Impact of COVID-19 Pandemic on the Diagnosis and Management of Infective Endocarditis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
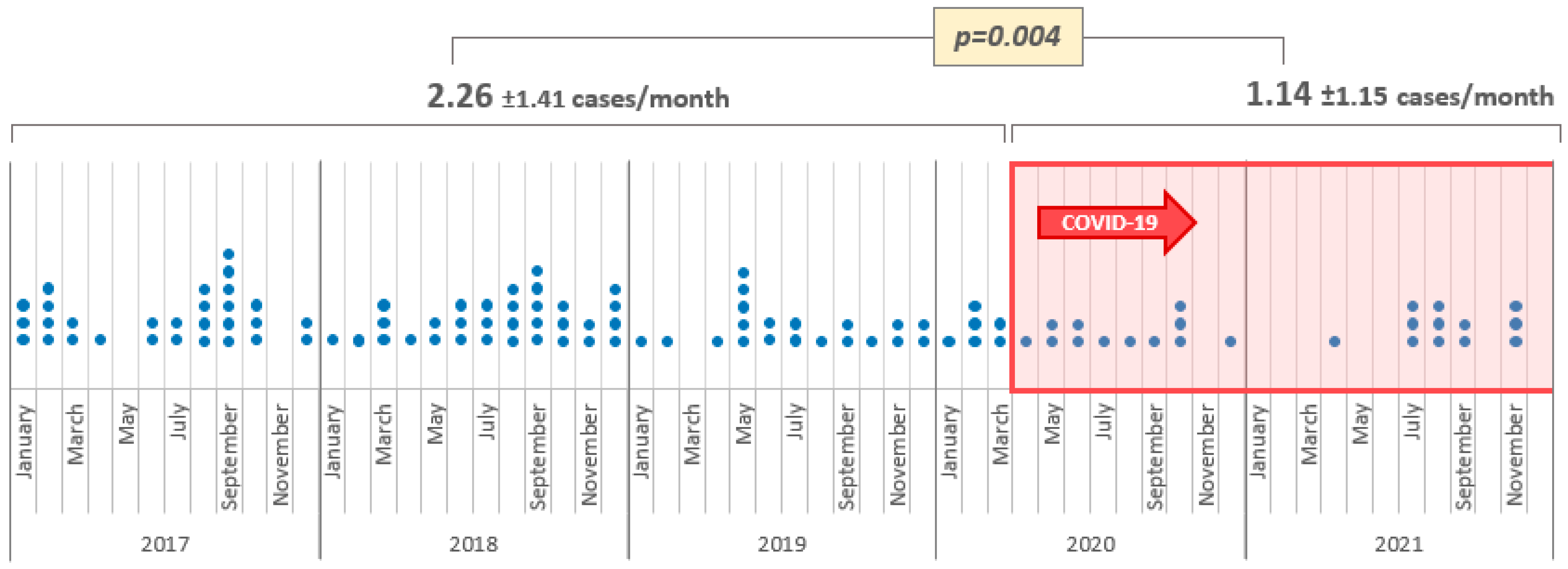
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| IE | Infective endocarditis |
| CT | Computed tomography |
| ICD | Implantable cardioverter defibrillator |
| CRT | Cardiac resynchronization therapy |
| CRP | C-reactive protein |
| NT-proBNP | N-terminal pro-brain natriuretic peptide |
| PCT | Procalcitonin |
References
- Chang, D.; Chang, X.; He, Y.; Tan, K.J.K. The determinants of COVID-19 morbidity and mortality across countries. Sci. Rep. 2022, 12, 5888. [Google Scholar] [CrossRef]
- Kalabikhina, I.E. Demographic and social issues of the pandemic. Popul. Econ. 2020, 4, 103–122. [Google Scholar] [CrossRef]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef]
- The Government of the Republic of Serbia. State of Emergency Declared Throughout Serbia. Published 15 March 2020. Available online: https://www.srbija.gov.rs/vest/en/151398/state-of-emergency-declared-throughout-serbia.php (accessed on 26 March 2023).
