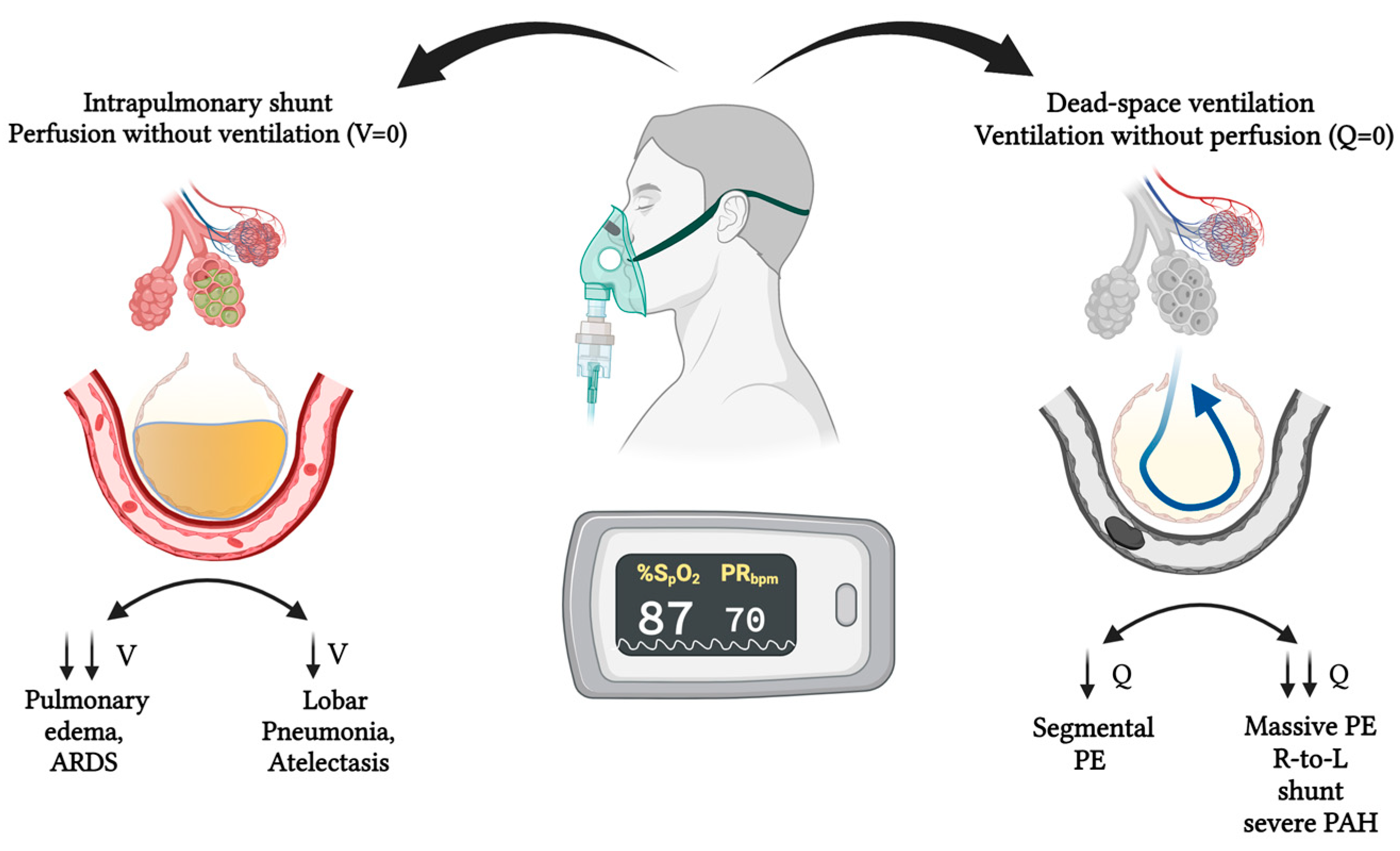
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Oxygen Titration in Clinical Practice
2.2.1. Eligibility Criteria
2.2.2. Inclusion Criteria
2.2.3. Exclusion Criteria
2.3. Data Collection
2.4. Oxygenation Metrics
2.5. Statistical Analysis
- FiO2 group (High ≥ 0.60 vs. Low < 0.60);
- P/F ratio (per 10 mmHg increment);
- A–a gradient (per 5 mmHg increment);
- C-reactive protein (per 10 mg/L increment);
- APACHE II score (per point).
