SARS-CoV-2 and HCoV IgG Antibodies in the Breast Milk of a Postpartum SARS-CoV-2 Patient Following Bamlanivimab Administration: A Case Report
Abstract
1. Introduction
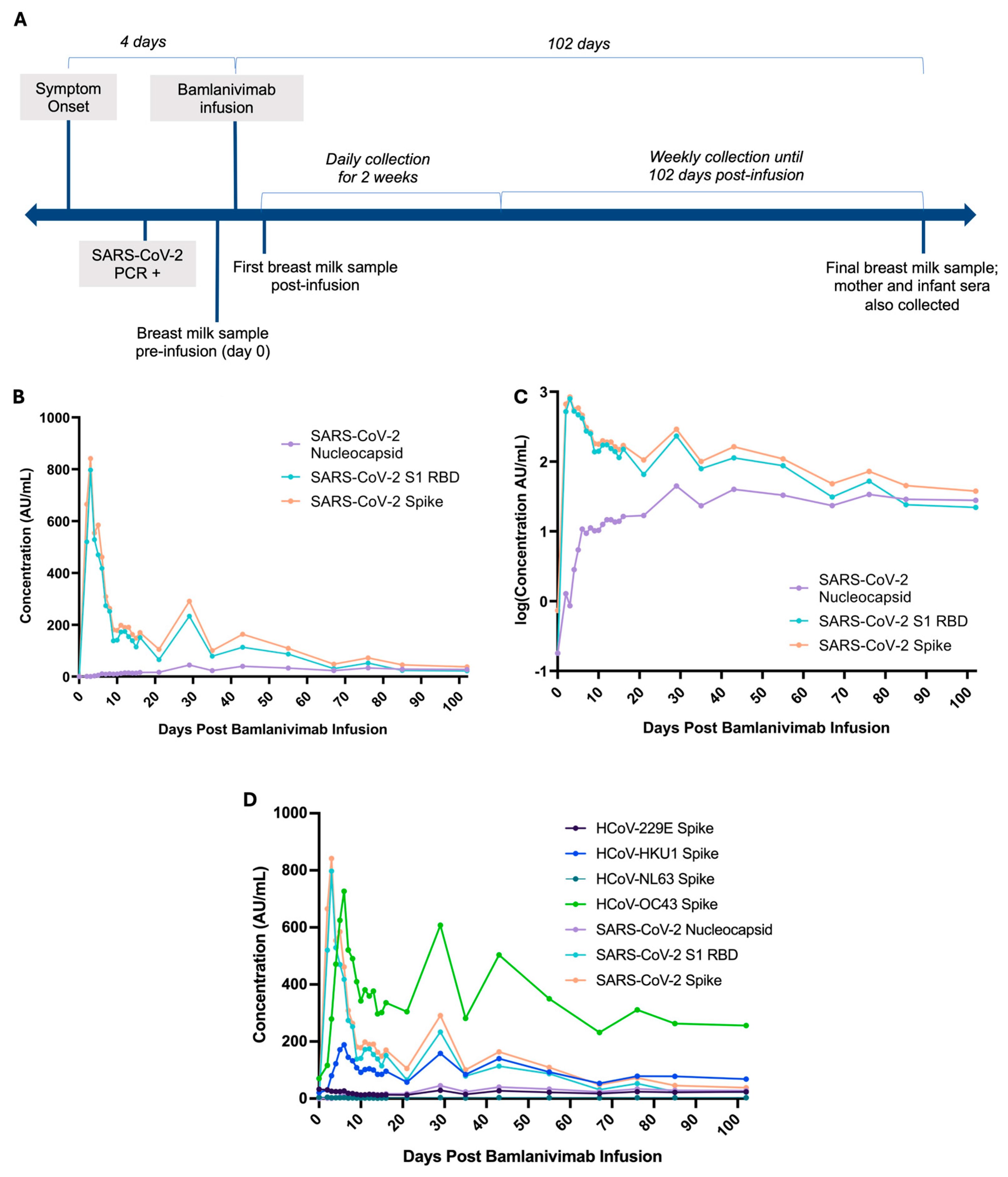
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| HCoV | Human coronaviruses |
| IgG | Immunoglobulin G |
| PCR | Polymerase chain reaction |
| mAb | Monoclonal antibody |
References
- Vassilopoulou, E.; Feketea, G.; Koumbi, L.; Mesiari, C.; Berghea, E.C.; Konstantinou, G.N. Breastfeeding and COVID-19: From Nutrition to Immunity. Front. Immunol 2021, 12, 661806. [Google Scholar] [CrossRef]
- Scrimin, F.; Campisciano, G.; Comar, M.; Ragazzon, C.; Davanzo, R.; Quadrifoglio, M.; Giangreco, M.; Stabile, G.; Ricci, G. IgG and IgA Antibodies Post SARS-CoV-2 Vaccine in the Breast Milk and Sera of Breastfeeding Women. Vaccines 2022, 10, 125. [Google Scholar] [CrossRef]
- Olearo, F.; Radmanesh, L.S.; Felber, N.; von Possel, R.; Emmerich, P.; Pekarek, N.; Pfefferle, S.; Nörz, D.; Hansen, G.; Diemert, A.; et al. Anti-SARS-CoV-2 antibodies in breast milk during lactation after infection or vaccination: A cohort study. J. Reprod. Immunol. 2022, 153, 103685. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.; Pentecost, B.T.; Schoen, C.N.; Alfandari, D.; Schneider, S.S.; Baker, R.; Arcaro, K.F. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obstet. Gynecol. 2022, 139, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2–Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef]
