Endothelial Activation and Stress Index (EASIX) to Predict the Outcome of Patients with COVID-19
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luft, T.; Benner, A.; Jodele, S.; Dandoy, C.E.; Storb, R.; Gooley, T.; Sandmaier, B.M.; Becker, N.; Radujkovic, A.; Dreger, P.; et al. EASIX in patients with acute graft-versus-host disease: A retrospective cohort analysis. Lancet Haematol. 2017, 4, e414–e423. [Google Scholar] [CrossRef] [PubMed]
- Luft, T.; Benner, A.; Terzer, T.; Jodele, S.; Dandoy, C.E.; Storb, R.; Kordelas, L.; Beelen, D.; Gooley, T.; Sandmaier, B.M.; et al. EASIX and mortality after allogeneic stem cell transplantation. Bone Marrow Transpl. 2020, 55, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, S.; Michonneau, D.; de Fontbrune, F.S.; del Galy, A.S.; Xhaard, A.; Robin, M.; de Latour, R.P.; Socie, G. Allogeneic reactivity–mediated endothelial cell complications after HSCT: A plea for consensual definitions. Blood Adv. 2019, 3, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-Y.; Jung, S.-H.; Kim, K.; Kim, S.J.; Yoon, S.E.; Lee, H.S.; Kim, M.; Ahn, S.-Y.; Ahn, J.-S.; Yang, D.-H.; et al. Endothelial activation and stress index (EASIX) is a reliable predictor for overall survival in patients with multiple myeloma. BMC Cancer 2020, 20, 803. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.; Meijers, J.; Brandjes, D.; van Gorp, E. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Heide, R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020, 8, 681–686. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Luft, T.; Wendtner, C.-M.; Kosely, F.; Radujkovic, A.; Benner, A.; Korell, F.; Kihm, L.; Bauer, M.F.; Dreger, P.; Merle, U. EASIX for Prediction of Outcome in Hospitalized SARS-CoV-2 Infected Patients. Front. Immunol. 2021, 12, 634416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-García, F.; Bailén, R.; Torres-Macho, J.; Fernández-Rodríguez, A.; Jiménez-Sousa, M.Á.; Jiménez, E.; Pérez-Butragueño, M.; Cuadros-González, J.; Cadiñanos, J.; García-García, I.; et al. Age-Adjusted Endothelial Activation and Stress Index for Coronavirus Disease 2019 at Admission Is a Reliable Predictor for 28-Day Mortality in Hospitalized Patients With Coronavirus Disease 2019. Front. Med. 2021, 8, 736028. [Google Scholar] [CrossRef] [PubMed]