3. Results
3.1. Baseline Characteristics
| High FiO2 (n = 102) | Low FiO2 (n = 99) | p-Value | |
|---|---|---|---|
| Sex, n (%) | <0.001 | ||
| Male | 84 (82.4%) | 42 (42.4%) | |
| Female | 18 (17.6%) | 57 (57.6%) | |
| Hypertension, n (%) | 39 (38.2%) | 22 (22.2%) | 0.01 |
| Smoking, n (%) | 51 (50.0%) | 36 (36.4%) | 0.24 |
| Age (years), mean ± SD | 55.3 ± 13.2 | 55.2 ± 15.3 | 0.98 |
| Height (cm), mean ± SD | 164 ± 7.9 | 166 ± 7.3 | 0.42 |
| Weight (kg), median (IQR) | 75 (68–85) | 78 (68–80) | 0.95 |
| BMI (kg/m2), median (IQR) | 27.3 (25.0–29.4) | 27.6 (24.3–29.5) | 0.91 |
| Heart rate (bpm), median (IQR) | 95 (82–109) | 95 (84–105) | 0.93 |
| Respiratory rate (rpm), median (IQR) | 25 (20–28) | 26 (24–28) | 0.10 |
| CO-RADS, median (IQR) | 5 (5–5) | 5 (5–5) | 0.55 |
| D-dimer (µg/mL), median (IQR) | 0.36 (0.23–0.56) | 0.36 (0.24–0.51) | 0.86 |
| Fibrinogen (g/L), median (IQR) | 5.5 (4.7–6.2) | 5.6 (4.2–6.1) | 0.59 |
| Ferritin (ng/mL), median (IQR) | 594 (268–1082) | 581 (355–1232) | 0.88 |
| CRP (mg/L), median (IQR) | 159 (47–259) | 139 (55–196) | 0.45 |
3.2. Arterial Blood Gas Parameters
| High FiO2 (n = 102), Median (IQR) | Low FiO2 (n = 99), Median (IQR) | p-Value | |
|---|---|---|---|
| Admission | |||
| pH | 7.44 (7.39–7.48) | 7.45 (7.44–7.47) | 0.18 |
| PaO2, mmHg | 55 (50–64) | 58 (55–61) | 0.29 |
| PaCO2, mmHg | 32 (27–35) | 30 (26–34) | 0.56 |
| SaO2, % | 85.6 (79.5–92) | 87 (84–88) | 0.79 |
| FiO2, % | 21 (21–21) | 21 (21–21) | 1.00 |
| A–a gradient, mmHg | 32 (25–48) | 34 (27–53) | <0.001 |
| P/F ratio | 242 (209–280) | 276 (261–290) | 0.01 |
| 24–48 h | |||
| pH | 7.41 (7.30–7.45) | 7.45 (7.42–7.47) | 0.01 |
| PaO2, mmHg | 67 (59–75) | 77 (72–85) | <0.001 |
| PaCO2, mmHg | 36 (31–43) | 34 (31–37) | 0.44 |
| SaO2, % | 93.5 (87–95) | 96 (94–97) | <0.001 |
| FiO2, % | 70 (60–90) | 40 (32–59) | <0.001 |
| A–a gradient, mmHg | 257 (170–369) | 198 (143–306) | 0.10 |
| P/F ratio | 95 (83–147) | 219 (140–282) | <0.001 |
3.3. Clinical Outcomes
| Outcome | High FiO2 (n = 102) | Low FiO2 (n = 99) | p-Value |
|---|---|---|---|
| Mechanical ventilation, n (%) | 44 (43.1%) | 16 (16.1%) | <0.001 |
| Days to mechanical ventilation, median (IQR) | 0 (0–1) | 0 (0–0) | 0.27 |
| In-hospital death, n (%) | 35 (34.3%) | 8 (8.1%) | <0.001 |
| Mechanical ventilation and in-hospital death, n (%) | 39 (38.2%) | 10 (10.1%) | <0.001 |
3.4. Multivariable Analysis
| Variable | OR (95% CI) | p-Value |
|---|---|---|
| Low FiO2 (<0.60 vs. ≥0.60) | 0.18 (0.08–0.39) | <0.001 |
| P/F ratio (per 10 mmHg increase) | 0.95 (0.92–0.98) | 0.002 |
| A–a gradient (per 5 mmHg increase) | 1.03 (1.01–1.05) | 0.010 |
| CRP (per 10 mg/L increase) | 1.01 (1.00–1.02) | 0.030 |
| APACHE II score (per point) | 1.12 (1.04–1.21) | 0.004 |
3.5. Kaplan–Meier Analysis
