Symptom Profile and Breakthrough Infections in Healthcare Workers Post Comirnaty Vaccine in a Tertiary General Hospital in Greece: A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
- A Rapid Test for the qualitative detection of SARS-CoV-2 antigen on nasopharyngeal swabs (by Biosynex Swiss SA Illkirch-Graffenstaden France). The Biosynex COVID-19 Antigen BSS (as of February 2021) is a qualitative membrane-based immunoassay that uses colloidal—gold conjugated particles with monoclonal antibodies to detect nucleocapsid protein of SARS–CoV-2. An internal control is included in the assay to indicate the proper volume of sample added to the test. The limit of detection of the assay is 1.15 × 102 TCID50/ML. External validation data reported a sensitivity of 96% and specificity of 98% in clinical settings [5]. It is important to note that the same test kits were consistently used throughout the 8-month study to ensure uniformity in results.
- A real-time PCR (RT-PCR) based on the qualitative determination of SARS-CoV-2 on an RT-PCR method using specific primers for N, E, or RdRp genes of SARS-CoV-2 (Youseq Winchester, UK). The detection limit of this method was 0.58 viral copies /mL of the sampling swab after saline buffer washing. However, the negative result cannot exclude the presence of inhibitors or the existence of a very low percentage of viral RNA below the detection limit of the method. External validation data indicated a sensitivity of 99.5% and a specificity of 99.9% in clinical evaluations [6].
- The RBD-specific antibody detection.
- The Anti-RBD-specific neutralization assay
Statistical Analysis
3. Results
4. Discussion
- COVID-19 patients were not hospitalized in private hospitals in Greece during the eight-month study period (1 February–30 September 2021), which may have reduced HCWs’ exposure to the virus. Prolonged intrafamilial exposure, particularly during the lockdown, and participation in crowded social events after lockdown restrictions were ended were identified as contributing factors to VBT infections.
- A comprehensive screening strategy was implemented to detect infections early. This included weekly rapid antigen testing for all asymptomatic personnel and hospitalized patients, weekly RT-PCR testing for physicians working in Emergency Rooms, surgeons, and anesthesiologists, and additional RT-PCR testing for any HCW exhibiting COVID-19 symptoms. This approach minimized exposure to asymptomatic HCWs in the incubation period and asymptomatic COVID-19-positive hospitalized patients admitted for non-COVID-related conditions. This is particularly important given that asymptomatic individuals can carry viral loads comparable to symptomatic cases, making them a significant source of virus transmission. However, studies on viral dynamics suggest that viral loads decline more rapidly in vaccinated individuals, and the virus they shed is less likely to be culture-positive. As a result, fully vaccinated individuals are not only at a lower risk of infection but, if infected, have a shorter period of contagiousness [19,20].
- The absence of significant underlying risk factors among the study group, except for five HCWs aged 61–65 with potentially higher risk, likely contributed to the findings. Notably, aside from three HCWs who experienced high fever and showed positive chest X-rays—one of whom required brief hospitalization—all others recovered at home and tested negative. Age distribution at least in those HCWs who became positive in the first three months post-vaccination seems to correlate with a threshold of ≥50 years of age.
