Effect of Human Immunodeficiency Virus (HIV) Infection on Mortality Among Hospitalised COVID-19 Patients at Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Site
2.3. Study Populations
2.3.1. Inclusion Criteria
2.3.2. Exclusion Criteria
2.4. Sample Size Determination and Sampling Methods
2.4.1. Sample Size Determination
2.4.2. Sampling Methods
2.5. Data Collection
2.6. Statistical Analysis
2.7. Handling of Missing Data
2.8. Ethical Considerations
3. Results
3.1. Participants’ Baseline Characteristics
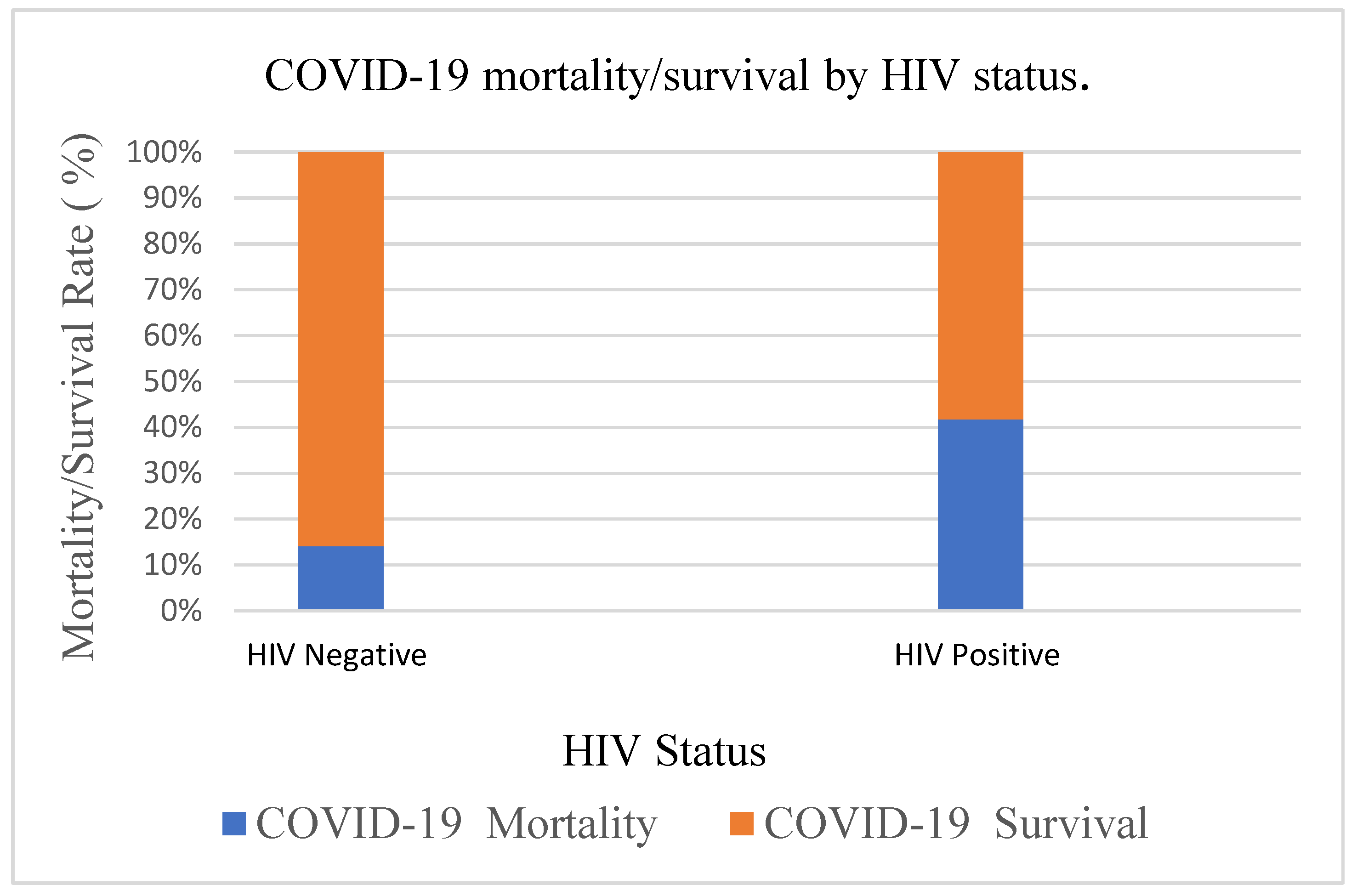
3.2. Mortality Among COVID-19 Patients Stratified by HIV Status
3.3. Baseline Characteristics Univariate Comparison
3.3.1. Univariable and Multivariable Logistic Regression Analysis
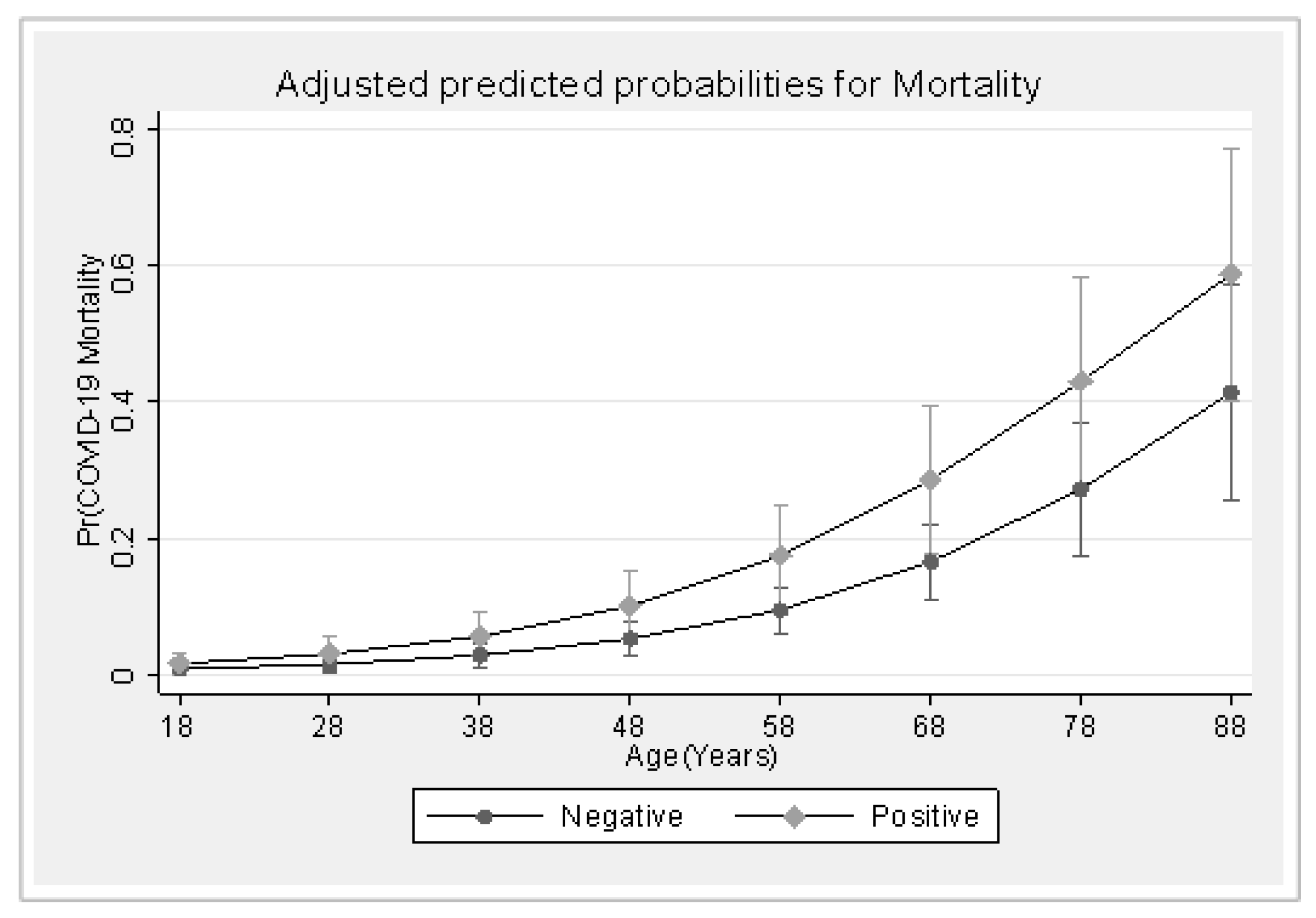
3.3.2. The Predicted Probabilities of COVID-19 Mortality by HIV Status Across Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Q.; Huang, D.; Ou, P.; Yu, H.; Zhu, Z.; Xia, Z.; Su, Y.; Ma, Z.; Zhang, Y.; Li, Z.; et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy 2020, 75, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.W.; Crawford, J.M.; Mospan, A.R.; E Watkins, S.; Munoz, B.; Zink, R.C.; Elliott, S.; Burleson, K.; Landis, C.; Reddy, K.R.; et al. Patient Characteristics and Outcomes of 11,721 Patients with Coronavirus Disease 2019 (COVID-19) Hospitalized Across the United States. Clin. Infect. Dis. 2020, 72, e558–e565. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Heilbrunn, E.S.; Ba, D.M.; Chinchilli, V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0238215. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Patidar, R.; Younis, K.; Desai, P. Comorbidity and Its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020, 2, 1069–1076. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 August 2021).
