Abstract
Introduction: Human metapneumovirus (HMPV), though commonly perceived as a pediatric pathogen, significantly impacts adults, yet its role in acute respiratory tract infections (ARTIs) remains underappreciated. The COVID-19 pandemic has reshaped respiratory virus epidemiology and amplified the need for comprehensive differential diagnosis. This study aimed to comprehensively investigate the prevalence, clinical characteristics, and post-COVID-19 trends of HMPV infection in adults and to elucidate its critical role in the differential diagnosis of ARTIs by distinguishing it from other common viral pathogens. Methods: This was a retrospective, multicenter study conducted across six hospitals within the Acibadem Hospitals Group in Istanbul, Turkey. Data were collected from two periods: January 2016 to January 2020 (pre-COVID-19) and January 2021 to September 2023 (post-COVID-19), excluding the peak pandemic phase (March 2020 to May 2021). Respiratory samples (sputum, BAL, nasopharyngeal/nasal/throat swabs) were analyzed using multiplex PCR (Seegene RV12-ACE), with an expanded panel including SARS-CoV-2 in the post-COVID-19 era. Demographic data, comorbidities, symptoms, hospitalization, and ICU admission rates were collected. Results: In the post-COVID-19 period, 2197 positive viral panels were recorded, an increase from 1357 in the pre-COVID period, reflecting enhanced testing. HMPV prevalence reached 9.7% post-COVID-19, making it the fourth most common respiratory virus in adults (8.7% of 644 positive adult tests), following SARS-CoV-2 (26.4%), influenza A (21.3%), and rhinovirus (17.5%). The average age of HMPV-infected adults was 52.14 years (18–90 years); 64% were female. While 52% had no comorbidities, common underlying conditions included hypertension (24%), cancer (12%), and diabetes (10%). Weakness (34%), lower respiratory symptoms (16%), and fever (12%) were frequent. A significant proportion of HMPV patients required hospitalization (34%) and ICU admission (18%), with 40% receiving antibiotics. Despite potential severity, the mortality rate was low (2.8%). No significant difference in severity was observed between HMPV monoinfection and co-infected groups (e.g., with influenza A, rhinovirus, SARS-CoV-2, parainfluenza virus 2). Conclusion: Our findings establish HMPV as a significant and increasingly prevalent respiratory pathogen among adults in Istanbul in the post-COVID-19 era. Its non-specific clinical presentation underscores the critical importance of multiplex PCR for accurate differential diagnosis, enabling appropriate patient management and antimicrobial stewardship. While HMPV can lead to severe outcomes requiring hospitalization and ICU admission, particularly in patients with comorbidities, the overall mortality rate remains low. Given the lack of specific antiviral treatments and vaccines, sustained surveillance and continued research into targeted interventions are crucial.
1. Introduction
Acute respiratory tract infections (ARTIs) represent a substantial global health burden, significantly contributing to morbidity and mortality across all age groups [1]. While numerous viral pathogens are implicated in these infections, comprehensive data on their specific etiology and epidemiology, particularly in adults, often remain limited [1]. The COVID-19 pandemic catalyzed a heightened awareness of viral infections, bringing previously underestimated pathogens into sharper focus [2]. Among the causative viruses, human metapneumovirus (HMPV) stands out; although commonly recognized as a childhood pathogen, its impact on vulnerable populations, such as frail elderly and immunocompromised adults, is increasingly acknowledged [3].
Globally identified as a seasonal infection 22 years ago [4], HMPV presents with clinical symptoms and infection patterns often indistinguishable from other respiratory viruses, including SARS-CoV-2 [5]. This clinical overlap underscores the critical challenge in differential diagnosis, as HMPV infection can resemble a wide array of respiratory illnesses. Despite its significant impact, routine screening for HMPV in adults is not widespread. Consequently, determining the true prevalence of adult HMPV infections is complicated by suboptimal surveillance systems, high rates of coinfections, and the lack of a universally precise definition for viral infection [6]. Nevertheless, existing studies indicate that asymptomatic infections are common in young adults [7], yet HMPV outbreaks can still contribute to increased mortality among elderly patients [8].