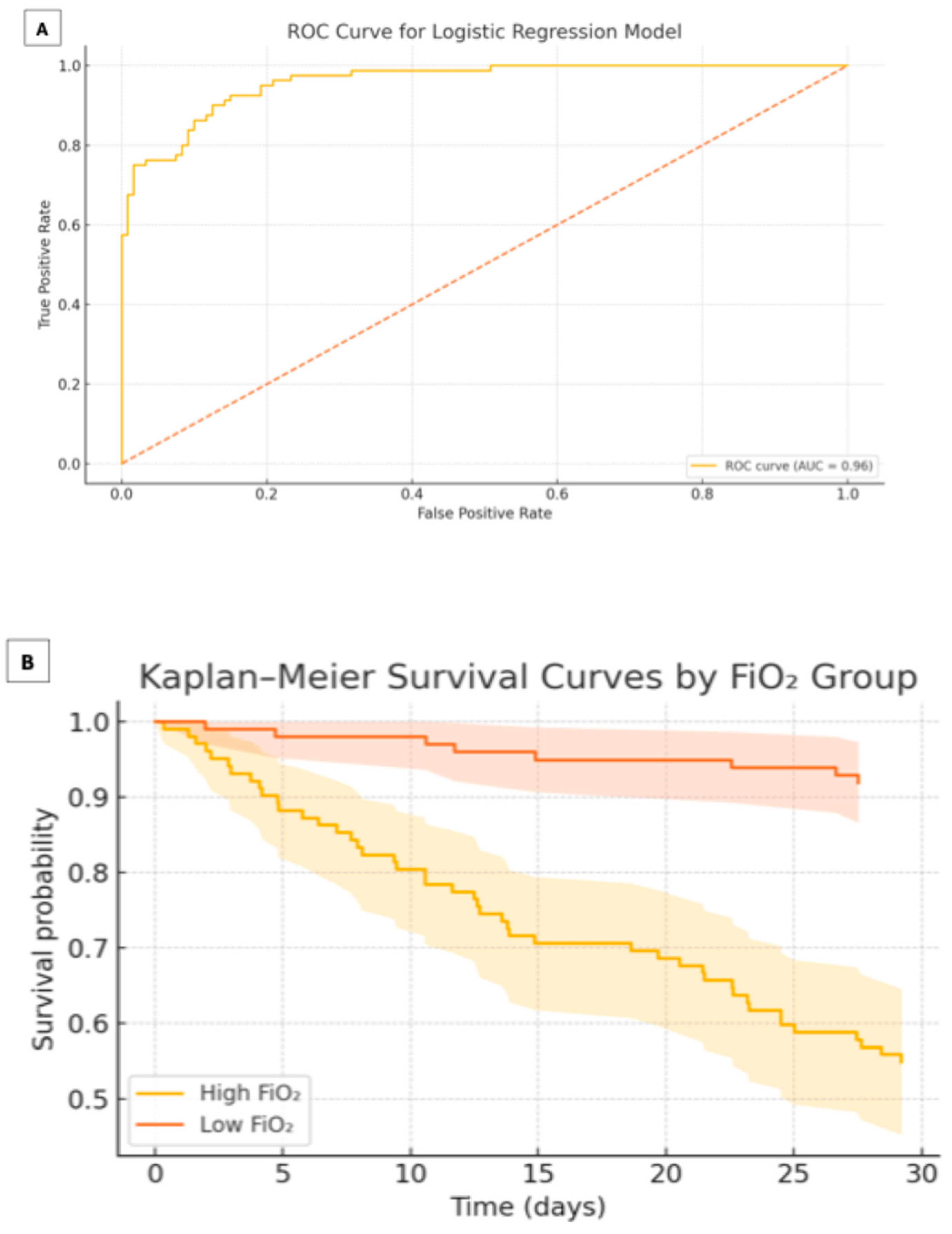
4. Discussion
5. Implications for Practice and Future Research
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.G.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstrictiction. Chest 2017, 1511, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tarry, D.; Powell, M. Hypoxic pulmonary vasoconstriction. BJA Educ. 2017, 17, 208–213. [Google Scholar] [CrossRef]
- Sommer, N.; Strielkov, I.; Pak, O.; Weissmann, N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur. Respir. J. 2016, 47, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.E.; Hanson, C.W.; Frasch, F.; Marshall, C. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. Intensive Care Med. 1994, 20, 379–389. [Google Scholar] [CrossRef]
- Lumb, A.B.; Slinger, P. Hypoxic pulmonary vasoconstriction: Physiology and anesthetic implications. Anesthesiology 2015, 122, 932–946. [Google Scholar] [CrossRef]
- Bradford, J.R.; Dean, H.P. Pulmonary circulation. J. Physiol. 1894, 16, 34–110. [Google Scholar] [CrossRef]
- Euler, U.S.V.; Liljestrand, G. Observations on the pulmonary arterial blood pressure in the cat. Acta Physiol. Scand. 1946, 12, 301–320. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-center descriptive study. Lancet 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–3. [Google Scholar] [CrossRef]
- Lang, M.; Som, A.; Mendoza, D.P.; Flores, E.J.; Reid, N.; Carey, D.; Li, M.D.; Witkin, A.; Rodriguez-Lopez, J.M.; Shepard, J.-A.O.; et al. Hypoxaemia related to COVID-19: Vascular and perfusion abnormalities on dual-energy CT. Lancet Infect. Dis. 2020, 20, 1365–1366. [Google Scholar] [CrossRef]
- Santamarina, M.G.; Boiser, D.; Contreras, R.; Baque, M.; Volpacchio, M.; Beddings, I. COVID-19: A hypothesis regarding the ventilation–perfusion mismatch. Crit. Care 2020, 24, 395. [Google Scholar] [CrossRef]
- Dhont, S.; Derom, E.; Van Braeckel, E.; Depuydt, P.; Lambrecht, B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir. Res. 2020, 21, 198. [Google Scholar] [CrossRef]
- Prokop, M.; Van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A categorical CT assessment scheme for patients suspected of having COVID-19—Definition and evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Abdo, W.F.; Heunks, L.M. Oxygen-induced hypercapnia in COPD: Myths and facts. Crit. Care 2012, 16, 323. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Forel, J.-M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.-M.; Perez, D.; Seghboyan, J.-M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. Extracorporeal membrane oxygenation for severe acute respiratory syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef]