- Chipimo, P.J.; Barradas, D.T.; Kayeyi, N.; Zulu, P.M.; Muzala, K.; Mazaba, M.L. MMWR, First 100 Persons with COVID-19—Zambia, March 18–April 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1547–1548. [Google Scholar] [CrossRef]
- John Hopkins—Zambia. Available online: https://coronavirus.jhu.edu/region/zambia (accessed on 1 August 2021).
- Chanda, D.; Minchella, P.A.; Kampamba, D.; Itoh, M.; Hines, J.Z.; Fwoloshi, S. COVID-19 Severity and COVID-19—Associated Deaths Among Hospitalized Patients with HIV Infection—Zambia, March–December 2020. Mmwr-Morb. Mortal. Wkly. Rep. 2021, 70, 807–810. [Google Scholar] [CrossRef]
- Survey, P.A.H.-F. Biomedical Equipment for COVID-19 Case Management. Available online: https://www.path.org/our-impact/resources/biomedical-equipment-covid-19-case-management-zambia-covid-19-treatment-facility-survey-report/ (accessed on 2 April 2021).
- Ssentongo, P.; Heilbrunn, E.S.; Ssentongo, A.E.; Advani, S.; Chinchilli, V.M.; Nunez, J.J.; Du, P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6283. [Google Scholar] [CrossRef]
- Allwood, B.W.; Koegelenberg, C.F.; Irusen, E.; Lalla, U.; Davids, R.; Chothia, Y.; Davids, R.; Prozesky, H.; Taljaard, J.; Parker, A.; et al. Clinical evolution, management and outcomes of patients with COVID-19 admitted at Tygerberg Hospital, Cape Town, South Africa: A research protocol. BMJ Open 2020, 10, e039455. [Google Scholar] [CrossRef]
- Shiau, S.; Krause, K.D.; Valera, P.; Swaminathan, S.; Halkitis, P.N. The Burden of COVID-19 in People Living with HIV: A Syndemic Perspective. AIDS Behav. 2020, 24, 0123456789. [Google Scholar] [CrossRef]
- ZAMPHIA. The Zambia Population-Based HIV/AIDS Impact Assessment 2021 (ZAMPHIA 2021). Available online: https://stacks.cdc.gov/view/cdc/133880 (accessed on 1 December 2022).
- Mweemba, C.; Hangoma, P.; Fwemba, I.; Mutale, W.; Masiye, F. Estimating district HIV prevalence in Zambia using small-area estimation methods (SAE). Popul. Health Metr. 2022, 20, 8. [Google Scholar] [CrossRef]
- Dai, M.; Tao, L.; Chen, Z.; Tian, Z.; Guo, X.; Lodola, F. Influence of Cigarettes and Alcohol on the Severity and Death of COVID-19: A Multicenter Retrospective Study in Wuhan, China. Front. Physiol. 2020, 11, 588553. [Google Scholar] [CrossRef] [PubMed]
- Albadawy, R.M.; Jadoon, B.A.; Mogahed, M.M.; Ibrahim, M.E.; Essawy, T.S.; Amin, A.M.A.; Abd-Elraouf, M.S.; Elawady, M.A. The Impact of Comorbidities on the Outcomes of Egyptian COVID-19 Patients: A Follow-Up Study. J. Environ. Public Health 2021, 2021, 6662476. [Google Scholar] [CrossRef] [PubMed]
- Tesoriero, J.M.; Swain, C.-A.E.; Pierce, J.L.; Zamboni, L.; Wu, M.; Holtgrave, D.R.; Gonzalez, C.J.; Udo, T.; Morne, J.E.; Hart-Malloy, R.; et al. COVID-19 Outcomes Among Persons Living with or Without Diagnosed HIV Infection in New York State. JAMA Netw. Open 2021, 4, e2037069. [Google Scholar] [CrossRef] [PubMed]
- WHO. Clinical Features and Prognostic Factors of COVID-19 in People Living with HIV Hospitalized with Suspected or Confirmed SARS-CoV-2 Infection; WHO: Geneva, Switzerland, 2021; pp. 1–20. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Clinical-HIV-2021.1 (accessed on 1 August 2022).
