Abstract
The COVID-19 pandemic has led to persistent complications beyond the respiratory system, with emerging evidence highlighting the role of gut dysbiosis in long COVID. Given the established gut–skin axis, alterations in gut microbiota post-COVID-19 may contribute to persistent dermatologic conditions such as eczema, acne, and rosacea. This review explores the mechanisms by which SARS-CoV-2 disrupts the gut microbiome, leading to systemic inflammation and skin disease. Furthermore, it examines potential interventions, including probiotics, prebiotics, and dietary modifications, as microbiome-targeted therapeutic strategies for post-COVID dermatologic recovery. Understanding this link may open new avenues for treating chronic inflammatory skin conditions in long COVID patients.
Keywords:
COVID-19; gut microbiome; gut–skin axis; dysbiosis; long COVID; eczema; acne; probiotics; inflammation 1. Introduction
The COVID-19 pandemic has resulted in long-term sequelae beyond acute infection. This phenomenon is often termed “long COVID”, which encompasses persistent symptoms affecting multiple organ systems, including the gastrointestinal (GI) tract and skin [1]. Increasing evidence suggests that SARS-CoV-2 disrupts gut microbiota composition, leading to gut dysbiosis, which is characterized by not only an increase in pathogenic microbes but also a loss of beneficial bacteria [2,3].
The gut–skin axis is a well-established concept in dermatology: it acts as a modulator for understanding the relationship between the gut microbiome and inflammatory skin diseases. Given that COVID-19-induced gut dysbiosis is linked to systemic inflammation, these changes may be associated with persistent—or even worsening—dermatologic conditions such as eczema, acne, and rosacea in long COVID patients.
This review aims to explore the mechanisms by which SARS-CoV-2-induced gut dysbiosis may contribute to persistent skin disorders, examine the clinical implications of the gut–skin axis in long COVID, and evaluate potential microbiome-targeted interventions for managing post-COVID dermatologic conditions.
2. COVID-19 and Gut Dysbiosis
COVID-19 affects the GI tract through multiple mechanisms and leads to significant alterations in gut microbiota composition, a condition known as “gut dysbiosis” (the imbalance in the microbial community). This is not to be confused with the gut microbiome, which refers to the collective genetic material of these microorganisms and plays a crucial role in maintaining gut health. This disruption arises from direct viral invasion, immune activation, and COVID-19-related treatments, all of which contribute to persistent gastrointestinal and systemic inflammation. Emerging evidence suggests that these microbial imbalances may play a key role in the long-term sequelae of COVID-19, including dermatologic manifestations.
2.1. Direct Effects of SARS-CoV-2 on the Gut Microbiome
SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) receptors, which are highly expressed in intestinal epithelial cells [3,4]. This interaction enables the virus to invade the gut lining, causing cell damage, impaired nutrient absorption, and local inflammation [3,4]. Studies have shown that this process alters the gut microbiota by reducing beneficial bacterial species such as Bifidobacterium and Faecalibacterium prausnitzii, which are essential for maintaining immune balance and intestinal barrier integrity [4,5]. Simultaneously, the loss of these protective bacteria allows the overgrowth of pathogenic microbes like Enterococcus faecalis and Escherichia coli, which further contribute to gut inflammation and increased intestinal permeability [4,5].
In addition to bacterial imbalances, SARS-CoV-2 may also impact the gut virome, which is a collection of viruses that naturally live in the intestine. Viral infections can shift the composition of the virome and potentially influence microbial interactions and immune responses. While research on the gut virome in cases of “long COVID” is still emerging, preliminary findings suggest that viral-induced disruptions could contribute to prolonged dysbiosis and immune dysregulation [6,7].
2.2. Immune Activation and Persistent Inflammation
Beyond direct viral invasion, COVID-19 triggers systemic immune dysregulation, which plays a significant role in gut dysfunction. The infection leads to a surge in pro-inflammatory cytokines, particularly interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), which disrupt gut homeostasis and contribute to chronic low-grade inflammation [8,9]. The presence of these microbial byproducts in circulation can trigger widespread systemic inflammation, which has been implicated in the exacerbation of inflammatory skin conditions.