The advent and widespread availability of PCR tests have revolutionized viral diagnostics, enabling multiple respiratory viruses to be simultaneously identified [9]. This technological advancement, further accelerated by the increased viral testing during the COVID-19 pandemic [10], has led to increased identification of various infections, including HMPV. For HMPV, establishing the etiology is crucial, even when specific etiology-based treatments are not available, primarily to prevent unnecessary antimicrobial use [11]. Furthermore, accurate etiological diagnosis is indispensable for effective public health surveillance, curbing infection spread, and informing the development of targeted vaccines and preventive measures.
Evidence suggests that behavioral changes during the COVID-19 pandemic significantly altered the transmission dynamics of various respiratory viruses. While some saw a decrease in circulation, others, including HMPV, might have experienced shifts in their epidemiological patterns, potentially influencing their diagnosis and presentation [12]. The aim of this study was to comprehensively investigate the prevalence, clinical characteristics, and post-COVID-19 trends of human metapneumovirus infection in adults and elucidate its critical role in the differential diagnosis of acute respiratory tract infections by distinguishing it from other common viral pathogens.
2. Materials and Methods
2.1. Study Design and Ethical Considerations
This was a retrospective, multicenter study conducted across six hospitals within the Acibadem Hospitals Group in Istanbul, Turkey. The study was approved by the local institutional review board (approval number 2023-20/687). Due to the retrospective nature of this study, the requirement for informed consent was waived.
2.2. Patient Population and Data Collection
Data were collected from adult patients with acute respiratory tract infections who sought medical treatment between January 2016 and September 2023. The period from March 2020 to May 2021 was deliberately excluded to avoid the confounding effects of the COVID-19 pandemic peak on epidemiological and clinical data for other respiratory viruses. In this study, we included adult patients who were treated for acute respiratory tract infections within the designated time periods. Patients were excluded if they were pediatric, did not have a viral panel requested, or had test results from other hospitals.
2.3. Sample Collection and Viral Panel Analysis
Various respiratory samples, including sputum, bronchoalveolar lavage (BAL) fluid, nasopharyngeal swabs, nasal swabs, and throat swabs, were collected from the patients. Viral nucleic acids were extracted from these samples using a commercially available kit, following the manufacturer’s protocol.
All extracted nucleic acids underwent analysis for the presence of multiple respiratory viruses using the Seeplex® RV12 ACE Detection Kit (Seegene, Seoul, Republic of Korea). This is a multiplex end-point reverse transcription polymerase chain reaction (RT-PCR) assay that utilizes proprietary dual-priming oligonucleotide (DPO) technology to enhance amplification specificity and sensitivity. The assays were performed on a thermal cycle according to the manufacturer’s instructions.
The initial viral panel, used from 2016 until the COVID-19 pandemic, simultaneously detected 16 respiratory viruses, including human adenovirus (AdV), human coronavirus (HCoV) 229E/NL63, HCoV OC43/HKU1, human metapneumovirus, human parainfluenza virus (PIV) 1, PIV 2, PIV 3, human respiratory syncytial virus (RSV) A, RSV B, human rhinovirus (HRV) A/B, influenza A virus (Flu A), and influenza B virus (Flu B).
Following PCR amplification, the products were analyzed via gel electrophoresis. The amplicons were visualized on agarose gel and stained, and the results were interpreted based on the size of the amplified DNA fragments.
After the onset of the COVID-19 pandemic, the diagnostic strategy was adapted. The expanded panel included SARS-CoV-2 and provided separate detection for HCoV 229E, HCoV HKU1, HCoV OC43, and bocavirus (BoV). The results from these comprehensive viral panels formed the exclusive basis for our analysis.