- Kalicińska, E.; Biernat, M.; Rybka, J.; Zińczuk, A.; Janocha-Litwin, J.; Rosiek-Biegus, M.; Morawska, M.; Waszczuk-Gajda, A.; Drozd-Sokołowska, J.; Szukalski, Ł.; et al. Endothelial Activation and Stress Index (EASIX) as an Early Predictor for Mortality and Overall Survival in Hematological and Non-Hematological Patients with COVID-19: Multicenter Cohort Study. J. Clin. Med. 2021, 10, 4373. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; Aggarwal, G.; Wong, J.; Benoit, S.; Vikse, J.; Plebani, M.; Lippi, G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am. J. Emerg. Med. 2020, 38, 1722–1726. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- Gandini, O.; Criniti, A.; Ballesio, L.; Giglio, S.; Galardo, G.; Gianni, W.; Santoro, L.; Angeloni, A.; Lubrano, C. Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19. J. Infect. 2020, 81, 979–997. [Google Scholar] [CrossRef]
- Cao, P.; Wu, Y.; Wu, S.; Wu, T.; Zhang, Q.; Zhang, R.; Wang, Z.; Zhang, Y. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers 2021, 26, 207–212. [Google Scholar] [CrossRef]
- Qeadan, F.; Tingey, B.; Gu, L.Y.; Packard, A.H.; Erdei, E.; Saeed, A.I. Prognostic Values of Serum Ferritin and D-Dimer Trajectory in Patients with COVID-19. Viruses 2021, 13, 419. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin—From iron, through inflammation and autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, J.; Wang, W.; Wang, X.; Zhou, J.; Chen, Z.; Li, J.; Chen, Y.; Yan, H.; Zhang, J.; et al. Case Fatality Risk of the First Pandemic Wave of Coronavirus Disease 2019 (COVID-19) in China. Clin. Infect. Dis. 2021, 73, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Carey, I.M.; Cook, D.G.; Harris, T.; DeWilde, S.; Chaudhry, U.A.R.; Strachan, D.P. Risk factors for excess all-cause mortality during the first wave of the COVID-19 pandemic in England: A retrospective cohort study of primary care data. PLoS ONE 2021, 16, e0260381. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.M.; Roelens, M.; Friker, B.; Thiabaud, A.; Iten, A.; Cusini, A.; Flury, D.; Buettcher, M.; Zucol, F.; Balmelli, C.; et al. Risk factors for severe outcomes for COVID-19 patients hospitalised in Switzerland during the first pandemic wave, February to August 2020: Prospective observational cohort study. Swiss Med. Wkly. 2021, 151, w20547. [Google Scholar] [CrossRef]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef]
- Demirelli, D.S.; Genc, G.; Basarir, C.I.; Bulut, S. Comparison of Clinical Characteristics of COVID-19-Related and Unrelated Acute Stroke Patients During the COVID-19 Pandemic in Turkey. Sisli Etfal Hast. Tip Bul. 2022, 56, 55–61. [Google Scholar] [CrossRef]
- Okuyucu, M.; Ozturk, O.; Atay, M.H.; Gullu, Y.T.; Temocin, F.; Terzi, O. Clinical Evaluation of Patients with COVID-19 Within the Framework of Comorbidities. Sisli Etfal Hast. Tip Bul. 2022, 56, 311–317. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Li, Y.; Chen, H.; Chen, T.; Su, N.; Huang, F.; Zhou, J.; Zhang, B.; Yan, F.; et al. Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 201, 1430–1434. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.J.; Subramaniam, A.; Reddy, M.P.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef] [PubMed]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef]