Additionally, the gut–lung axis—which is a bidirectional relationship between gut microbiota and respiratory health—may further complicate post-COVIDsymptoms. Evidence from both human and animal studies suggests that gut dysbiosis can worsen pulmonary inflammation and vice versa, creating a cycle of persistent immune activation that prolongs recovery. In humans, long COVID patients with gut dysbiosis show increased systemic inflammation, particularly in the lungs and gastrointestinal tract. Animal studies further support this by demonstrating that gut dysbiosis leads to immune dysregulation, affecting immune cells like Th17 cells and macrophages, which contribute to inflammation in both the gut and lungs. This interplay may explain why many patients who have had long COVID experience both respiratory and GI symptoms, along with dermatologic issues linked to systemic inflammation [3,10].
2.3. The Role of COVID-19 Treatments in Gut Dysbiosis
Moreover, several treatments commonly used for COVID-19 may exacerbate gut microbiome imbalances. For example, broad-spectrum antibiotics are frequently administered to prevent secondary bacterial infections in hospitalized COVID-19 patients and can lead to significant microbial depletion. This is because they reduce bacterial diversity and thus increase one’s susceptibility to opportunistic pathogens [11]. The use of corticosteroids, which help control hyperinflammatory responses in severe COVID-19 cases, has also been associated with alterations in gut microbiota composition, including an increased risk of fungal overgrowth, particularly with Candida species [12].
Additionally, antiviral medications (such as Paxlovid) have been critical in reducing COVID-19 severity, but their impact on the gut microbiome is still under investigation. Some studies suggest that antiviral treatments may alter bacterial metabolism or disrupt microbial diversity, although the long-term consequences of these changes are not yet fully understood [13]. Understanding the role of COVID-19 therapies in shaping the gut microbiome is essential, as interventions aimed at restoring microbial balance may help mitigate some of the persistent symptoms seen in long COVID.
2.4. Clinical Evidence of Gut Dysbiosis in COVID-19 Survivors
Multiple studies have demonstrated that COVID-19 survivors experience persistent alterations in their gut microbiome, even months after recovering from the acute infection [10,14]. Stool analyses have revealed a significant reduction in beneficial bacteria and an increase in markers of intestinal permeability, indicating a compromised gut barrier [10,14]. Many individuals with long COVID report prolonged GI symptoms, such as bloating, diarrhea, constipation, and food sensitivities, which resemble patterns observed in irritable bowel syndrome (IBS) [10,15].
Interestingly, a subset of long COVID patients has also reported worsening skin conditions along with GI symptoms, which reinforces the relevance of the gut–skin axis [1] (more on this in Section 3). This connection suggests that systemic inflammation associated with gut dysbiosis may play a role in dermatologic manifestations such as eczema, acne, and psoriasis. Emerging evidence supports this link, with studies showing that gut dysbiosis in long COVID patients is associated with elevated levels of circulating inflammatory markers, such as IL-6 and TNF-α, which are known to exacerbate skin conditions [8,16]. As research into post-COVID gut health continues, identifying strategies to restore microbial balance is important in managing both GI and skin-related complications in long COVID patients.
3. The Gut–Skin Axis and Post-COVID Dermatologic Manifestations
The gut–skin axis is a well-recognized concept in dermatology, describing the bidirectional communication between the gut microbiome and skin health. This connection is primarily mediated through immune regulation, microbial metabolites, and intestinal permeability. In the context of COVID-19, viral-induced gut dysbiosis may contribute to systemic inflammation and immune dysfunction, exacerbating dermatologic conditions [17]. Many long COVID patients have reported persistent or new-onset skin issues, which may be linked to underlying gut microbial imbalances [18]. This section will explore this topic in more detail.
3.1. Mechanisms Linking Gut Dysbiosis to Skin Inflammation
The gut microbiome plays a crucial role in regulating systemic immune responses, and disruptions in microbial balance have been linked to chronic inflammation that may affect various organs, including the skin. One of the primary mechanisms driving this connection is the production of inflammatory cytokines in response to gut dysbiosis. When beneficial bacteria such as Bifidobacterium and Faecalibacterium prausnitzii are depleted, the gut environment shifts toward a more pro-inflammatory state [19]. This imbalance leads to increased levels of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and other cytokines that promote skin inflammation and worsen conditions such as eczema, acne, and psoriasis.