2.4. Definitions
A patient was defined as positive for a specific viral pathogen if at least one multiplex PCR panel submitted during their hospitalization yielded a positive result for that pathogen. A patient’s viral panel result was considered negative for all tested pathogens if all submitted viral panels during that hospitalization returned negative results.
2.5. Statistical Analysis
All data were collected and entered into a dedicated database for analysis. Descriptive statistics were used to summarize patient characteristics and prevalence data. Continuous variables are presented as means, while categorical variables are presented as frequencies and percentages. To compare hospitalized and non-hospitalized patient groups, The chi-square test was used to compare categorical variables. The independent-samples t-test was used to compare continuous variables. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 26.0.
3. Results
A total of 7708 viral panels were evaluated. During the first study period, 1357 respiratory samples were positive for at least one respiratory tract virus, and 2197 were positive in the post-COVID period. The bar chart shown in Figure 1 illustrates the annual trends in HMPV prevalence across the specified years. The prevalence of HMPV infection varied by year, being lowest in 2017 and highest during the post-COVID period. Initially, HMPV prevalence exhibited a minor year-on-year fluctuation, being approximately 6.4% in 2016, followed by a slight decrease to 5.3% in 2017. Subsequently, the data indicate an increasing trend in HMPV prevalence from 2017 onwards, culminating in the highest recorded prevalence during the most recent period (2022–2023).
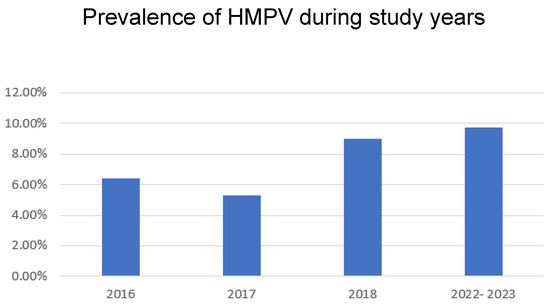
Figure 1.
Prevalence of HMPV in all ages during study years.
Table 1 shows the prevalence of HMPV and other respiratory viruses in adults, based on data from 644 positive viral tests. SARS-CoV-2 was the most frequently detected pathogen, accounting for a quarter of all positive results (n = 170, 26.4%). This was followed by influenza A (H1; H3) (n = 137, 21.3%) and rhinovirus (n = 113, 17.5%). Respiratory syncytial virus (RSV) was the fourth most common virus, detected in 62 adults (9.6%), and human metapneumovirus was the fifth most common virus, identified in 50 adults (8.7%). All other viruses, including various coronaviruses, parainfluenza viruses, adenoviruses, influenza B, and bocavirus, were each detected in less than 10% of the positive samples.

Table 1.
Prevalence of HMPV and other respiratory viruses in adults.
The average age of the patients was 52.14 years, with a range of 18 to 90 years. Overall, 36% (18) of the patients were male and 64% (32) were female (Table 2). The most common comorbidities were hypertension (24%), cancer (12%), and diabetes mellitus (10%), but 52% (26) of patients had no underlying medical conditions. The most common symptoms were weakness (34%), lower respiratory symptoms (16%), and fever (12%), with 4% of patients showing no symptoms. Seventeen (34%) patients were admitted to hospital and nine (18%) were admitted to an intensive care unit (ICU) (Table 3).

Table 2.
Human metapneumovirus-positive patients’ characteristics.

Table 3.
Characteristics of patients with HMPV infection.
Table 4 shows that the hospitalized patients with HMPV infection have no significant difference in age but have more frequent ischemic heart disease. However, severe HMPV infection can be a serious illness, with significant proportions of patients requiring hospitalization (17, 33.0%), ICU admission (9, 18.0%), and antibiotic treatment (20, 40.0%). Nevertheless, the mortality rate is relatively low, at only 2.8%.

Table 4.
Outcomes among patients with severe HMPV infection.