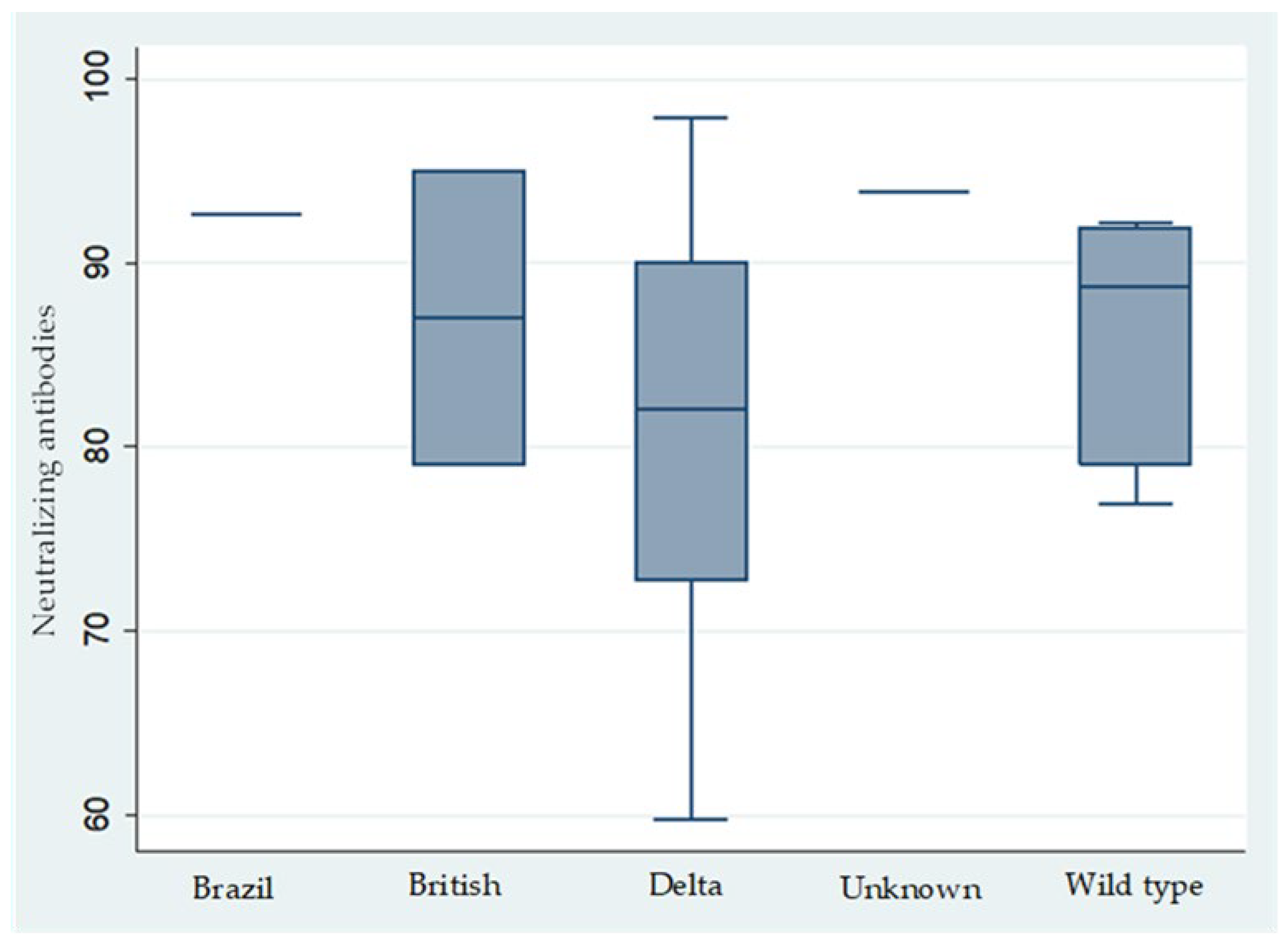
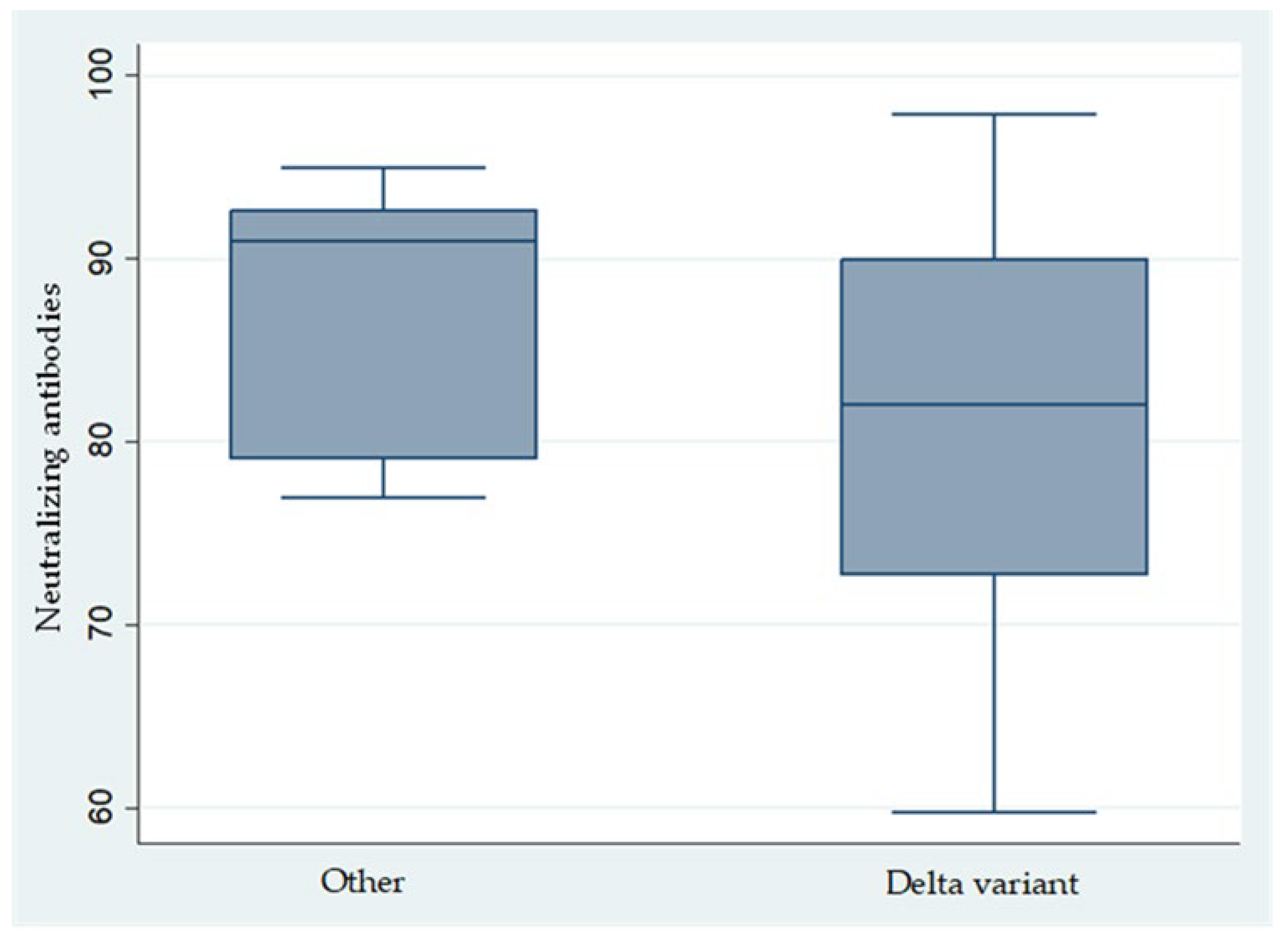
- Since July 2021, the Delta variant became the predominant strain, yet no significant differences were observed in the clinical presentation of infected HCWs, who generally experienced mild symptoms. This supports that the Comirnaty vaccine was equally effective against the Wild, British, and Delta variants [21].
- As observed in this study and corroborated by other researchers, the incidence of VBT infections increased as the time since the administration of the second vaccine dose lengthened, with a notable threshold emerging around seven months (Table 4), despite that, the levels of both IgG anti-spike and neutralizing antibodies remained consistently high, showing no significant variation beyond the seven-month mark and being evenly elevated among physicians, nurses, and other medical personnel. Notably, previous studies have indicated that anti-spike IgG levels exceeding 1000 AU/mL are predictive of infection within the preceding three months, while levels below this threshold demonstrated a 99.5% negative predictive value for COVID-19 infection during the same period [22]. Thus, anti-IgG serology can be a valuable tool for confirming or excluding prior infections within the previous three months when assessing vaccination timing. It is important to note that VBT infections, as observed in this study and reported elsewhere, have also been documented within the initial weeks after vaccination. Furthermore, with the emergence of the BA2 and BA5 Omicron variants—against which the effectiveness of both mRNA vaccines is reduced—it remains imperative to maintain preventive measures. Social distancing, mask-wearing, and regular handwashing are essential to mitigate the risk of COVID-19 infections caused by these variants among healthcare workers.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.E.W.; Caban-Martinez, A.J.; Fowlkes, A.; Lutrick, K.; et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID-19 Response Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, January 1–April 30, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Biosynex Swiss SA. Biosynex COVID-19 Ag BSS Rapid Test Product Information. Available online: https://www.biosynex.com/products/pharmacy/diagnosis/rdt/covid-ag-bss/?lang=en (accessed on 16 March 2025).