- Al-Azzawi, M.; Douedi, S.; Alshami, A.; Al-Saoudi, G.; Mikhail, J. Spontaneous Subcutaneous Emphysema and Pneumomediastinum in COVID-19 Patients: An Indicator of Poor Prognosis? Am. J. Case Rep. 2020, 21, e925557. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.-N.; Sivanushanthan, S.; Etling, E.; Hockstein, M.; Yohannes, S.; Clark, P. Incidence of barotrauma in patients with COVID-19 (alpha- and beta-predominant period) requiring mechanical ventilation: Single-center retrospective study. SAGE Open Med. 2023, 11, 20503121231159479. [Google Scholar] [CrossRef]
- Kahn, M.R.; Watson, R.L.; Thetford, J.T.; Wong, J.I.; Kamangar, N. High Incidence of Barotrauma in Patients With Severe Coronavirus Disease 2019. J. Intensiv. Care Med. 2021, 36, 646–654. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; del Barrio, I.D.M.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef]
- Caravita, S.; Baratto, C.; Di Marco, F.; Calabrese, A.; Balestrieri, G.; Russo, F.; Faini, A.; Soranna, D.; Perego, G.B.; Badano, L.P.; et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur. J. Heart Fail. 2020, 22, 2228–2237. [Google Scholar] [CrossRef]
- Zou, F.; Qian, Z.; Wang, Y.; Zhao, Y.; Bai, J. Cardiac Injury and COVID-19: A Systematic Review and Meta-analysis. CJC Open 2020, 2, 386–394. [Google Scholar] [CrossRef]
- Khan, W.; Safi, A.; Muneeb, M.; Mooghal, M.; Aftab, A.; Ahmed, J. Complications of invasive mechanical ventilation in critically Ill Covid-19 patients—A narrative review. Ann. Med. Surg. 2022, 80, 104201. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Sakellari, I.; Chatzikonstantinou, T.; Mallouri, D.; Batsis, I.; Vardi, A.; Bousiou, Z.; Koravou, E.-E.; Masmanidou, M.; Touloumenidou, T.; et al. Endothelial and Complement Activation As Predictors of Survival in Adult Allogeneic Hematopoietic Cell Transplantation. HemaSphere 2020, 5, e487. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, A.; Salas, M.Q.; Rodríguez-Lobato, L.G.; Escribano-Serrat, S.; Suárez-Lledo, M.; Martínez-Cebrian, N.; Solano, M.T.; Arcarons, J.; Rosiñol, L.; Gutiérrez-García, G.; et al. Easix Score Correlates With Endothelial Dysfunction Biomarkers and Predicts Risk of Acute Graft-Versus-Host Disease After Allogeneic Transplantation. Transplant. Cell. Ther. 2024, 30, 187.e1–187.e12. [Google Scholar] [CrossRef]