In addition to cytokine production, the disruption of the intestinal barrier allows bacterial endotoxins such as lipopolysaccharides (LPS) to enter the bloodstream. LPS activates toll-like receptor 4 (TLR4) on immune cells, including macrophages and dendritic cells, leading to the release of additional pro-inflammatory cytokines such as IL-1β and IL-17 [20]. These cytokines can migrate to the skin, where they promote inflammation by activating keratinocytes and recruiting T cells, particularly Th17 and Th22 cells, which are known to play a role in inflammatory skin diseases like psoriasis and eczema [21]. Animal models have shown that increased intestinal permeability results in elevated levels of circulating LPS, which correlates with exacerbated skin inflammation and impaired epidermal barrier function [22]. In human studies, patients with inflammatory skin conditions such as atopic dermatitis and psoriasis have been found to have higher levels of circulating LPS and Th17-related cytokines, suggesting a direct link between gut dysbiosis and skin inflammation [23].
Another important factor is the role of regulatory T cells (Tregs) in maintaining immune tolerance [24]. In a healthy gut, Tregs help suppress excessive immune responses and maintain barrier integrity. However, in the context of gut dysbiosis, Treg function is impaired, leading to unchecked inflammation. Studies in mice have demonstrated that the depletion of Tregs exacerbates skin inflammation, while the restoration of Treg function can mitigate these effects [25,26]. In humans, reduced Treg activity has been observed in patients with chronic inflammatory skin conditions, further supporting the role of immune dysregulation in the gut–skin axis [17,27].
Additionally, the gut microbiome influences sebum production and hormonal balance, both of which play key roles in acne and other inflammatory skin disorders [28]. Certain gut bacteria regulate androgen metabolism, and an imbalance in these microbes may contribute to increased sebum production and clogged pores, leading to acne flares. Dysbiosis also affects short-chain fatty acid (SCFA) production, particularly butyrate, which has anti-inflammatory properties and supports skin barrier function [29]. A reduction in SCFAs due to microbial imbalances may impair skin health, leading to increased dryness, irritation, and susceptibility to conditions like eczema [29].
3.2. Post-COVID Dermatologic Conditions Associated with Gut Dysbiosis
Many dermatologic conditions have been reported in long COVID patients, with emerging evidence suggesting a strong link to underlying gut dysbiosis [30,31]. One of the most common conditions observed is eczema (also known as atopic dermatitis), which is characterized by a heightened Th2-driven inflammatory response [22]. In post-COVID patients, gut microbial imbalances may promote Th2-skewed immunity which can exacerbate eczema symptoms. Studies have shown that individuals with atopic dermatitis often have lower levels of beneficial gut bacteria and increased intestinal permeability, both of which can be worsened by COVID-19-induced dysbiosis [22].
Acne and rosacea have also been reported in long COVID patients [16,32]. Some evidence suggests that gut dysbiosis may be linked to acne by increasing systemic inflammation and altering hormonal regulation [33]. Changes in gut bacteria may influence insulin sensitivity and androgen levels, leading to increased sebum production and a higher likelihood of acne breakouts. Similarly, rosacea—a chronic inflammatory skin disorder—has been associated with small intestinal bacterial overgrowth (SIBO), a condition linked to gut dysbiosis [33]. Post-COVID disruptions in gut microbiota may predispose individuals to SIBO, potentially worsening rosacea symptoms.
Another dermatologic condition linked to post-COVID gut dysbiosis is psoriasis, which is an autoimmune disorder characterized by the hyperproliferation of skin cells and chronic inflammation [34]. Recent research has highlighted the role of the gut microbiome in modulating immune responses in psoriasis patients [35,36]. Studies have found that individuals with psoriasis often have reduced levels of Akkermansia muciniphila, a beneficial gut bacterium involved in maintaining gut barrier integrity [37]. Post-COVID dysbiosis may further impair gut barrier function, increasing systemic inflammation and triggering psoriasis flares.
Beyond these conditions, some long COVID patients have reported new onset urticaria (hives), alopecia (hair loss), and vitiligo, suggesting broader immune dysregulation influenced by gut health [38]. While more research is needed to establish definitive links, these findings emphasize the potential role of gut dysbiosis in post-COVID dermatologic manifestations.