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Gatechompol, S.; Avihingsanon, A.; Putcharoen, O.; Ruxrungtham, K. COVID-19 and HIV infection co-pandemics and their impact: A review of the literature. AIDS Res. Ther. 2021, 18, 28. [Google Scholar] [CrossRef]
- Alcorn, K. COVID-19 and coronavirus in people living with HIV Key points Are people with HIV at higher risk of infection? Br. Med. Bull. 2022, 147, 20–30. [Google Scholar]
- Aborode, A.T.; Olotu, T.M.; Oyetunde, O.B.; Ajagbe, A.O.; Mustapha, M.A.; Karra-Aly, A.; Oko, C.I. COVID-19 outcomes in HIV patients: A review. Ann. Med. Surg. 2022, 78, 103768. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Sun, K.; Ma, Y.; Hsue, P.Y. Association of HIV infection with outcomes among adults hospitalized with COVID-19. AIDS 2022, 36, 391–398. [Google Scholar] [CrossRef]
- Id, A.I.; Shah, J.; Id, R.S. The burden and characteristics of HIV-infected COVID-19 patients at a tertiary care hospital in sub-Saharan Africa—A retrospective cohort study. PLoS ONE 2022, 17, e0273859. [Google Scholar] [CrossRef]
- Minchella, P.A.; Chanda, D.; Hines, J.Z.; Fwoloshi, S.; Itoh, M.; Kampamba, D.; Chirwa, R.; Sivile, S.; Zyambo, K.D.; Agolory, S.; et al. Clinical Characteristics and Outcomes of Patients Hospitalized With COVID-19 During the First 4 Waves in Zambia. JAMA Netw. Open 2022, 5, e2246152. [Google Scholar] [CrossRef]
- Collaborators, E.M. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020-21. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Hoffmann, C. Immune de fi ciency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2020, 22, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Gervasoni, C.; Meraviglia, P.; Riva, A.; Giacomelli, A.; Oreni, L.; Minisci, D.; Atzori, C.; Ridolfo, A.; Cattaneo, D. Clinical Features and Outcomes of Patients with Human Immunodeficiency Virus with COVID-19. Clin. Infect. Dis. 2020, 71, 2276–2278. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.R.; Benjamin-neelon, S.E.; Sonnenschein, S. Alcohol Consumption during the COVID-19 Pandemic: A Cross-Sectional Survey of US Adults. Int. J. Environ. Res. Public Health 2020, 17, 9189. [Google Scholar] [CrossRef]
- Menon, J.A.; Kusanthan, T.; Mwaba, S.O.C. Alcohol and Tobacco Usage among Students in a Higher Education Institution in Lusaka, Zambia. Transl. Biomed. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Yaya, S.; Bishwajit, G. Alcohol and Tobacco Use among Men in Zambia and Zimbabwe. J. Lifestyle Med. 2019, 9, 67–73. [Google Scholar] [CrossRef]
- Silumbwe, A.; Sabastian, M.S.; Michelo, C.; Zulu, J.M.; Johansson, K. Sociodemographic factors associated with daily tobacco smoking and binge drinking among Zambians: Evidence from the 2017 STEPS survey. BMC Public Health 2022, 22, 205. [Google Scholar] [CrossRef]
- Nzala, S.H.; Songolo, P.; Rudatsikira, E. Alcohol Consumption in Lusaka Urban District, Zambia: A Population Based Survey, 2007. J. Public Health Epidemiol. 2011, 3, 419–423. [Google Scholar]
- Calina, D.; Hartung, T.; Mardare, I.; Mitroi, M.; Poulas, K.; Tsatsakis, A.; Rogoveanu, I.; Docea, A.O. COVID-19 pandemic and alcohol consumption: Impacts and interconnections. Toxicol. Rep. 2021, 8, 529–535. [Google Scholar] [CrossRef]
- OECD. The Effect of COVID-19 on Alcohol Consumption, and Policy Responses to Prevent Harmful Alcohol Consumption; OECD: Paris, France, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Sigel, K.; Swartz, T.; Golden, E.; Paranjpe, I.; Somani, S.; Richter, F.; De Freitas, J.K.; Miotto, R.; Zhao, S.; Polak, P.; et al. Coronavirus 2019 and People Living With Human Immunodeficiency Virus: Outcomes for Hospitalized Patients in New York City. Clin. Infect. Dis. 2020, 71, 2933–2938. [Google Scholar] [CrossRef]
- Jassat, L.B.W.; Cohen, C.; Masha, M.; Goldstein, S.; Kufa, T.; Savulescu, D.; Walaza, S.; Bam, J.-L.; Davies, M.-A.; Prozesky, H.W.; et al. COVID-19 in-hospital mortality in South Africa: The intersection of communicable and non-communicable chronic diseases in a high HIV Research in context. Preprint 2020. Available online: https://www.medrxiv.org/content/10.1101/2020.12.21.20248409v1 (accessed on 1 June 2021).