Figure 2, presented as a horizontal bar chart, comprehensively compares the seasonal prevalence of HMPV during two distinct epidemiological phases, namely "pre-COVID" and "post-COVID". A notable observation is the highest prevalence recorded in the post-COVID period, specifically in January, reaching approximately 20%, which surpasses the prevalence observed in any single month during the pre-COVID period. This figure strongly suggests that the COVID-19 pandemic and its associated public health interventions (e.g., lockdowns, mask-wearing, social distancing) have likely altered the typical seasonal patterns of HMPV. Overall, Figure 2 effectively demonstrates a significant alteration in the seasonal epidemiology of HMPV in the wake of the COVID-19 pandemic, transitioning from a more distinct winter/early spring peak to a potentially broader or shifted seasonal distribution.
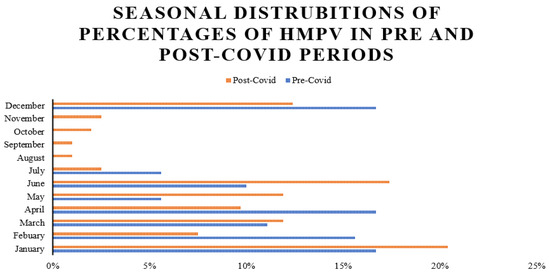
Figure 2.
Seasonal distributions of percentages of HMPV in pre- and post-COVID periods.
4. Discussion
A retrospective analysis of local surveillance data indicates that human metapneumovirus is predominant during the winter and early spring months, with sporadic detection throughout the year. This observation aligns with initial reports from the Netherlands, where HMPV was exclusively identified during winter [13]. Subsequent international investigations have further substantiated a winter-to-spring seasonal distribution [13]. While HMPV seasonality is not definitively established, temperate climates typically experience heightened HMPV prevalence during winter, often co-circulating with influenza and respiratory syncytial virus. However, one study noted peak HMPV activity extending into spring and early summer [14]. Variations in prevalence studies, localized outbreaks, and living conditions likely contribute to these observed differences.
The COVID-19 pandemic has demonstrably altered the epidemiology of respiratory viruses, with heterogeneous effects across different viral types and geographical regions [15,16]. Our data suggest that the pandemic influenced HMPV prevalence, elevating it to the fourth most common respiratory virus among adults in Istanbul. The post-COVID period appears to show an increased percentage of HMPV infections, potentially attributable to factors such as expanded testing capabilities, the implementation of public health interventions [17], and possible competitive dynamics between circulating viruses [12]. As the pandemic wanes, a return to established seasonal patterns for other respiratory viruses is anticipated. Historically, viral pneumonia in adults has been primarily attributed to adenoviruses, metapneumoviruses, parainfluenza group viruses, RSVs, and influenza viruses [18]. Prior to the COVID-19 pandemic, rhinovirus was the most frequent etiologic agent of viral pneumonia in adults; however, our data indicate a reduction in its prevalence post-COVID-19 [19]. Most existing studies focus on the pandemic period [19], underscoring the need for longitudinal studies to ascertain the re-establishment of pre-pandemic viral epidemiology. Of note, COVID-19 demonstrated rapid community spread during periods of peak community-acquired pneumonia virus activity. Preliminary research suggests that SARS-CoV-2 has a potential competitive inhibitory effect on the circulation of other respiratory viruses [12].