- YouSeq Ltd. YouSeq SARS-CoV-2 Multiplex RT-qPCR Kit Instructions for Use. Available online: https://youseq.com/img/products/CE-qP-COVID19C%20IFU%20version%201.0.pdf (accessed on 16 March 2025).
- National Public Health Organization. Reports of Epidemiological Surveillance of COVID-19 in Greece. Epidemiological Surveillance of Infections by SARS-CoV-2 2021. Available online: https://eody.gov.gr/epidimiologika-statistika-dedomena/ektheseis-epidimiologikis-epitirisis-loimoxis-apo-ton-sars-cov-2/ektheseis-covid-19-2021/ (accessed on 20 July 2023).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard > Cases [Dashboard]. 2023. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 20 July 2023).
- Hasibuan, A.S.; Koesnoe, E.S.; Putri, A.C.; Prabowo, S.; Gunawan, W.; Mappangara, I.; Wungu, C.D.K. Incidence and Associated Factors of SARS-CoV-2 Infection Post-mRNA-1273 Booster Vaccination in Health-Care Workers. Vaccines 2023, 11, 481. [Google Scholar] [CrossRef]
- Gopinath, S.; Ishak, A.; Trivedi, N.; Nyachieo, A.; Mulenga, F. Characteristics of COVID-19 Breakthrough Infections among Vaccinated Individuals and Associated Risk Factors: A Systematic Review. Trop. Med. Infect. Dis. 2022, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Liossi, S.; Tsiambas, E.; Papatheodoridis, G.; Kottonis, P.; Kalogeropoulos, C.; Daviti, P. Mathematical Modeling for Delta and Omicron Variant of SARS-CoV-2 Transmission Dynamics in Greece. Infect. Dis. Model. 2023, 8, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Keehner, J.; Horton, L.E.; Binkin, N.; Laurent, L.C.; Pride, D.; Longhurst, C.A.; Abeles, S.R.; Torriani, F.J. SARS-CoV-2 Infection after Vaccination in Health Care Workers in California. N. Engl. J. Med. 2021, 384, 1774–1775. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Reves, J.J.; Hollandsworth, H.M.; Torriani, F.J.; Taplitz, R.; Abeles, S.R.; Tai-Seale, M.; Millen, M.; Clay, B.J.; Longhurst, C.A. Rapid Response to COVID-19: Health Informatics Support for Outbreak Management in an Academic Health System. J. Am. Med. Inform. Assoc. 2020, 27, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Amit, S.; Beni, S.A.; Biber, A.; Grinberg, A.; Leshem, E.; Regev-Yochay, G. Postvaccination COVID-19 among Healthcare Workers, Israel. Emerg. Infect. Dis. 2021, 27, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Hirabara, S.M.; Serdan, T.D.A.; Gorjao, R.; Masi, L.N.; Pithon-Curi, T.C.; Curi, R.; Durigon, E.L.; Sampaio, S.C.; Fiamoncini, J. SARS-CoV-2 Variants: Differences and Potential of Immune Evasion. Front. Cell. Infect. Microbiol. 2022, 11, 781429. [Google Scholar] [CrossRef] [PubMed]
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 Vaccine Coverage in Health-Care Workers in England and Effectiveness of BNT162b2 mRNA Vaccine Against Infection (SIREN): A Prospective, Multicentre, Cohort Study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination. JAMA 2021, 326, 2018–2020. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of Covid-19 Vaccines Against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Gao, L.; Zhou, Q.; Yu, T.; Sun, F.; Li, H.; Shi, Y. Effectiveness of COVID-19 Vaccines Against SARS-CoV-2 Variants of Concern: A Systematic Review and Meta-Analysis. BMC Med. 2022, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Abu Jabal, K.; Edelstein, M.; Bouhnik, E.; Dagan, N.; Martinez, D.; Mizrahi, B.; Ben-Tov, A.; Cohen, M.J. Using SARS-CoV-2 Anti-S IgG Levels as a Marker of Previous Infection: Example from an Israeli Healthcare Worker Cohort. Int. J. Infect. Dis. 2022, 120, 22–24. [Google Scholar] [CrossRef] [PubMed]