| Characteristic | COVID-19 Outcome | Sig. | |
|---|---|---|---|
| Died | Survived | ||
| Continuous Variables | |||
| Age (years) | |||
| Median (IQR) | 67.5 (61, 75.5) | 53 (41, 63) | <0.0001 W |
| CRP | |||
| Median (IQR) | 26.8 (18, 34) | 3.4 (2.3, 5.1) | <0.0001 W |
| Categorical Variables, n (%) | |||
| Sex | |||
| Female | 51 (20.8) | 194 (79.2) | 0.513 C |
| Male | 85 (18.8) | 368 (81.2) | |
| HIV Status | |||
| Negative | 70 (14.1) | 426 (85.9) | <0.0001 C |
| Positive | 66 (41.8) | 92 (58.2) | |
| Smoking | |||
| No | 95 (16.9) | 467 (83.1) | <0.0001 C |
| Yes | 39 (30.7) | 88 (69.3) | |
| Alcohol drinking | |||
| No | 49 (14.8) | 282 (85.2) | 0.003 C |
| Yes | 87 (23.8) | 279 (76.2) | |
| Comorbidity | |||
| None | 3 (1.9) | 152 (98.1) | <0.0001 C |
| One | 20 (6.7) | 280 (93.3) | |
| Two and more | 113 (46.5) | 130 (53.5) | |
| Characteristic | COVID-19 Outcome | Sig. | |
|---|---|---|---|
| Died | Survived | ||
| Continuous Variables | |||
| CD4 (Cells/µL) | |||
| Median (IQR) | 217.6 (167, 270.5) | 393.4 (347.9, 509.3) | <0.0001 W |
| Categorical Variables, n (%) | |||
| ART | |||
| No | 4 (80.0) | 1 (20) | 0.163 F |
| Yes | 61 (40.9) | 88 (59.1) | |
| Variables | Univariable Estimates | Multivariable Estimates | ||||
|---|---|---|---|---|---|---|
| cOR | CI (95%) | p-Value | aOR | CI (95%) | p-Value | |
| Age in years | 1.08 | 1.07, 1.10 | <0.0001 | 1.07 | 1.04, 1.09 | <0.0001 |
| HIV Status | ||||||
| Negative | Ref | Ref | ||||
| Positive | 4.37 | 2.91, 6.54 | <0.0001 | 2.02 | 1.21, 3.37 | 0.007 |
| Smoking | ||||||
| No | Ref | Ref | ||||
| Yes | 2.18 | 1.41, 3.37 | <0.0001 | 1.38 | 0.76, 2.51 | 0.288 |
| Drinking alcohol | ||||||
| No | Ref | Ref | ||||
| Yes | 1.79 | 1.22, 2.64 | 0.003 | 2.46 | 1.42, 4.24 | 0.001 |
| Comorbidity | ||||||
| None | Ref | Ref | ||||
| One | 3.62 | 1.06, 12.37 | 0.040 | 1.92 | 0.54, 6.82 | 0.312 |
| Two or more | 44.04 | 13.67, 141.92 | <0.0001 | 12.97 | 3.77, 44.58 | <0.0001 |
| Sex | ||||||
| Female | Ref | |||||
| Male | 0.88 | 0.60, 1.30 | 0.514 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nsakulula, J.; Moonga, G.; Banda, J.; Musonda, P. Effect of Human Immunodeficiency Virus (HIV) Infection on Mortality Among Hospitalised COVID-19 Patients at Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia. COVID 2025, 5, 88. https://doi.org/10.3390/covid5060088
Nsakulula J, Moonga G, Banda J, Musonda P. Effect of Human Immunodeficiency Virus (HIV) Infection on Mortality Among Hospitalised COVID-19 Patients at Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia. COVID. 2025; 5(6):88. https://doi.org/10.3390/covid5060088
Chicago/Turabian StyleNsakulula, John, Given Moonga, Jeremiah Banda, and Patrick Musonda. 2025. "Effect of Human Immunodeficiency Virus (HIV) Infection on Mortality Among Hospitalised COVID-19 Patients at Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia" COVID 5, no. 6: 88. https://doi.org/10.3390/covid5060088
APA StyleNsakulula, J., Moonga, G., Banda, J., & Musonda, P. (2025). Effect of Human Immunodeficiency Virus (HIV) Infection on Mortality Among Hospitalised COVID-19 Patients at Levy Mwanawasa University Teaching Hospital, Lusaka, Zambia. COVID, 5(6), 88. https://doi.org/10.3390/covid5060088