Our findings demonstrate that HMPV infection affects adults, particularly those with underlying medical comorbidities. Consistent with the extant literature, the most frequently reported symptoms in adults include weakness, lower respiratory tract symptoms, and fever. While HMPV is commonly associated with mild upper respiratory tract illnesses, it can progress to respiratory distress [20,21,22]. Compared to other respiratory viruses, HMPV is associated with comparable rates of intensive care unit (ICU) admission, mechanical ventilation, and length of hospital stay [21,23] In our cohort, while some patients required hospitalization and antibiotic treatment, the majority achieved recovery without complications. The severity and comprehensive clinical spectrum of HMPV infection can be influenced by diverse HMPV serotypes [21] and, to a greater extent, by the host’s humoral immunity [7]. Frail elderly individuals and immunocompromised patients are particularly susceptible to severe infections and exhibit higher mortality rates [24]. Furthermore, HMPV has been concurrently detected in the respiratory tracts of numerous encephalitis cases of unknown etiology [25]. Acute HMPV infection can also exacerbate pre-existing conditions such as asthma and chronic obstructive pulmonary disease (COPD) [26], being detected in up to 12% of patients experiencing COPD exacerbations [27]. Additionally, our analysis of the 50 HMPV-positive patients revealed a gender difference, with a higher proportion of females. While this finding suggests a higher incidence of HMPV in women in our specific cohort, it is important to interpret this result with caution due to the study’s limitations. The small sample size of our HMPV-positive group means that this gender distribution may be a statistical fluctuation and not necessarily representative of a larger population. The existing literature on gender differences in respiratory virus infections is mixed. In the future, larger-scale studies with a defined population will be needed to examine this gender distribution and to explore the contributing factors more definitively.
Although most elderly individuals possess neutralizing antibodies [28], these do not confer complete protection [29]. Antibody titers wane over time, predisposing individuals to reinfection throughout life [30]. HMPV co-infections are frequently observed, with their prevalence varying based on the study population [31], co-pathogen type [17], and season of study [32]. The pathophysiology of co-infection severity is intricate [13]. While some studies correlate co-infections with severe disease [17], other reports indicate no significant difference in outcomes between patients infected solely with HMPV versus those with a co-infection of HMPV and another respiratory virus [21]. Data on HMPV co-infections in adults are limited, though a case report of SARS-CoV-2 infection complicated by HMPV exists [33]. Our data do not indicate increased infection severity with viral co-infection. Specifically, no significant difference was observed between HMPV-only-infected and co-infected groups in our cohort, although a larger study would be required to thoroughly evaluate this aspect.
The clinical presentation, laboratory findings, and radiological results are non-specific and insufficient for a definitive differentiation between HMPV and other viral agents [34] necessitating microbiological confirmation [21,35]. Approximately 5% of specimens initially negative for other respiratory viruses were subsequently found positive for HMPV [21]. In clinical settings, viral culture is inefficient [4]. Immunofluorescent staining offers moderate sensitivity and high specificity [36]. Enzyme-linked immunosorbent assays were initially developed utilizing HMPV-infected cells as antigens [4,37], and recombinant proteins have been employed to enhance sensitivity and specificity [38]. Real-time reverse transcription-polymerase chain reaction (RT-PCR) is the most sensitive method for confirmation [39], and multiplex RT-PCR is recommended for routine clinical practice to augment diagnostic capacity for respiratory pathogens [40].
Currently, there is no specific antiviral treatment for HMPV infection. Clinical management primarily involves preventive and control measures, alongside supportive care, such as supplemental oxygen or mechanical ventilatory support, administered as clinically indicated [18,41,42]. The efficacy of polyclonal intravenous immunoglobulin-based immunotherapy, which has shown success in certain respiratory infections, remains inconclusive for HMPV [43]. Monoclonal antibodies targeting the HMPV F protein demonstrate promise for future clinical application [44]. While no vaccine is currently available for HMPV prevention, various immunization strategies are being investigated [45,46].
While our study provides valuable insights into HMPV prevalence and clinical characteristics in a post-pandemic setting, it is not without limitations. The retrospective design and the relatively small number of HMPV-positive patients (n = 50) restrict our ability to perform robust statistical comparisons between different subgroups and to draw definitive conclusions about the broader population of adults with HMPV infection. While our findings provide valuable descriptive insights into the clinical characteristics and outcomes of these patients, the small sample size may limit the generalizability of these observations. Future studies with larger patient cohorts are warranted to validate these findings and to more thoroughly investigate factors associated with disease severity. Despite this limitation, the findings from this study have significant real-world implications. By highlighting the substantial prevalence of HMPV, particularly in the post-pandemic era, our data underscore the importance of including HMPV in differential diagnoses for adult patients with ARTIs. This insight is crucial for guiding clinical suspicion, informing appropriate diagnostic testing strategies, and improving patient management, especially in high-risk groups. Furthermore, the detailed epidemiological data from multiple centers contribute to a broader understanding of how respiratory virus circulation is re-establishing itself following the profound disruptions of the COVID-19 pandemic, providing a foundation for future public health surveillance and resource allocation.