- Perez, S.E.; Luna Centeno, L.D.; Cheng, W.A.; Marentes Ruiz, C.J.; Lee, Y.; Congrave-Wilson, Z.; Powell, R.L.; Stellwagen, L.; Pannaraj, P.S. Human Milk SARS-CoV-2 Antibodies up to 6 Months After Vaccination. Pediatrics 2022, 149, e2021054260. [Google Scholar] [CrossRef]
- Graciliano, N.G.; Goulart, M.O.F.; de Oliveira, A.C.M. Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk. Int. J. Mol. Sci. 2025, 26, 2600. [Google Scholar] [CrossRef]
- Armistead, B.; Jiang, Y.; Carlson, M.; Ford, E.S.; Jani, S.; Houck, J.; Wu, X.; Jing, L.; Pecor, T.; Kachikis, A.; et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. Mucosal Immunol. 2023, 16, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Blanton, M.B.; Doratt, B.M.; Malherbe, D.C.; Rincon, M.; Messaoudi, I. Monoclonal Antibody Therapy of Breastfeeding Patient Infected with SARS-CoV-2: A Case Report. Breastfeed. Med. 2023, 18, 626–630. [Google Scholar] [CrossRef]
- Eli Lilly Canada, Inc. Bamlanivimab Product Monograph; Eli Lilly Canada, Inc.: Toronto, ON, Canada, 2021; pp. 1–19. [Google Scholar]
- Regulatory Decision Summary—Bamlanivimab—Health Canada [Internet]. Available online: https://covid-vaccine.canada.ca/info/regulatory-decision-summary-detailTwo.html?linkID=RDS00719& (accessed on 30 May 2025).