| Personnel Category | Total No of Personnel | Vaccinated | Non Vaccinated | Vaccination Rate (%, 95% CI) |
|---|---|---|---|---|
| Physicians | 1057 | 1048 | 9 | 99.15% (98.15–99.6%) |
| Non medical staff * | 313 | 298 | 15 | 95.21% (95.21–97.4%) |
| Nurses | 523 | 468 | 55 | 89.48% (87.00–91.8%) |
| GDA ** | 157 | 145 | 12 | 92.36% (89.5–94.9%) |
| Total | 2049 | 1958 | 91 | 95.56% (94.9–96.2%) |
| Case No | Gender | Personnel Category | Age | Case No | Gender | Personnel Category | Age | Case No | Gender | Personnel Category | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | nurses | 51 | 19 | M | doctors | 42 | 37 | F | nurses | 29 |
| 2 | F | nurses | 42 | 20 | F | nurses | 52 | 38 | M | doctors | 29 |
| 3 | F | doctors | 65 | 21 | F | GDAs | 56 | 39 | F | non medical staff | 53 |
| 4 | M | non medical staff | 52 | 22 | F | non medical staff | 51 | 40 | F | nurses | 35 |
| 5 | M | doctors | 49 | 23 | M | non medical staff | 65 | 41 | F | doctors | 34 |
| 6 | M | doctors | 49 | 24 | F | nurses | 43 | 42 | F | nurses | 32 |
| 7 | M | doctors | 61 | 25 | F | nurses | 39 | 43 | F | non medical staff | 35 |
| 8 | F | nurses | 52 | 26 | M | nurses | 26 | 44 | F | nurses | 48 |
| 9 | M | doctors | 61 | 27 | M | non medical staff | 46 | 45 | F | nurses | 27 |
| 10 | F | doctors | 60 | 28 | F | non medical staff | 41 | 46 | F | non medical staff | 51 |
| 11 | F | nurses | 39 | 29 | M | nurses | 38 | 47 | M | doctors | 56 |
| 12 | F | GDAs | 53 | 30 | M | doctors | 46 | 48 | F | doctors | 44 |
| 13 | M | doctors | 48 | 31 | F | nurses | 36 | 49 | M | doctors | 41 |
| 14 | F | nurses | 62 | 32 | M | GDAs | 29 | 50 | F | nurses | 49 |
| 15 | M | doctors | 52 | 33 | M | doctors | 40 | 51 | M | doctors | 53 |
| 16 | M | doctors | 65 | 34 | F | non medical staff | 45 | 52 | F | non medical staff | 48 |
| 17 | F | nurses | 63 | 35 | M | doctors | 65 | 53 | M | GDAs | 50 |
| 18 | M | doctors | 48 | 36 | M | nurses | 26 | 54 | F | non medical staff | 56 |
| Month (2021) | No of Cases | |
|---|---|---|
| HCWs | Community | |
| February | 2 | 34,143 |
| March | 5 | 72,589 |
| April | 14 | 81,344 |
| May | 3 | 57,273 |
| June | 1 | 20,150 |
| July | 11 | 70,850 |
| August | 14 | 94,650 |
| September | 6 | 67,803 |
| Antibodies | |||||||
|---|---|---|---|---|---|---|---|
| Gender | Personnel Category | Age (Years) | Onset of Illness After Vaccination (Days) | AntiSpike (U/mL) | Neutralizing (%) | COVID Variant | Reason for na |
| M | non medical staff * | 52 | 32 | 52 | na | BRITISH (B.1.1.7) | assay failure |
| F | physician | 60 | 54 | 4150 | 95 | BRITISH (B.1.1.7) | |
| M | physician | 42 | 61 | 1248 | 93.81 | UNKOWN | |
| M | non medical staff * | 65 | 67 | 973 | 92.14 | WILD TYPE (WIV04) | |
| F | non medical staff * | 51 | 94 | 320.3 | 79.05 | BRITISH (B.1.1.7) | |