- Williams, M.L.; Doyle, M.P.; McNamara, N.; Tardo, D.; Mathew, M.; Robinson, B. Epidemiology of infective endocarditis before versus after change of international guidelines: A systematic review. Ther. Adv. Cardiovasc. Dis. 2021, 15, 17539447211002688. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- Redzek, A.; Preveden, A.; Kaloci, S.R.; Samardzija, G.; Preveden, M.; Golubovic, M.; Velicki, L. Unusual non-valvular left ventricular endocarditis presenting as multiple brain embolism. Acta Clin. Belg. 2022, 77, 142–146. [Google Scholar] [CrossRef]
- Havers-Borgersen, E.; Fosbøl, E.L.; Butt, J.H.; Petersen, J.K.; Dalsgaard, A.; Kyhl, F.; Schou, M.; Phelps, M.; Kragholm, K.; Gislason, G.H.; et al. Incidence of infective endocarditis during the coronavirus disease 2019 pandemic: A nationwide study. IJC Heart Vasc. 2020, 31, 100675. [Google Scholar] [CrossRef] [PubMed]
- Feldman, I.; Natsheh, A.; Nesher, G.; Breuer, G.S. Social distancing and bacteraemia in the time of COVID-19. Intern. Med. J. 2022, 52, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N. The Impact of the COVID-19 Pandemic on Physician Visits in Japan. Front. Public Health 2021, 9, 743371. [Google Scholar] [CrossRef] [PubMed]
- Arbune, M.; Iancu, A.V.; Lupasteanu, G.; Vasile, M.C.; Stefanescu, V. A Challenge of COVID-19: Associated Infective Endocarditis with Streptococcus gordonii in a Young Immunocompetent Patient. Medicine 2021, 57, 1298. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Ghosal, S.; Bhattacharyya, R.; Majumder, M. Impact of complete lockdown on total infection and death rates: A hierarchical cluster analysis. Diabetes Metab. Syndr. 2020, 14, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Krishna, N.; Jose, R.; Biju, S.S.C.; Pichandi, J.S.; Varma, P.K. Effects of the COVID-19 Pandemic on Cardiac Surgery Practice and Outcomes. J. Chest Surg. 2022, 55, 61–68. [Google Scholar] [CrossRef]
- COVIDSurg Collaborative. Elective surgery cancellations due to the COVID-19 pandemic: Global predictive modelling to inform surgical recovery plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar] [CrossRef]