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.T.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 4704. [Google Scholar] [CrossRef]
- Tanunliong, G.; Liu, A.C.; Kaweski, S.; Irvine, M.; Reyes, R.C.; Purych, D.; Krajden, M.; Morshed, M.; Sekirov, I.; Gantt, S.; et al. Age-Associated Seroprevalence of Coronavirus Antibodies: Population-Based Serosurveys in 2013 and 2020, British Columbia, Canada. Front. Immunol. 2022, 13, 836449. [Google Scholar] [CrossRef]
- Aydillo, T.; Rombauts, A.; Stadlbauer, D.; Aslam, S.; Abelenda-Alonso, G.; Escalera, A.; Amanat, F.; Jiang, K.; Krammer, F.; Carratala, J.; et al. Immunological imprinting of the antibody response in COVID-19 patients. Nat. Commun. 2021, 12, 3781. [Google Scholar] [CrossRef]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Shrwani, K.; Sharma, R.; Krishnan, M.; Jones, T.; Mayora-Neto, M.; Cantoni, D.; Temperton, N.J.; Dobson, S.L.; Subramaniam, K.; McNamara, P.S.; et al. Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post–COVID-19 Convalescent Samples. J. Infect. Dis. 2021, 224, 1305–1315. [Google Scholar] [CrossRef]
- Aguilar-Bretones, M.; Westerhuis, B.M.; Raadsen, M.P.; de Bruin, E.; Chandler, F.D.; Okba, N.M.; Haagmans, B.L.; Langerak, T.; Endeman, H.; Akker, J.P.v.D.; et al. Seasonal coronavirus–specific B cells with limited SARS-CoV-2 cross-reactivity dominate the IgG response in severe COVID-19. J. Clin. Investig. 2021, 131, e150613. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040.e14. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; DaPra, C.; Mathijssen, G.; Sela, D.A.; Järvinen, K.M.; Seppo, A.; Fels, S.; Medo, E. Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers. Int. J. Mol. Sci. 2021, 22, 1749. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; DaPra, C.; Mathijssen, G.B.; Medo, E. Previous viral symptoms and individual mothers influenced the leveled duration of human milk antibodies cross-reactive to S1 and S2 subunits from SARS-CoV-2, HCoV-229E, and HCoV-OC43. J. Perinatol. 2021, 41, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Liu, A.; Gibbs, E.; Tanunliong, G.; Marquez, A.C.; Gantt, S.; Frykman, H.; Krajden, M.; Morshed, M.; Prystajecky, N.A.; et al. A novel multiplex electrochemiluminescent immunoassay for detection and quantification of anti-SARS-CoV-2 IgG and anti-seasonal endemic human coronavirus IgG. J. Clin. Virol. 2022, 146, 105050. [Google Scholar] [CrossRef]
- Korchinski, I.; Marquez, C.; McClymont, E.; Av-Gay, G.; Andrade, J.; Elwood, C.; Jassem, A.; Krajden, M.; Morshed, M.; Sadarangani, M.; et al. Maternal-infant transfer of SARS-CoV-2 antibodies following vaccination in pregnancy: A prospective cohort study. Vaccine 2024, 42, 126123. [Google Scholar] [CrossRef]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. [Google Scholar] [CrossRef]
- Chigutsa, E.; O’Brien, L.; Ferguson-Sells, L.; Long, A.; Chien, J. Population Pharmacokinetics and Pharmacodynamics of the Neutralizing Antibodies Bamlanivimab and Etesevimab in Patients With Mild to Moderate COVID-19 Infection. Clin. Pharmacol. Ther. 2021, 110, 1302–1310. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef]
- Kim, P.S.; Dimcheff, D.E.; Siler, A.; Schildhouse, R.J.; Chensue, S.W. Effect of monoclonal antibody therapy on the endogenous SARS-CoV-2 antibody response. Clin. Immunol. 2022, 236, 108959. [Google Scholar] [CrossRef]
- Petro, C.D.; Hooper, A.T.; Peace, A.; Mohammadi, K.; Eagan, W.; Elbashir, S.M.; DiPiazza, A.; Makrinos, D.; Pascal, K.; Bandawane, P.; et al. Monoclonal antibodies against the spike protein alter the endogenous humoral response to SARS-CoV-2 vaccination and infection. Sci. Transl. Med. 2024, 16, eadn0396. [Google Scholar] [CrossRef]