- de Nooijer, A.H.; Grondman, I.; Janssen, N.A.F.; Netea, M.G.; Willems, L.; van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.J.; Toonen, E.J.M.; Joosten, L.A.B.; RCI-COVID-19 study group. Complement Activation in the Disease Course of Coronavirus Disease 2019 and Its Effects on Clinical Outcomes. J. Infect. Dis. 2020, 223, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Narayan, R.K.; Kumari, C.; Faiq, M.A.; Kulandhasamy, M.; Kant, K.; Pareek, V. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med. Hypotheses 2020, 145, 110320. [Google Scholar] [CrossRef]
- Chang, J. COVID-19 Sepsis: Pathogenesis and Endothelial Molecular Mechanisms Based on “Two-Path Unifying Theory” of Hemostasis and Endotheliopathy-Associated Vascular Microthrombotic Disease, and Proposed Therapeutic Approach with Antimicrothrombotic Therapy. Vasc. Health Risk Manag. 2021, 17, 273–298. [Google Scholar] [CrossRef]

| Survivors (n = 31) | Non-Survivors (n = 58) | p | ||
|---|---|---|---|---|
| Age (years) | 60.5 ± 20.3 | 69.3 ± 14.7 | 0.039 b | |
| Gender | Female | 13 (41.9%) | 30 (51.7%) | 0.511 a |
| Male | 18 (58.1%) | 28 (48.3%) | ||
| APACHE II score | 14.9 ± 7.9 | 31.2 ± 10.5 | <0.001 b | |
| Comorbidity | No | 11 (35.5%) | 8 (13.8%) | 0.035 a |
| Yes | 20 (64.5%) | 50 (86.2%) | ||
| Diabetes mellitus | 9 (29.0%) | 24 (41.4%) | 0.358 a | |
| Hypertension | 15 (48.4%) | 37 (63.8%) | 0.238 a | |
| Coronary artery disease | 7 (22.6%) | 14 (24.1%) | 1.000 a | |
| Chronic heart failure | 3 (9.7%) | 13 (22.4%) | 0.230 a | |
| Stroke | 2 (6.5%) | 0 (0.0%) | 0.119 a | |
| Thyroid disorders | 2 (6.5%) | 4 (6.9%) | 1.000 a | |
| Liver disease | 0 (0.0%) | 0 (0.0%) | -- | |
| Chronic kidney disease | 0 (0.0%) | 8 (13.8%) | <0.001 a | |
| Chronic obstructive pulmonary disease | 4 (12.9%) | 15 (25.9%) | 0.250 a | |
| Cancer | 2 (6.5%) | 8 (13.8%) | 0.484 a | |
| Length of ICU stay (days) | 7.0 (4.0–12.0) | 12.5 (6.8–21.3) | 0.005 c | |
| Length of hospital stay (days) | 17.2 ± 9.8 | 17.8 ± 12.3 | 0.820 b | |
| Respiratory support | Invasive mechanical ventilation | 2 (6.5%) | 58 (100.0%) | <0.001 a |
| High-flow | 17 (54.8%) | 0 (0.0%) | ||
| Mask | 12 (38.7%) | 0 (0.0%) | ||
| Lactate dehydrogenase (U/L) | 449.5 ± 225.1 | 559.6 ± 223.0 | 0.030 b | |
| Creatinine (mg/dL) | 0.77 (0.60–0.90) | 1.09 (0.75–1.38) | 0.004 c | |
| Troponin I (ng/L) | 5.0 (2.5–12.0) | 17.5 (9.3–72.0) | <0.001 c | |
| Prothrombin time (sec) | 12.6 (11.6–13.0) | 13.1 (12.6–14.6) | 0.006 c | |
| Prothrombin activity (%) | 87.8 ± 15.3 | 76.3 ± 20.3 | 0.007 b | |
| INR | 1.09 ± 0.12 | 1.21 ± 0.30 | 0.032 b | |
| Activated partial thromboplastin time (sec) | 22.2 (20.6–24.6) | 24.6 (21.9–27.7) | 0.011 c | |