3.3. Clinical Evidence Supporting the Gut–Skin Axis in Long COVID
Several studies have reinforced the connection between gut dysbiosis and skin disorders in long COVID patients [39,40]. Clinical analyses of COVID-19 survivors have consistently shown altered gut microbiome profiles, with reduced bacterial diversity and increased inflammatory markers [41,42]. Many of these individuals experience prolonged GI symptoms, which often correlate with worsening skin conditions.
One study analyzing stool samples from long COVID patients found significantly lower levels of Bifidobacterium and Lactobacillus species, which are known to play protective roles in both gut and skin health [5]. The same study reported increased gut permeability markers, suggesting a direct link between microbial imbalances and systemic inflammation [5].
Moreover, research on pre-existing gut–skin interactions supports the hypothesis that COVID-19-induced dysbiosis may exacerbate dermatologic conditions. Previous studies have demonstrated that individuals with psoriasis, eczema, and acne tend to have distinct gut microbiome signatures, with reduced beneficial bacterial species and increased inflammatory markers. Given that COVID-19 disrupts similar microbial pathways, it is plausible that post-COVID gut disturbances play a significant role in skin health.
However, not all studies support a strong gut–skin connection in long COVID. Some research has found that while gut dysbiosis is common in long COVID patients, the correlation with skin conditions is less clear. One 2024 study found no significant association between gut microbiome alterations and the severity of skin conditions in long COVID patients, suggesting that other factors, such as direct viral effects or immune dysregulation, may play a more prominent role in dermatologic manifestations [10,43]. Similarly, a meta-analysis concluded that while gut dysbiosis is prevalent in long COVID, its impact on skin health may be overstated, with only weak correlations observed between gut microbiome changes and specific skin conditions like eczema and psoriasis [20,44].
Moreover, it is important to consider alternative explanations for post-COVID skin manifestations beyond gut microbiome changes. Systemic inflammation, driven by the persistent immune activation seen in long COVID, is a key factor that can directly impact skin health. Elevated levels of pro-inflammatory cytokines such as IL-6, TNF-α, and IL-17 have been observed in long COVID patients, and these cytokines are known to exacerbate inflammatory skin conditions like eczema, psoriasis, and acne [8,18]. Endothelial dysfunction may further contribute to these dermatologic manifestations, as SARS-CoV-2 has been shown to directly infect endothelial cells, leading to vascular damage, impaired microcirculation, and increased vascular permeability [45,46]. These effects can manifest in the skin as rashes, chilblains, and livedo reticularis, which have been frequently reported in long COVID patients [47]. Endothelial injury also promotes a pro-thrombotic state, potentially contributing to vasculopathy-related skin conditions such as purpura and necrosis [48].
Additionally, autoimmune dysregulation triggered by SARS-CoV-2 infection may contribute to skin manifestations. For example, molecular mimicry between viral proteins and self-antigens can lead to the production of autoantibodies, which may target skin tissues and result in conditions such as urticaria, vitiligo, or alopecia areata [38]. Furthermore, endothelial dysfunction exacerbates systemic inflammation by increasing the release of pro-inflammatory cytokines and reactive oxygen species, creating a cycle of immune activation that worsens skin conditions [49]. A recent study found that long COVID patients with persistent skin symptoms exhibited elevated markers of endothelial activation, including von Willebrand factor and soluble thrombomodulin, highlighting the potential role of vascular pathology in post-COVID dermatologic changes [50].
Similarly, direct viral effects on the skin cannot be ruled out. SARS-CoV-2 has been shown to infect endothelial cells and disrupt vascular function, which may contribute to skin manifestations such as rashes, chilblains, and livedo reticularis [39]. These vascular changes, combined with systemic inflammation and immune dysregulation, provide alternative pathways for post-COVID skin conditions that are independent of gut microbiome alterations. These vascular changes, combined with systemic inflammation and immune dysregulation, provide additional mechanisms linking endothelial dysfunction to post-COVID skin conditions.
3.4. Implications for Treatment and Management
The role of the gut–skin axis in long COVID means there is the potential for microbiome-targeted interventions in managing post-COVID dermatologic conditions. Given that gut dysbiosis is a modifiable factor, restoring microbial balance could alleviate both GI and skin symptoms.