- LaHue, S.C.; Anderson, A.; Krysko, K.M.; Rutatangwa, A.; Dorsey, M.J.; Hale, T.; Mahadevan, U.; Rogers, E.E.; Rosenstein, M.G.; Bove, R. Transfer of monoclonal antibodies into breastmilk in neurologic and non-neurologic diseases. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e769. [Google Scholar] [CrossRef]
- Sagar, M.; Reifler, K.; Rossi, M.; Miller, N.S.; Sinha, P.; White, L.F.; Mizgerd, J.P. Recent endemic coronavirus infection is associated with less-severe COVID-19. J. Clin. Investig. 2021, 131, e143380. [Google Scholar] [CrossRef]
- Fillmore, N.R.; Szalat, R.E.; La, J.; Branch-Elliman, W.; Monach, P.A.; Nguyen, V.; Samur, M.K.; Brophy, M.T.; Do, N.V.; Munshi, N.C. Recent common human coronavirus infection protects against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A Veterans Affairs cohort study. Proc. Natl. Acad. Sci. USA 2022, 119, e2213783119. [Google Scholar] [CrossRef]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y.; Ellebedy, A.; Schultz-Cherry, S. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio 2020, 11, e01991-20. [Google Scholar] [CrossRef]
- van den Elsen, L.W.J.; Kollmann, T.R.; Verhasselt, V. Microbial antigen in human milk: A natural vaccine? Mucosal Immunol. 2022, 15, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71, S112–S120. [Google Scholar] [CrossRef]
| Days Post Infusion | Sample Type | Description | HCoV-229E Spike (AU/mL) | HCoV-HKU1 Spike (AU/mL) | HCoV-NL63 Spike (AU/mL) | HCoV-OC43 Spike (AU/mL) | SARS-CoV-2 Nucleocapsid (AU/mL) | SARS-CoV-2 S1 RBD (AU/mL) | SARS-CoV-2 Spike (AU/mL) |
|---|---|---|---|---|---|---|---|---|---|
| 0 | milk | Pre-infusion baseline, 4 days post-symptom onset | 32.69 | 20.73 | 4.57 | 70.23 | 0.18 | 0.18 | 0.74 |
| 102 | milk | mother milk | 23.62 | 68.38 | 3.08 | 255.79 | 27.92 | 22.13 | 37.86 |
| serum | mother serum | 17,712.70 | 46,143.88 | 2009.79 | 165,563.13 | 18,506.28 | 34,046.59 | 31,833.55 | |
| serum | infant serum | 2336.92 | 1311.99 | 245.42 | 4257.35 | 6836.31 | 14,140.66 | 16,418.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanunliong, G.; Condin, C.; Márquez, A.C.; Li, S.; Binning, N.; Gibson, M.; Griffiths, B.; Wright, A.; Money, D.; Krajden, M.; et al. SARS-CoV-2 and HCoV IgG Antibodies in the Breast Milk of a Postpartum SARS-CoV-2 Patient Following Bamlanivimab Administration: A Case Report. COVID 2025, 5, 123. https://doi.org/10.3390/covid5080123
Tanunliong G, Condin C, Márquez AC, Li S, Binning N, Gibson M, Griffiths B, Wright A, Money D, Krajden M, et al. SARS-CoV-2 and HCoV IgG Antibodies in the Breast Milk of a Postpartum SARS-CoV-2 Patient Following Bamlanivimab Administration: A Case Report. COVID. 2025; 5(8):123. https://doi.org/10.3390/covid5080123
Chicago/Turabian StyleTanunliong, Guadalein, Christopher Condin, Ana Citlali Márquez, Susan Li, Nimrat Binning, Miriam Gibson, Brayden Griffiths, Alissa Wright, Deborah Money, Mel Krajden, and et al. 2025. "SARS-CoV-2 and HCoV IgG Antibodies in the Breast Milk of a Postpartum SARS-CoV-2 Patient Following Bamlanivimab Administration: A Case Report" COVID 5, no. 8: 123. https://doi.org/10.3390/covid5080123
APA StyleTanunliong, G., Condin, C., Márquez, A. C., Li, S., Binning, N., Gibson, M., Griffiths, B., Wright, A., Money, D., Krajden, M., Morshed, M., Jassem, A. N., Haljan, G., & Sekirov, I. (2025). SARS-CoV-2 and HCoV IgG Antibodies in the Breast Milk of a Postpartum SARS-CoV-2 Patient Following Bamlanivimab Administration: A Case Report. COVID, 5(8), 123. https://doi.org/10.3390/covid5080123