| Fibrinogen (g/L) | 5.8 ± 1.8 | 6.2 ± 1.6 | 0.306 b | |
| D-dimer (mg/L) | 1.21 (0.72–1.79) | 1.75 (0.99–3.58) | 0.033 c | |
| Procalcitonin (µg/L) | 0.09 (0.05–0.17) | 0.22 (0.10–0.61) | 0.002 c | |
| Ferritin (µg/L) | 355.0 (150.0–628.0) | 526.0 (279.8–1152.8) | 0.012 c | |
| Leukocyte (×109/L) | 11.0 ± 5.5 | 10.7 ± 5.3 | 0.799 b | |
| Lymphocyte (×109/L) | 0.72 (0.46–0.98) | 0.58 (0.34–0.93) | 0.753 c | |
| Neutrophil (×109/L) | 9.6 (5.5–12.8) | 8.9 (5.7–12.3) | 0.935 c | |
| Monocyte (×109/L) | 0.38 (0.24–0.54) | 0.34 (0.23–0.53) | 0.555 c | |
| Eosinophile (×109/L) | 0.02 (0.01–0.05) | 0.02 (0.01–0.06) | 0.843 c | |
| Hemoglobin (g/dL) | 12.8 ± 1.7 | 12.2 ± 2.0 | 0.141 b | |
| Haematocrit (%) | 39.5 ± 4.2 | 38.3 ± 6.0 | 0.273 b | |
| Platelet (×109/L) | 301.2 ± 124.5 | 255.6 ± 100.7 | 0.064 b | |
| Albumin (g/L) | 35.7 ± 3.4 | 34.7 ± 4.5 | 0.295 b | |
| Lactate (mmol/L) | 2.5 (1.8–3.0) | 2.2 (1.6–2.9) | 0.218 c | |
| C-reactive protein (g/L) | 0.07 (0.04–0.15) | 0.11 (0.04–0.18) | 0.225 c | |
| IL-6 (pg/mL) | 14.1 (6.1–29.7) | 50.7 (17.5–154.6) | 0.001 c | |
| EASIX score | 1.2 (0.7–2.0) | 2.5 (1.6–4.2) | <0.001 c | |
| Log2-EASIX score | 0.2 ± 0.9 | 1.3 ± 1.2 | <0.001 b | |
| Mortality | ||
|---|---|---|
| r | p | |
| Age (years) | 0.237 | 0.018 |
| APACHE II score | 0.636 | <0.001 |
| Length of ICU stay (days) | 0.264 | 0.008 |
| Method of oxygen support | −0.936 | <0.001 |
| Lactate dehydrogenase (U/L) | 0.242 | 0.016 |
| Creatinine (mg/dL) | 0.292 | 0.003 |
| Troponin I (ng/L) | 0.350 | <0.001 |
| Prothrombin time (sec) | 0.279 | 0.005 |
| Prothrombin activity (%) | −0.271 | 0.007 |
| INR | 0.278 | 0.005 |
| Activated partial thromboplastin time (sec) | 0.237 | 0.018 |
| D-dimer (mg/L) | 0.238 | 0.017 |
| Procalcitonin (µg/L) | 0.296 | 0.003 |
| Ferritin (µg/L) | 0.212 | 0.035 |
| IL-6 (pg/mL) | 0.313 | 0.002 |
| EASIX score | 0.317 | 0.001 |
| Log2-EASIX score | 0.317 | 0.001 |
| Risk Factor | B | SE | Wald | Odds | 95% CI | p * |
|---|---|---|---|---|---|---|
| Age (years) | 0.036 | 0.021 | 2.860 | 1.04 | 0.99–1.08 | 0.091 |
| APACHE II | 0.223 | 0.057 | 15.114 | 1.25 | 1.12–1.40 | <0.001 |
| Length of ICU stay (days) | 0.136 | 0.061 | 4.929 | 1.15 | 1.02–1.29 | 0.026 |
| Prothrombin time (sec) | 0.562 | 0.382 | 2.161 | 1.75 | 0.83–3.71 | 0.142 |
| Log2-EASIX score | 1.394 | 0.466 | 8.954 | 4.03 | 1.62–10.04 | 0.003 |
| Constant | −16.779 | 6.145 | 7.457 | 0.006 |
| Risk Factor | B | SE | Wald | Odds | 95% CI | p * |
|---|---|---|---|---|---|---|
| APACHE II | 0.186 | 0.042 | 19.630 | 1.20 | 1.11–1.31 | <0.001 |