Dietary modifications—such as adopting an anti-inflammatory diet rich in fiber, polyphenols, and fermented foods—may support gut microbiome diversity and reduce systemic inflammation [51]. Probiotics, particularly strains from the Lactobacillus and Bifidobacterium genera, have shown promise in improving both gut and skin health. Prebiotics, which serve as nutrients for beneficial bacteria, may further enhance microbiome restoration [52]. Additionally, emerging therapies such as postbiotics (bacterial metabolites) and fecal microbiota transplantation (FMT) are being explored as potential interventions for severe gut dysbiosis [53].
Ultimately, addressing post-COVID gut dysbiosis may offer a novel approach to managing persistent skin conditions in long COVID patients, some of which are outlined in Table 1. Further research is needed to determine optimal treatment strategies, but current findings suggest that interventions targeting the gut microbiome could play a crucial role in dermatologic recovery.

Table 1.
Common dermatologic conditions linked to post-COVID gut dysbiosis.
4. Potential Microbiome-Targeted Interventions for Post-COVID Skin Health
Given the emerging evidence linking gut dysbiosis to dermatologic manifestations in long COVID, microbiome-targeted interventions represent a promising avenue for therapeutic development (Table 1 and Table 2). These interventions aim to restore the balance of the gut microbiota and reduce systemic inflammation with the goal of improving skin health. Several approaches, including dietary modifications, probiotics, prebiotics, and emerging therapies like fecal microbiota transplantation (FMT), are being explored as potential treatments for post-COVID skin complications [51,53]. This section delves deeper into the mechanisms, evidence, and potential benefits of these interventions.
4.1. Dietary Modifications and the Role of Anti-Inflammatory Diets
Diet plays a pivotal role in shaping the gut microbiome, and dietary modifications can be an effective strategy for promoting gut health and alleviating dermatologic symptoms in long COVID patients. An anti-inflammatory diet, rich in fiber, polyphenols, and fermented foods, can help restore gut microbiome balance by supporting the growth of beneficial bacteria as well as reducing inflammation [51].
The Mediterranean diet, for example, is known for its anti-inflammatory properties and has been shown to promote microbial diversity in the gut. This diet emphasizes the consumption of fruits, vegetables, whole grains, healthy fats (e.g., olive oil), and lean proteins, all of which provide nutrients that support gut health [60]. The Mediterranean diet is also rich in polyphenols—plant compounds found in foods like berries, nuts, and olive oil—that have antioxidant properties and help reduce systemic inflammation. In a study focusing on the Mediterranean diet’s effects on the gut microbiome, participants exhibited higher levels of beneficial bacteria, including Bifidobacterium and Lactobacillus, which are associated with improved immune function and gut barrier integrity [61].
Additionally, fiber-rich foods such as whole grains, legumes, and vegetables are essential for maintaining gut health. Fiber serves as a prebiotic, providing nourishment for beneficial gut bacteria. The fermentation of fiber by gut microbes produces short-chain fatty acids (SCFAs), particularly butyrate, which have anti-inflammatory properties and help maintain the integrity of the intestinal barrier [62]. Increased SCFA production may also have direct benefits for skin health, as these metabolites help reduce systemic inflammation that could otherwise exacerbate skin conditions such as eczema, acne, and psoriasis [63].
Conversely, it is important to avoid diets high in processed foods, refined sugars, and high-glycemic-index carbohydrates, as these can promote the growth of pathogenic bacteria and increase gut inflammation. Evidence suggests that such diets contribute to microbial imbalances that can worsen both gastrointestinal and dermatologic symptoms [17]. Thus, dietary changes aimed at reducing inflammation and promoting microbial diversity may offer significant benefits for long COVID patients.
4.2. Probiotics and Prebiotics for Restoring Gut Balance
Probiotics and prebiotics are two microbiome-targeted interventions that have shown promise in restoring gut balance and alleviating post-COVID skin conditions. Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefits to the host. Specific strains of probiotics have been shown to modulate immune responses, reduce inflammation, and improve gut barrier function, all of which may help alleviate skin conditions associated with gut dysbiosis [64].
Among the most studied probiotic strains for gut–skin health are Lactobacillus and Bifidobacterium species. Research has demonstrated that these probiotics can help reduce inflammation in individuals with conditions like eczema, acne, and rosacea [64]. For example, a study involving patients with eczema found that supplementation with Lactobacillus rhamnosus GG led to a reduction in skin inflammation and improvement in symptoms [65]. Similarly, Bifidobacterium strains have been shown to promote gut health and reduce systemic inflammation, which could benefit skin conditions driven by immune dysregulation [66].