5. Conclusions
This multicenter retrospective study conducted in Istanbul reveals that human metapneumovirus is the fourth most common respiratory virus in adults, with increased prevalence post-COVID-19. HMPV can cause severe acute respiratory tract infections, leading to hospitalizations and ICU admissions, especially in those with comorbidities. The non-specific clinical presentation necessitates an accurate microbiological diagnosis via multiplex PCR, and the evolving epidemiology highlights the need for continued surveillance. As no specific treatments or vaccines exist, further research into therapies and prevention is crucial. This study reinforces HMPV’s importance in adult ARTIs and differential diagnosis.
Author Contributions
L.D. conceptualized the research, performed the statistical analysis, and was responsible for the final writing and editing; N.Y.U. oversaw the data collection process and performed all laboratory experiments; S.K. supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the local institutional review board (approval number 2023-20/687, 14 December 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request with permission from Ethics Committee.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Niederman, M.S.; Torres, A. Respiratory infections. Eur. Respir. Rev. 2022, 31, 220150. [Google Scholar] [CrossRef]
- Esposito, S.; Noviello, S.; Pagliano, P. Update on treatment of COVID-19: Ongoing studies between promising and disappointing results. Infez. Med. 2020, 28, 198–211. [Google Scholar]
- Haas, L.E.M.; Thijsen, S.F.T.; van Elden, L.; Heemstra, K.A. Human metapneumovirus in adults. Viruses 2013, 5, 87–110. [Google Scholar] [CrossRef]
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Bai, W.-Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Jain, S. Epidemiology of Viral Pneumonia. Clin. Chest Med. 2017, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Erdman, D.; Anderson, L.J.; Walsh, E.E. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 2003, 187, 785–790. [Google Scholar] [CrossRef]
- Boivin, G.; De Serres, G.; Hamelin, M.-E.; Côté, S.; Argouin, M.; Tremblay, G.; Maranda-Aubut, R.; Sauvageau, C.; Ouakki, M.; Boulianne, N.; et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin. Infect. Dis. 2007, 44, 1152–1158. [Google Scholar] [CrossRef]
- Brittain-Long, R.; Nord, S.; Olofsson, S.; Westin, J.; Anderson, L.M.; Lindh, M. Multiplex real-time PCR for detection of respiratory tract infections. J. Clin. Virol. 2008, 41, 53–56. [Google Scholar] [CrossRef]
- Mercer, T.R.; Salit, M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021, 22, 415–426. [Google Scholar] [CrossRef]
- Ruuskanen, O.; Lahti, E.; Jennings, L.C.; Murdoch, D.R. Viral pneumonia. Lancet 2011, 377, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B. Epidemiology of SARS-CoV-2. Infection 2021, 49, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Greensill, J.; McNamara, P.S.; Dove, W.; Flanagan, B.; Smyth, R.L.; Hart, C.A. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg. Infect. Dis. 2003, 9, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Tang, W.-H.; Chan, K.-H.; Khong, P.-L.; Guan, Y.; Lau, Y.-L.; Chiu, S.-S. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg. Infect. Dis. 2003, 9, 628–633. [Google Scholar] [CrossRef]