- El–Hamamsy, I.; Brinster, D.R.; DeRose, J.J.; Girardi, L.N.; Hisamoto, K.; Imam, M.N.; Itagaki, S.; Kurlansky, P.A.; Lau, C.; Nemeth, S.; et al. The COVID-19 Pandemic and Acute Aortic Dissections in New York. J. Am. Coll. Cardiol. 2020, 76, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Mesnier, J.; Cottin, Y.; Coste, P.; Ferrari, E.; Schiele, F.; Lemesle, G.; Thuaire, C.; Angoulvant, D.; Cayla, G.; Bouleti, C.; et al. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: A registry study. Lancet Public Health 2020, 5, e536–e542. [Google Scholar] [CrossRef]
- Milovančev, A.; Petrović, M.; Popadić, V.; Miljković, T.; Klašnja, S.; Djuran, P.; Ilić, A.; Kovačević, M.; Stojšić Milosavljević, A.; Brajković, M.; et al. Characteristics and Outcomes of Patients with Acute Coronary Syndrome and COVID-19. J. Clin. Med. 2022, 11, 1791. [Google Scholar] [CrossRef]
- Hall, M.E.; Vaduganathan, M.; Khan, M.S.; Papadimitriou, L.; Long, R.C.; Hernandez, G.A.; Moore, C.K.; Lennep, B.W.; Mcmullan, M.R.; Butler, J. Reductions in Heart Failure Hospitalizations During the COVID-19 Pandemic. J. Card. Fail. 2020, 26, 462–463. [Google Scholar] [CrossRef]
- Hartnett, K.P.; Kite-Powell, A.; DeVies, J.; Coletta, M.A.; Boehmer, T.K.; Adjemian, J.; Gundlapalli, A.V.; National Syndromic Surveillance Program Community of Practice. Impact of the COVID-19 Pandemic on Emergency Department Visits—United States, January 1, 2019–May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 699–704. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Cuervo, G.; de Alarcón, A.; Sousa, D.; Barca, L.V.; Fernández-Hidalgo, N. Impact of the COVID-19 pandemic on the diagnosis, management and prognosis of infective endocarditis. Clin. Microbiol. Infect. 2021, 27, 660–664. [Google Scholar] [CrossRef]
- Cosyns, B.; Motoc, A.; Arregle, F.; Habib, G. A Plea Not to Forget Infective Endocarditis in COVID-19 Era. JACC Cardiovasc. Imaging 2020, 13, 2470–2471. [Google Scholar] [CrossRef]
- Van Camp, G.; De Beenhouwer, H.; Beles, M.; Collet, C.; Nasser, R.; Schelfaut, D.; Penicka, M. Disturbing effect of lockdown for COVID-19 on the incidence of infective endocarditis: A word of caution. Clin. Res. Cardiol. 2020, 109, 1573–1576. [Google Scholar] [CrossRef]