Prebiotics, on the other hand, are non-digestible food components (often fibers) that selectively stimulate the growth or activity of beneficial gut bacteria. Prebiotics serve as fuel for probiotic bacteria and can promote the growth of protective species that enhance gut health. Common prebiotics include inulin, resistant starch, and fructooligosaccharides (FOS), which have been shown to support the growth of beneficial bacteria such as Bifidobacterium and Lactobacillus. These beneficial bacteria, in turn, help maintain intestinal barrier function and regulate systemic immune responses. In clinical studies, prebiotic supplementation has been shown to improve gut microbiota composition and reduce markers of systemic inflammation, which may have downstream effects on skin health [17,67].
When used together, probiotics and prebiotics can have a synergistic effect, promoting the restoration of a balanced microbiome and reducing the chronic inflammation that often underlies dermatologic conditions in long COVID patients [68]. This combined approach may provide an effective strategy for managing post-COVID skin complications.
4.3. Fecal Microbiota Transplantation (FMT) and Emerging Therapies
Fecal microbiota transplantation (FMT) is an innovative therapy that involves transferring stool from a healthy donor into the gastrointestinal tract of a patient with gut dysbiosis. The goal of FMT is to restore microbial diversity and re-establish a healthy microbiome. While FMT has been primarily used to treat Clostridium difficile infections, there is growing interest in its potential for treating a wide range of diseases, including long COVID and associated dermatologic conditions [69].
Animal models have provided key insights into the mechanisms by which FMT restores gut homeostasis [70,71]. Studies in germ-free mice have shown that FMT can reduce systemic inflammation, improve gut barrier integrity, and modulate immune responses, leading to improved skin health in models of inflammatory skin diseases such as psoriasis and atopic dermatitis [17,72]. Additionally, FMT-treated animals exhibited increased levels of beneficial short-chain fatty acid (SCFA)-producing bacteria, suggesting a potential link between microbiota restoration and reduced skin inflammation [73].
In patients with severe gut dysbiosis, FMT has been shown to restore microbiome balance, increase microbial diversity, and improve gut barrier function [74]. Clinical trials have demonstrated its effectiveness in conditions like inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS), but data on dermatologic applications remain limited [75]. Small-scale human studies suggest that FMT may reduce systemic inflammation and improve symptoms in conditions such as psoriasis and atopic dermatitis [70].
Despite its potential, FMT has significant limitations. Variability in donor microbiota composition, a lack of standardized treatment protocols, and concerns about long-term safety present challenges for widespread clinical use [76]. Additionally, adverse effects such as transient gastrointestinal discomfort and, in rare cases, serious infections, highlight the need for rigorous screening and regulation [69]. Future research should focus on optimizing donor selection, treatment standardization, and long-term follow-up in clinical trials to determine the feasibility of FMT as a dermatologic therapy for long COVID.
In addition to FMT, emerging pharmacological therapies that target gut inflammation are also being explored as potential treatments for post-COVID skin health. For example, rifaximin—a non-absorbable antibiotic that targets the gut microbiome—has shown promise in treating conditions like irritable bowel syndrome (IBS) and could potentially benefit long COVID patients with GI symptoms [77]. Butyrate supplements, which are used to increase SCFA production and support gut barrier integrity, are also being investigated for their potential to reduce inflammation and improve skin health [62]. Preclinical studies suggest that butyrate supplementation enhances intestinal barrier function and decreases inflammatory cytokine production, but human trials specific to dermatologic conditions in long COVID are still needed.
4.4. The Role of Personalized Microbiome-Based Approaches
One of the key challenges in microbiome-based interventions is the variability in individual responses to treatment. Microbial composition is influenced by numerous factors, including genetics, diet, lifestyle, and pre-existing health conditions, meaning that there is no one-size-fits-all solution [78,79]. As such, personalized microbiome-based approaches offer a more effective means of managing post-COVID skin health.