- Chu, D.K.; Kim, L.H.-Y.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Ohshimo, S. Oxygen administration for patients with ARDS. J. Intensive Care 2021, 9, 17. [Google Scholar] [CrossRef]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir. Res. 2017, 4, e000170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alhazzani, W.; Evans, L.; Alshamsi, F.; Møller, M.H.; Ostermann, M.; Prescott, H.C.; Arabi, Y.M.; Loeb, M.; Ng Gong, M.; Fan, E.; et al. Surviving Sepsis Campaign Guidelines on the Management of Adults With Coronavirus Disease 2019 (COVID-19) in the ICU: First Update. Crit. Care Med. 2021, 49, e219–e234. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.W.; Glenny, R. Gas exchange and ventilation–perfusion relationships in the lung. Eur. Respir. J. 2014, 44, 1023–1041. [Google Scholar] [CrossRef]
- Busana, M.; Giosa, L.; Cressoni, M.; Gasperetti, A.; Di Girolamo, L.; Martinelli, A.; Sonzogni, A.; Lorini, L.; Palumbo, M.M.; Romitti, F.; et al. The impact of ventilation–perfusion inequality in COVID-19: A computational model. J. Appl. Physiol. 2021, 130, 865–876. [Google Scholar] [CrossRef]
- Dantzker, D.R.; Brook, C.J.; Dehart, P.; Lynch, J.P.; Weg, J.G. Ventilation–perfusion distributions in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1979, 120, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Girardis, M.; Busani, S.; Damiani, E.; Donati, A.; Rinaldi, L.; Marudi, A.; Morelli, A.; Antonelli, M.; Singer, M. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: The Oxygen-ICU randomized clinical trial. JAMA 2016, 316, 1583–1589. [Google Scholar] [CrossRef]
- Mackle, D.; Bellomo, R.; Bailey, M.; Beasley, R.; Deane, A.; Eastwood, G.; Finfer, S.; Freebairn, R.; King, V.; Linke, N.; et al. Conservative oxygen therapy during mechanical ventilation in the ICU. N. Engl. J. Med. 2019, 381, 989–998. [Google Scholar] [CrossRef]
- Schjørring, O.L.; Klitgaard, T.L.; Perner, A.; Wetterslev, J.; Lange, T.; Siegemund, M.; Bäcklund, M.; Keus, F.; Laake, J.H.; Morgan, M.; et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N. Engl. J. Med. 2021, 384, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.; Steinlechner, B.; Gruber, E.; Simon, P.; Wollenek, G. The oxygen dissociation curve: Quantifying the shift. Perfusion 2004, 19, 141–144. [Google Scholar] [CrossRef]
- Woyke, S.; Rauch, S.; Ströhle, M.; Gatterer, H. Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients. Clin. Nutr. 2021, 40, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, B.; Hachenberg, T.; Feyerherd, F.; Wendt, M. Ventilation–perfusion distribution with volume-reduced, pressure-limited ventilation with permissive hypercapnia. Anasthesiol. Intensivmed. Notfallmedizin Schmerzther. 1998, 33, 367–372. [Google Scholar] [CrossRef]