| F | nurse | 39 | 96 | 3201 | 92.62 | BRASIL (P.1) | |
| M | nurse | 26 | 108 | na | na | DELTA (B.1.617.2) | insufficient serum volume |
| F | nurse | 43 | 116 | 836.5 | 87.38 | WILD TYPE (WIV04) | |
| M | non medical staff * | 46 | 120 | 136.4 | 77.17 | DELTA (B.1.617.2) | |
| M | nurse | 38 | 134 | 1862 | 90 | WILD TYPE (WIV04) | |
| M | physician | 46 | 136 | 643.5 | 81.91 | DELTA (B.1.617.2) | |
| F | non medical staff * | 41 | 140 | 665.7 | 82.14 | DELTA (B.1.617.2) | |
| M | physician | 40 | 149 | 1615 | 79.05 | WILD TYPE (WIV04) | |
| F | non medical staff * | 45 | 151 | 418.6 | 59.76 | DELTA (B.1.617.2) | |
| M | physician | 65 | 154 | 913.2 | 91.91 | WILD TYPE (WIV04) | |
| M | physician | 29 | 164 | 588.8 | 82.14 | DELTA (B.1.617.2) | |
| F | nurse | 35 | 171 | 2784 | 97.86 | DELTA (B.1.617.2) | |
| M | nurse | 32 | 176 | 641.1 | 76.91 | WILD TYPE (WIV04) | |
| M | nurse | 48 | 180 | 91 | 61.19 | DELTA (B.1.617.2) | |
| M | non medical staff * | 51 | 187 | 25,000 | 92.5 | DELTA (B.1.617.2) | |
| M | physician | 41 | 193 | na | na | DELTA (B.1.617.2) | assay failure |
| F | nurse | 49 | 196 | na | na | DELTA (B.1.617.2) | insufficient serum volume |
| M | GDA ** | 50 | 215 | 772.4 | 72.72 | DELTA (B.1.617.2) | |
| F | non medical staff * | 56 | 218 | 14,875 | 90 | DELTA (B.1.617.2) | |
| Delta Variant Present | OR | Standard Error | p-Value | [95% CI] |
|---|---|---|---|---|
| Female | 0.6 | 0.7 | 0.63 | 0.1–5.6 |
| Age | 0.9 | 0.1 | 0.39 | 0.8–1.1 |
| Neutralizing antibodies | 0.9 | 0.1 | 0.04 | 0.7–1.0 |
| Spike protein antibodies | 1.0 | 0.0 | 0.23 | 1.0–1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giamarellou, H.; Karavasilis, T.; Sakka, V.; Pechlivanidou, E.; Syriopoulou, V.; Dasyras, F.; Michos, A.; Spanakis, N.; Karaiskos, I.; Galani, L.; et al. Symptom Profile and Breakthrough Infections in Healthcare Workers Post Comirnaty Vaccine in a Tertiary General Hospital in Greece: A Narrative Review. COVID 2025, 5, 63. https://doi.org/10.3390/covid5050063
Giamarellou H, Karavasilis T, Sakka V, Pechlivanidou E, Syriopoulou V, Dasyras F, Michos A, Spanakis N, Karaiskos I, Galani L, et al. Symptom Profile and Breakthrough Infections in Healthcare Workers Post Comirnaty Vaccine in a Tertiary General Hospital in Greece: A Narrative Review. COVID. 2025; 5(5):63. https://doi.org/10.3390/covid5050063
Chicago/Turabian StyleGiamarellou, Helen, Theodoros Karavasilis, Vissaria Sakka, Evmorfia Pechlivanidou, Vasiliki Syriopoulou, Fragiskos Dasyras, Athanasios Michos, Nikolaos Spanakis, Ilias Karaiskos, Lambrini Galani, and et al. 2025. "Symptom Profile and Breakthrough Infections in Healthcare Workers Post Comirnaty Vaccine in a Tertiary General Hospital in Greece: A Narrative Review" COVID 5, no. 5: 63. https://doi.org/10.3390/covid5050063
APA StyleGiamarellou, H., Karavasilis, T., Sakka, V., Pechlivanidou, E., Syriopoulou, V., Dasyras, F., Michos, A., Spanakis, N., Karaiskos, I., Galani, L., & Papadogeorgaki, E. (2025). Symptom Profile and Breakthrough Infections in Healthcare Workers Post Comirnaty Vaccine in a Tertiary General Hospital in Greece: A Narrative Review. COVID, 5(5), 63. https://doi.org/10.3390/covid5050063