- Pommier, T.; Benzenine, E.; Bernard, C.; Mariet, A.S.; Béjot, Y.; Giroud, M.; Morgant, M.C.; Steinmetz, E.; Guenancia, C.; Bouchot, O.; et al. Trends of Myocarditis and Endocarditis Cases Before, During, and After the First Complete COVID-19-Related Lockdown in 2020 in France. Biomedicines 2022, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Morawiec, R.; Matuszewska-Brycht, O.; Maeser, P.; Kośny, M.; Krejca, M.; Drożdż, J. Decreasing numbers of valve-related infective endocarditis cases. An urgent call to action to improve diagnostic pathways: A retrospective tertiary center perspective (2015–2022). Kardiol. Pol. 2024, 82, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Fernandes, S.; Santos, L.G.; Carvalho, R.; Sa, F.M.; Martins, H.; Pernencar, S.; Ruivo, C.; Santos, B.; Morais, J. An outbreak of infective endocarditis during the COVID-19 pandemic?—An observational retrospective single centre study. Eur. Heart J. Acute Cardiovasc. Care 2021, 10 (Suppl. 1), zuab020.184. [Google Scholar] [CrossRef]
- Spatafora, F.; Matos Fialho, P.M.; Busse, H.; Helmer, S.M.; Zeeb, H.; Stock, C.; Wendt, C.; Pischke, C.R. Fear of Infection and Depressive Symptoms among German University Students during the COVID-19 Pandemic: Results of COVID-19 International Student Well-Being Study. Int. J. Environ. Res. Public Health 2022, 19, 1659. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M. Recommendations for the practice of echocardiography in infective endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219. [Google Scholar] [CrossRef]
- Skulstad, H.; Cosyns, B.; Popescu, B.A.; Galderisi, M.; Salvo, G.D.; Donal, E.; Petersen, S.; Gimelli, A.; Haugaa, K.H.; Muraru, D.; et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 592–598. [Google Scholar] [CrossRef]
- Pang, L.; Stahl, E.P.; Fujikura, K.; Chen, M.; Li, W.; Zhang, M.; Levsky, J.M.; Travin, M.I.; Ho, E.C.; Goldberg, Y.; et al. Echocardiography Abnormal Findings and Laboratory Operations during the COVID-19 Pandemic at a High Volume Center in New York City. Healthcare 2020, 8, 534. [Google Scholar] [CrossRef]
- Mikus, E.; Fiorentino, M.; Sangiorgi, D.; Fiaschini, C.; Tenti, E.; Tremoli, E.; Calvi, S.; Costantino, A.; Tripodi, A.; Zucchetta, F.; et al. Surgical Treatment of Active Endocarditis Pre- and Post-COVID-19 Pandemic Onset. Biomedicines 2024, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Zecchin, M.; Ciminello, E.; Mari, V.; Proclemer, A.; D’Onofrio, A.; Zanotto, G.; De Ponti, R.; Capovilla, T.M.; Laricchiuta, P.; Biondi, A.; et al. A global analysis of implants and replacements of pacemakers and cardioverter-defibrillators before, during, and after the COVID-19 pandemic in Italy. Intern. Emerg. Med. 2024, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]