- Swenson, E.R. Hypoxic pulmonary vasoconstriction. High. Alt. Med. Biol. 2013, 14, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, S.H.; Golestanian, E.; Binks, A.P.; Lansing, R.W.; Brown, R.; Banzett, R.B. Hypoxic and hypercapnic drives to breathe generate equivalent levels of air hunger in humans. J. Appl. Physiol. 2003, 94, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, C.J.; Eastwood, P.R.; O’Driscoll, G.; Green, D.J. Abnormal ventilatory responses to hypoxia in Type 2 diabetes. Diabet. Med. 2005, 22, 563–568. [Google Scholar] [CrossRef]
- Nishimura, M.; Miyamoto, K.; Suzuki, A.; Yamamoto, H.; Tsuji, M.; Kishi, F.; Kawakami, Y. Ventilatory and heart rate responses to hypoxia and hypercapnia in patients with diabetes mellitus. Thorax 1989, 44, 251–257. [Google Scholar] [CrossRef] [PubMed]
| CO-RADS | Description |
|---|---|
| 0 | Incomplete or technically insufficient CT scan preventing evaluation. |
| 1 | Very low level of suspicion: normal findings or findings clearly unrelated to infection (e.g., emphysema, fibrosis). |
| 2 | Low level of suspicion: findings typical of other infectious etiologies (e.g., “tree-in-bud” pattern, lobar consolidation). |
| 3 | Indeterminate level of suspicion: equivocal findings compatible with viral pneumonia or non-infectious pathology (e.g., peripheral ground-glass). |
| 4 | High level of suspicion: imaging features typical of COVID-19 but with atypical distribution or overlap with other diseases (e.g., unilateral). |
| 5 | Very high level of suspicion: multifocal bilateral peripheral ground-glass opacities with or without consolidation, predominantly subpleural. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Ruiz, F.J.; Broca-García, B.E.; Manzur-Sandoval, D.; Santos-Martínez, L.E.; Encarnación-Martínez, U.; Lazcano-Díaz, E.A.; Ramos-Enriquez, A. Breaking the Oxygen Dogma: How High FiO2 May Disrupt Pulmonary Physiology in COVID-19. COVID 2025, 5, 139. https://doi.org/10.3390/covid5080139
González Ruiz FJ, Broca-García BE, Manzur-Sandoval D, Santos-Martínez LE, Encarnación-Martínez U, Lazcano-Díaz EA, Ramos-Enriquez A. Breaking the Oxygen Dogma: How High FiO2 May Disrupt Pulmonary Physiology in COVID-19. COVID. 2025; 5(8):139. https://doi.org/10.3390/covid5080139
Chicago/Turabian StyleGonzález Ruiz, Francisco Javier, Blanca Estela Broca-García, Daniel Manzur-Sandoval, Luis Efrén Santos-Martínez, Uriel Encarnación-Martínez, Emmanuel Adrián Lazcano-Díaz, and Angel Ramos-Enriquez. 2025. "Breaking the Oxygen Dogma: How High FiO2 May Disrupt Pulmonary Physiology in COVID-19" COVID 5, no. 8: 139. https://doi.org/10.3390/covid5080139
APA StyleGonzález Ruiz, F. J., Broca-García, B. E., Manzur-Sandoval, D., Santos-Martínez, L. E., Encarnación-Martínez, U., Lazcano-Díaz, E. A., & Ramos-Enriquez, A. (2025). Breaking the Oxygen Dogma: How High FiO2 May Disrupt Pulmonary Physiology in COVID-19. COVID, 5(8), 139. https://doi.org/10.3390/covid5080139