Precision probiotics tailored to an individual’s specific microbiome profile may optimize the therapeutic effects of probiotics and prebiotics. Advances in microbiome sequencing technologies now allow for the identification of unique microbial signatures, which can inform the selection of targeted probiotics and prebiotics to restore gut balance [80]. In the future, microbiome testing may become a standard part of long COVID treatment protocols, helping to guide personalized interventions for both gut and skin health.
Similarly, dietary interventions could be customized based on an individual’s gut microbiome profile, ensuring that dietary changes are aligned with the specific needs of the patient’s microbiota.
By incorporating personalized nutrition, precision medicine, and microbiome-based therapies, it may be possible to develop highly effective, individualized treatment plans for long COVID patients suffering from dermatologic issues linked to gut dysbiosis (Table 2).

Table 2.
Potential microbiome-targeted interventions for skin health.
Table 2.
Potential microbiome-targeted interventions for skin health.
| Intervention | Mechanism | Evidence for Effectiveness |
|---|---|---|
| Probiotics (Lactobacillus, Bifidobacterium) | Restores gut balance, reduces inflammation [81] | Shown to improve acne, eczema, and rosacea symptoms |
| Prebiotics (inulin, resistant starch) | Promotes beneficial bacterial growth [82] | Supports microbiome restoration, reduces systemic inflammation |
| Fecal microbiota transplantation (FMT) | Restores gut microbial diversity [83] | Experimental but promising for treating gut dysbiosis and associated conditions |
| Anti-inflammatory diet (Mediterranean, fiber-rich) | Reduces systemic inflammation, supports gut health [84] | Shown to promote gut microbial diversity, improve immune function |
| Butyrate supplementation | Supports gut barrier function, reduces gut inflammation [85] | Enhances SCFA production, reduces inflammation, supports skin health |
5. Discussion
The evidence supporting the link between COVID-19-induced gut dysbiosis and persistent dermatologic conditions in long COVID is compelling (Figure 1), but further exploration is needed [20,43].
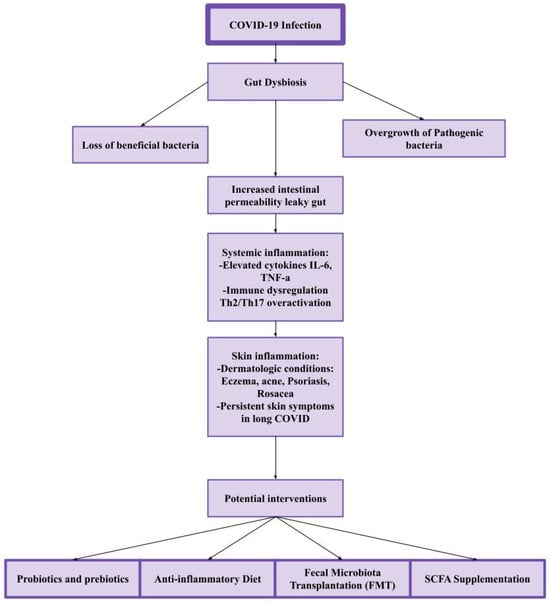
Figure 1.
The gut–skin axis in long COVID. COVID-19 can disrupt gut microbiota, leading to gut dysbiosis, which causes increased intestinal permeability and systemic inflammation. This inflammatory response contributes to skin conditions such as eczema, acne, psoriasis, and rosacea, with persistent symptoms in long COVID. Potential interventions, including probiotics, an anti-inflammatory diet, fecal microbiota transplantation (FMT), and SCFA supplementation, may help restore gut balance and reduce skin-related symptoms.
The gut–skin axis presents an intriguing target for potential therapies, yet much remains unclear about the precise mechanisms at play. Increased intestinal permeability and the release of pro-inflammatory cytokines highlight the complexity of this relationship, with conditions such as eczema, acne, and psoriasis emerging as potential manifestations of this dysbiosis [8,9,86].
Therapeutically, the promise of microbiome-targeted interventions is evident, with approaches like probiotics, prebiotics, and dietary modifications showing potential for improving both gut and skin health. While dietary changes, such as adopting an anti-inflammatory Mediterranean diet, are beneficial, the role of FMT remains experimental, and its long-term effects are still uncertain. Likewise, while probiotics like Lactobacillus and Bifidobacterium demonstrate benefits, particularly in conditions like eczema and acne, the variability in patient responses underscores the need for personalized approaches [5].