| Log2-EASIX score | 1.062 | 0.333 | 10.155 | 2.89 | 1.51–5.56 | 0.001 |
| Constant | −4.334 | 1.052 | 16.972 | 0.01 | 0.00–0.00 | <0.001 |
| AUC | 95% CI | Cut-Off | Sensitivity | Specificity | Youden Index | +PV | −PV | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 0.634 | 0.525–0.733 | >55 | 84.5 | 48.4 | 0.329 | 75.4 | 62.5 | 0.055 |
| APACHE II score | 0.886 | 0.801–0.943 | >18 | 87.9 | 74.2 | 0.621 | 86.4 | 76.7 | 0.001 |
| Length of ICU stay (days) | 0.681 | 0.574–0.776 | >11 | 56.9 | 74.2 | 0.311 | 80.5 | 47.9 | 0.002 |
| Lactate dehydrogenase (U/L) | 0.674 | 0.566–0.769 | >471 | 55.2 | 77.4 | 0.326 | 82.1 | 48.0 | 0.005 |
| Creatinine (mg/dL) | 0.688 | 0.581–0.782 | >0.9 | 65.5 | 77.4 | 0.429 | 84.4 | 54.5 | 0.001 |
| Troponin I (ng/L) | 0.754 | 0.651–0.839 | >9 | 75.9 | 74.2 | 0.501 | 84.6 | 62.2 | 0.001 |
| Prothrombin time (sec) | 0.677 | 0.569–0.772 | >13.1 | 50.0 | 80.7 | 0.307 | 82.9 | 46.3 | 0.003 |
| Prothrombin activity (%) | 0.668 | 0.560–0.764 | ≤79.7 | 58.6 | 74.2 | 0.328 | 81.0 | 48.9 | 0.005 |
| INR | 0.659 | 0.551–0.757 | >1.1 | 48.3 | 80.7 | 0.289 | 82.4 | 45.5 | 0.006 |
| aPTT (sn) | 0.664 | 0.556–0.761 | >25.2 | 48.3 | 80.7 | 0.289 | 82.4 | 45.5 | 0.005 |
| D-dimer (mg/L) | 0.638 | 0.529–0.737 | >1.49 | 62.1 | 71.0 | 0.330 | 80.0 | 50.0 | 0.025 |
| Procalcitonin (µg/L) | 0.701 | 0.595–0.794 | >0.14 | 63.8 | 74.2 | 0.380 | 82.2 | 52.3 | 0.001 |
| Ferritin (µg/L) | 0.662 | 0.554–0.759 | >689 | 43.1 | 83.9 | 0.270 | 83.3 | 44.1 | 0.008 |
| IL-6 (pg/mL) | 0.715 | 0.609–0.806 | >26.5 | 67.2 | 74.2 | 0.414 | 83.0 | 54.8 | <0.001 |
| EASIX score | 0.764 | 0.662–0.847 | >2.05 | 70.7 | 83.9 | 0.546 | 89.1 | 60.5 | 0.001 |
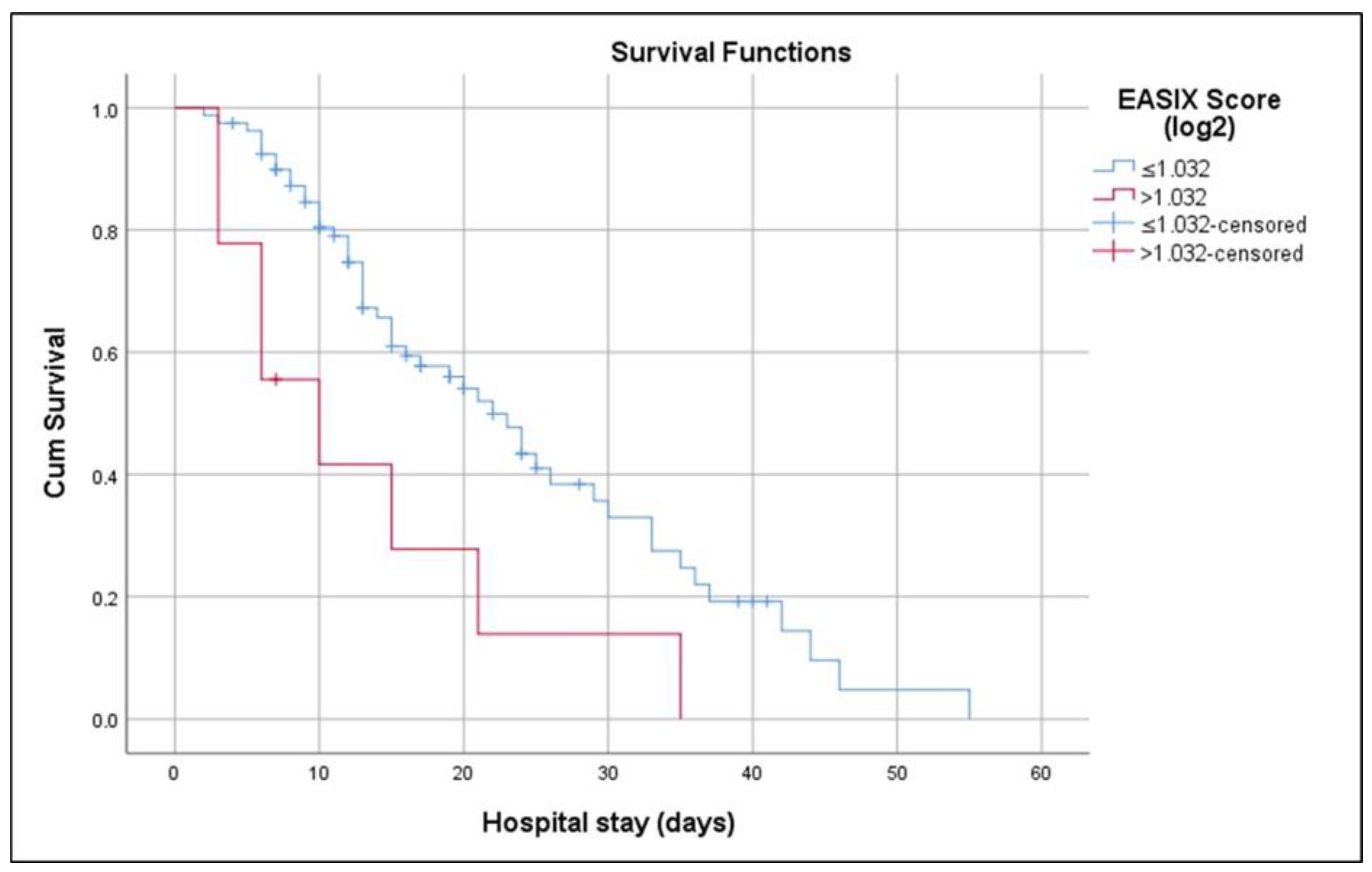
| Log2-EASIX score | 0.764 | 0.662–0.847 | >1.032 | 70.7 | 83.9 | 0.546 | 89.1 | 60.5 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokcinar, D.; Lafci, A.; Izdes, S. Endothelial Activation and Stress Index (EASIX) to Predict the Outcome of Patients with COVID-19. COVID 2025, 5, 89. https://doi.org/10.3390/covid5060089
Gokcinar D, Lafci A, Izdes S. Endothelial Activation and Stress Index (EASIX) to Predict the Outcome of Patients with COVID-19. COVID. 2025; 5(6):89. https://doi.org/10.3390/covid5060089
Chicago/Turabian StyleGokcinar, Derya, Ayse Lafci, and Seval Izdes. 2025. "Endothelial Activation and Stress Index (EASIX) to Predict the Outcome of Patients with COVID-19" COVID 5, no. 6: 89. https://doi.org/10.3390/covid5060089
APA StyleGokcinar, D., Lafci, A., & Izdes, S. (2025). Endothelial Activation and Stress Index (EASIX) to Predict the Outcome of Patients with COVID-19. COVID, 5(6), 89. https://doi.org/10.3390/covid5060089