| Overall n = 111 | Before COVID-19 n = 85 | During COVID-19 n = 26 | p-Value | |
|---|---|---|---|---|
| Females, n (%) | 40 (35.7%) | 35 (41.2%) | 5 (18.5%) | 0.032 |
| Age (years) | 65 [46–65] | 68 [52–74] | 53 [41–65] | 0.002 |
| Body mass index (kg/m2) | 25.3 [23.7–27.8] | 24.9 [23.4–27.9] | 25.7 [24.2–27.7] | 0.373 |
| Comorbidities | ||||
| Hypertension, n (%) | 65 (58.0%) | 53 (62.4%) | 12 (44.4%) | 0.100 |
| Prior MI, n (%) | 9 (8.0%) | 8 (9.4%) | 1 (3.7%) | 0.342 |
| Heart failure, n (%) | 24 (21.4%) | 15 (17.6%) | 9 (33.3%) | 0.084 |
| Stroke, n (%) | 16 (14.3%) | 13 (15.3%) | 3 (11.1%) | 0.588 |
| COPD, n (%) | 7 (6.2%) | 5 (5.9%) | 2 (7.4%) | 0.775 |
| Diabetes mellitus, n (%) | 28 (25.0%) | 23 (27.1%) | 5 (18.5%) | 0.372 |
| Renal failure, n (%) | 25 (22.3%) | 18 (21.2%) | 7 (25.9%) | 0.606 |
| Intravenous drug use, n (%) | 6 (5.4%) | 3 (3.5%) | 3 (11.1%) | 0.127 |
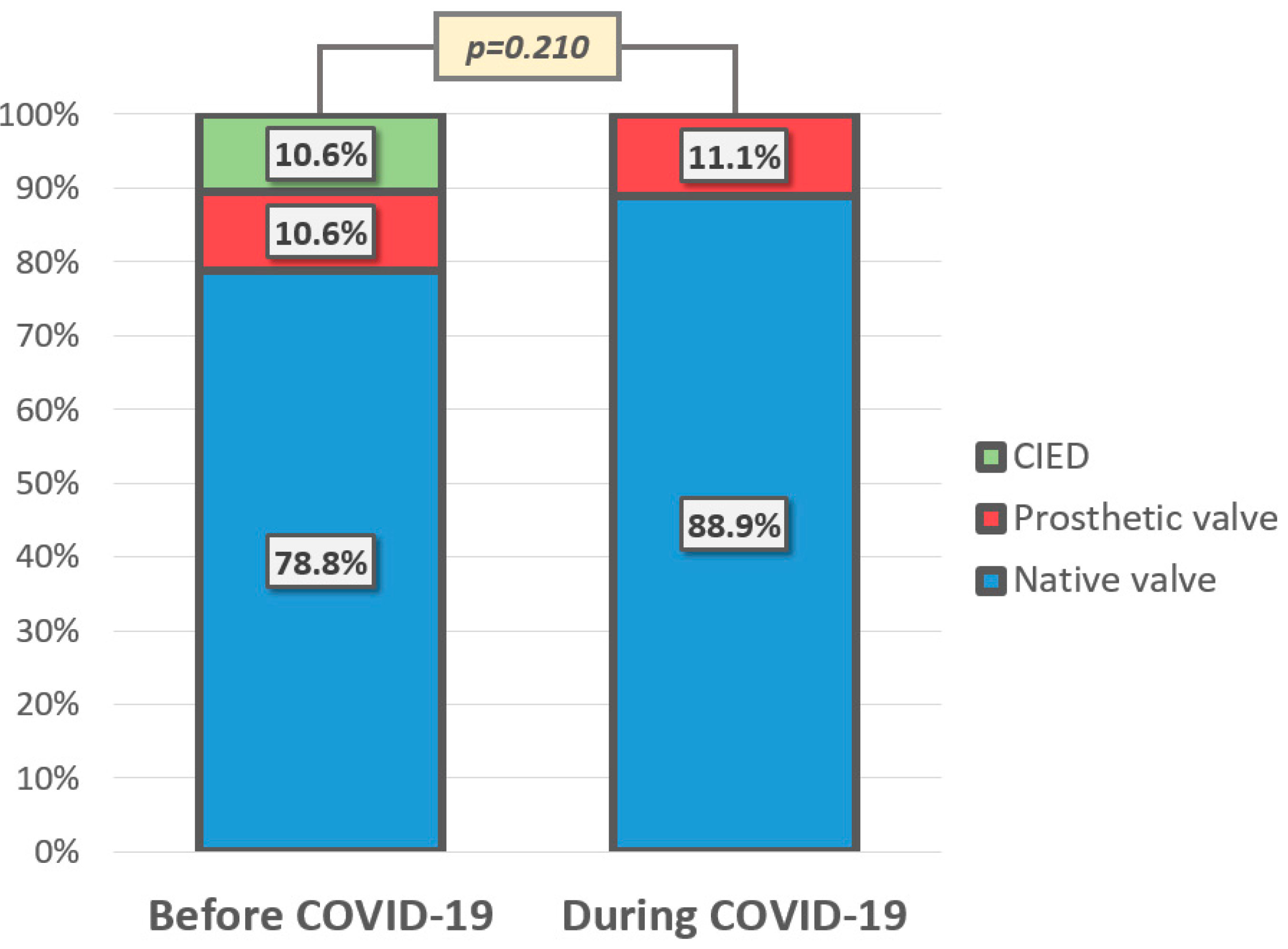
| Prosthetic heart valve, n (%) | 12 (10.7%) | 9 (10.6%) | 3 (11.1%) | 0.591 |
| Pacemaker/ICD/CRT, n (%) | 9 (8.0%) | 9 (10.6%) | 0 | 0.075 |
| Overall n = 111 | Before COVID-19 n = 85 | During COVID-19 n = 26 | p-Value | |
|---|---|---|---|---|
| Fever, n (%) | 21 (19.1%) | 18 (21.7%) | 3 (11.1%) | 0.225 |
| Laboratory | ||||
| CRP (mg/L) | 59.1 [25.5–98.5] | 59.9 [27.3–95.9] | 53.5 [19.6–101.4] | 0.568 |
| PCT (µg/L) | 0.28 [0.09–0.79] | 0.24 [0.09–0.96] | 0.30 [0.20–0.51] | 0.671 |
| NT-proBNP (ng/L) | 13,141 [2315–25,000] | 9023 [2219–25,000] | 17,664 [3695–25,000] | 0.096 |
| Performed imaging studies | ||||
| TTE, n (%) | 111 (100%) | 85 (100%) | 26 (100%) | 1.000 |
| TEE, n (%) | 23 (20.7%) | 20 (23.3%) | 3 (12.0%) | 0.222 |
| Cardiac CT, n (%) | 5 (4.5%) | 3 (3.5%) | 2 (8.0%) | 0.338 |
| Endocarditis finding | ||||
| Vegetation, n (%) | 107 (97.3%) | 83 (97.6%) | 24 (96.0%) | 0.657 |
| Abscess, n (%) | 12 (10.8%) | 9 (10.5%) | 3 (12.0%) | 0.828 |
| Pseudoaneurysm, n (%) | 1 (0.9%) | 0 (0.0%) | 1 (4.0%) | 0.062 |
| Prosthetic valve dehiscence, n (%) | 4 (3.6%) | 3 (3.5%) | 1 (4.0%) | 0.904 |
| Affected valve | ||||
| Aortic, n (%) | 46 (41.4%) | 31 (36.0%) | 15 (60.0%) | 0.032 |
| Mitral, n (%) | 49 (44.1%) | 39 (45.3%) | 10 (40.0%) | 0.635 |
| Tricuspid, n (%) | 14 (12.6%) | 10 (11.6%) | 4 (16.0%) | 0.562 |