- Chow, E.J.; Uyeki, T.M.; Chu, H.Y. The effects of the COVID-19 pandemic on community respiratory virus activity. Nat. Rev. Microbiol. 2023, 21, 195–210. [Google Scholar] [CrossRef]
- Liu, P.; Xu, M.; Cao, L.; Su, L.; Lu, L.; Dong, N.; Jia, R.; Zhu, X. Impact of COVID-19 pandemic on the prevalence of respiratory viruses in children with lower respiratory tract infections in China. Virol. J. 2021, 18, 159. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Papanikolopoulou, A.; Vassiliu, S.; Theodoridou, K.; Nikolopoulou, G.; Sipsas, N.V. COVID-19 and respiratory virus co-infections: A systematic review of the literature. Viruses 2023, 15, 865. [Google Scholar] [CrossRef]
- Pagliano, P.; Sellitto, C.; Conti, V.; Ascione, T.; Esposito, S. Characteristics of viral pneumonia in the COVID-19 era: An update. Infection 2021, 49, 607–616. [Google Scholar] [CrossRef]
- Kreger, J.E.; Hershenson, M.B. Effects of COVID-19 and Social Distancing on Rhinovirus Infections and Asthma Exacerbations. Viruses 2022, 14, 2340. [Google Scholar] [CrossRef]
- Mullins, J.A.; Erdman, D.D.; Weinberg, G.A.; Edwards, K.; Hall, C.B.; Walker, F.J.; Iwane, M.; Anderson, L.J. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 2004, 10, 700–705. [Google Scholar] [CrossRef]
- Williams, J.V.; Harris, P.A.; Tollefson, S.J.; Halburnt-Rush, L.L.; Pingsterhaus, J.M.; Edwards, K.M.; Wright, P.F.; Crowe, J.E., Jr. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 2004, 350, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Boivin, G.; Abed, Y.; Pelletier, G.; Ruel, L.; Moisan, D.; Côté, S.; Peret, T.C.T.; Erdman, D.D.; Anderson, L.J. Virological features and clinical manifestations associated with human metapneumovirus: A new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 2002, 186, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Hermos, C.R.; Vargas, S.O.; McAdam, A.J. Human metapneumovirus. Clin. Lab. Med. 2010, 30, 131–148. [Google Scholar] [CrossRef]
- Sánchez Fernández, I.; Rebollo Polo, M.; Muñoz-Almagro, C.; Carretero, L.M.; Ureña, S.F.; Muñoz, A.R.; Roura, R.C.; Dueñas, B.P. Human Metapneumovirus in the Cerebrospinal Fluid of a Patient with Acute Encephalitis. Arch. Neurol. 2012, 69, 649–652. [Google Scholar] [CrossRef]
- Hamelin, M.E.; Côté, S.; Laforge, J.; Lampron, N.; Bourbeau, J.; Weiss, K.; Gilca, R.; DeSerres, G.; Boivin, G. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin. Infect. Dis. 2005, 41, 498–502. [Google Scholar] [CrossRef]
- Falsey, A.R. Human metapneumovirus infection in adults. Pediatr. Infect. Dis. J. 2008, 27, S80–S83. [Google Scholar] [CrossRef]
- Lüsebrink, J.; Wiese, C.; Thiel, A.; Tillmann, R.L.; Ditt, V.; Mu, A.; Schildgen, O.; Schildgen, V. High seroprevalence of neutralizing capacity against human metapneumovirus in all age groups studied in Bonn, Germany. Clin. Vaccine Immunol. 2010, 17, 481–484. [Google Scholar] [CrossRef]
- Okamoto, M.; Sugawara, K.; Takashita, E.; Muraki, Y.; Hongo, S.; Nishimura, H.; Matsuzaki, Y. Longitudinal course of human metapneumovirus antibody titers and reinfection in healthy adults. J. Med. Virol. 2010, 82, 2092–2096. [Google Scholar] [CrossRef]
- Williams, J.V.; Tollefson, S.J.; Johnson, J.E.; Crowe, J.E., Jr. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J. Virol. 2005, 79, 10944–10951. [Google Scholar] [CrossRef]