| Pulmonary, n (%) | 3 (2.7%) | 3 (3.5%) | 0 (0.0%) | 0.344 |
| Multiple valves, n (%) | 11 (9.9%) | 7 (8.1%) | 4 (16.0%) | 0.247 |
| Treatment modality | ||||
| Conservative treatment, n (%) | 49 (43.8%) | 40 (47.1%) | 9 (33.3%) | 0.210 |
| Surgery, n (%) | 63 (56.2%) | 45 (52.9%) | 18 (66.7%) | |
| Time until surgery (days) | 5 [3–8] | 5 [2–8] | 7 [4–13] | 0.202 |
| Hospital stay (days) | 18 [12–24] | 16 [12–24] | 21 [15–27] | 0.095 |
| 30-day all-cause mortality, n (%) | 30 (26.8%) | 24 (28.2%) | 6 (22.2%) | 0.539 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Stroke | 3.364 (1.132–9.999) | 0.029 | - | ns |
| Fever | 3.136 (1.165–8.445) | 0.024 | - | ns |
| Treatment modality | 3.467 (1.435–8.376) | 0.006 | - | ns |
| Hospital stay | 0.911 (0.862–0.963) | 0.001 | 0.907 (0.852–0.965) | 0.002 |
| CRP | 1.021 (1.011–1.031) | <0.0005 | 1.025 (1.013–1.036) | <0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preveden, A.; Bandulaja, M.; Drljevic Todic, V.; Zdravkovic, R.; Golubovic, M.; Pantic, T.; Crnomarkovic, B.; Mladenovic, N.; Maletin, S.; Jarakovic, M.; et al. Impact of COVID-19 Pandemic on the Diagnosis and Management of Infective Endocarditis. COVID 2025, 5, 138. https://doi.org/10.3390/covid5080138
Preveden A, Bandulaja M, Drljevic Todic V, Zdravkovic R, Golubovic M, Pantic T, Crnomarkovic B, Mladenovic N, Maletin S, Jarakovic M, et al. Impact of COVID-19 Pandemic on the Diagnosis and Management of Infective Endocarditis. COVID. 2025; 5(8):138. https://doi.org/10.3390/covid5080138
Chicago/Turabian StylePreveden, Andrej, Marina Bandulaja, Vanja Drljevic Todic, Ranko Zdravkovic, Miodrag Golubovic, Teodora Pantic, Branislav Crnomarkovic, Nikola Mladenovic, Srdjan Maletin, Milana Jarakovic, and et al. 2025. "Impact of COVID-19 Pandemic on the Diagnosis and Management of Infective Endocarditis" COVID 5, no. 8: 138. https://doi.org/10.3390/covid5080138
APA StylePreveden, A., Bandulaja, M., Drljevic Todic, V., Zdravkovic, R., Golubovic, M., Pantic, T., Crnomarkovic, B., Mladenovic, N., Maletin, S., Jarakovic, M., Dabovic, D., Andric, D., Milosavljevic, A., Mladenovic, A., Maletin, S., Andric, S., & Preveden, M. (2025). Impact of COVID-19 Pandemic on the Diagnosis and Management of Infective Endocarditis. COVID, 5(8), 138. https://doi.org/10.3390/covid5080138