- Kanji, J.N.; Zelyas, N.; Pabbaraju, K.; Granger, D.; Wong, A.; Murphy, S.A.; Buss, E.; MacDonald, C.; Berenger, B.M.; Diggle, M.A.; et al. Respiratory virus coinfections with severe acute respiratory coronavirus virus 2 (SARS-CoV-2) continue to be rare one year into the coronavirus disease 2019 (COVID-19) pandemic in Alberta, Canada (June 2020–May 2021). Infect. Control Hosp. Epidemiol. 2023, 44, 805–808. [Google Scholar] [CrossRef]
- Uhteg, K.; Amadi, A.; Forman, M.; Mostafa, H.H. Circulation of non-SARS-CoV-2 respiratory pathogens and coinfection with SARS-CoV-2 amid the COVID-19 pandemic. Open Forum Infect. Dis. 2022, 9, ofab618. [Google Scholar] [CrossRef] [PubMed]
- Alharthy, A.; Faqihi, F.; Karakitsos, D. SARS-CoV-2 Complicated by Sinusitis and Co-Infection with Human Metapneumovirus. RI Med. J. 2020, 103, 23–24. [Google Scholar]
- Eccles, R.; Weber, O. Common Cold; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Samransamruajkit, R.; Thanasugarn, W.; Prapphal, N.; Theamboonlers, A.; Poovorawan, Y. Human metapneumovirus in infants and young children in Thailand with lower respiratory tract infections; molecular characteristics and clinical presentations. J. Infect. 2006, 52, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, T.; Endo, R.; Kikuta, H.; Ishiguro, N.; Ishiko, H.; Hara, M.; Takahashi, Y.; Kobayashi, K. Human metapneumovirus infection in Japanese children. J. Clin. Microbiol. 2004, 42, 126–132. [Google Scholar] [CrossRef]
- Ebihara, T.; Endo, R.; Kikuta, H.; Ishiguro, N.; Yoshioka, M.; Ma, X.; Kobayashi, K. Seroprevalence of human metapneumovirus in Japan. J. Med. Virol. 2003, 70, 281–283. [Google Scholar] [CrossRef]
- Hamelin, M.-E.; Boivin, G. Development and validation of an enzyme-linked immunosorbent assay for human metapneumovirus serology based on a recombinant viral protein. Clin. Diagn. Lab. Immunol. 2005, 12, 249–253. [Google Scholar] [CrossRef]
- Mackay, I.M.; Jacob, K.C.; Woolhouse, D.; Waller, K.; Syrmis, M.W.; Whiley, D.M.; Siebert, D.J.; Nissen, M.; Sloots, T.P. Molecular assays for detection of human metapneumovirus. J. Clin. Microbiol. 2003, 41, 100–105. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L.; Hay, A.J.; Cao, B.; Cox, R.J.; Dunning, J.; Moen, A.C.; Olson, D.; Pizzorno, A.; Hayden, F.G. COVID-19, Influenza and RSV: Surveillance-informed prevention and treatment—Meeting report from an isirv-WHO virtual conference. Antiviral Res. 2022, 197, 105227. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.-G.; Gu, L.; Zhang, Y.; Yan, X.-X.; Liang, Z.-A.; Zhang, W.; Jia, H.-Y.; Chen, W.; Liu, M.; et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respi. Viruses 2017, 11, 345–354. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Dexamethasone, A.U.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- Liu, X.; Cao, W.; Li, T. High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Front. Immunol. 2020, 11, 1660. [Google Scholar] [CrossRef] [PubMed]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998, 102, 531–537. [Google Scholar] [CrossRef]
- Schuster, J.E.; Williams, J.V. Human Metapneumovirus. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Skiadopoulos, M.H.; Biacchesi, S.; Buchholz, U.J.; Amaro-Carambot, E.; Surman, S.R.; Collins, P.L.; Murphy, B.R. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology 2006, 345, 492–501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).