Dermatologists can integrate microbiome-targeted interventions into clinical practice by evaluating patients’ gut health as part of a comprehensive approach to managing dermatologic conditions. This could involve recommending probiotic and prebiotic supplements, dietary adjustments, and, where appropriate, referrals for further microbiome-based therapies like FMT. As the evidence base grows, dermatologists may also incorporate microbiome sequencing to personalize treatments, targeting specific microbial imbalances contributing to skin conditions.
A key challenge is the inherent individual variability in microbiota composition, which complicates the application of one-size-fits-all interventions. Advances in microbiome sequencing will be critical for refining personalized treatment plans, allowing for more effective targeting of gut–skin interactions. For long COVID patients with persistent skin disorders, it may be beneficial to consider gut microbiome testing as part of a diagnostic workup, especially when traditional treatments fail. This could help identify underlying dysbiosis contributing to skin symptoms and guide targeted microbiome-based therapies.
Ultimately, integrating precision medicine into long COVID care will offer the best potential for addressing the complex interplay between the gut and skin, but more research is needed to establish clear guidelines and optimize therapeutic strategies.
Limitations and Future Research Directions
Despite the promising potential of microbiome-targeted therapies, several limitations remain. Much of the research on the gut–skin axis in long COVID is observational, with controlled clinical trials still lacking. Most studies rely on stool samples, but the complex relationship between gut microbiota and dermatologic outcomes is influenced by factors such as genetics, medication use, and pre-existing health conditions. Additionally, the roles of the gut virome and fungal microbiome in post-COVID dysbiosis remain unclear, and while bacterial imbalances have been the primary focus, viral and fungal dysbiosis may also contribute to inflammation and dermatologic symptoms.
A key limitation is the lack of large-scale, longitudinal studies tracking microbiome changes in long COVID, making it difficult to establish causative links to dermatologic outcomes. Furthermore, there are too few clinical trials assessing microbiome-targeted interventions for skin conditions, hindering the development of evidence-based treatment protocols. Long-term studies are essential to evaluate the durability and effectiveness of these interventions in managing long-term dermatologic symptoms in long COVID patients.
Future research should prioritize longitudinal studies to track microbiome changes over time and their relationship to dermatologic outcomes. Clinical trials assessing the efficacy of probiotics, prebiotics, dietary modifications, and FMT in treating both gastrointestinal and dermatologic symptoms are crucial. In particular, randomized controlled trials on probiotic treatments for post-COVID dermatologic conditions are needed to establish standardized therapeutic guidelines. Additionally, studies should explore personalized microbiome-based therapies using advances in metagenomics and metabolomics to tailor interventions. The ultimate goal is to develop integrated, multi-disciplinary treatment approaches that address the interconnected nature of gut, skin, and immune health in long COVID (Figure 1).
6. Conclusions
COVID-19-induced gut dysbiosis has emerged as a key factor in the persistence of gastrointestinal and dermatologic symptoms in long COVID. Disruptions in the gut microbiome have been associated with systemic inflammation, increased intestinal permeability, and immune dysregulation, which may influence skin conditions such as eczema, acne, rosacea, and psoriasis. Addressing these imbalances through microbiome-targeted therapies—such as dietary interventions, probiotics, prebiotics, and fecal microbiota transplantation (FMT)—may offer a more effective, holistic approach to managing post-COVID complications.
Beyond long COVID, understanding the gut–skin axis has significant implications for other chronic diseases linked to gut dysbiosis. Conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), psoriasis, and atopic dermatitis share common pathways of immune dysfunction and microbial imbalance. Research into COVID-19’s impact on the gut may provide valuable insights into these conditions, potentially leading to microbiome-based therapies that can improve both gut and skin health.
Additionally, studying the long-term effects of viral infections on the microbiome could reshape how we approach chronic conditions following illnesses like HIV, hepatitis C, and influenza. Understanding how viruses alter microbial ecosystems and contribute to prolonged inflammation may pave the way for novel therapeutic strategies, including precision probiotics, dietary interventions, and targeted microbiome restoration.
By further exploring the gut–skin axis in long COVID, researchers can develop more personalized and preventative approaches for managing post-viral syndromes and chronic inflammatory conditions, ultimately improving long-term patient outcomes. As it turns out, what happens in the gut does not stay in the gut—so maybe the best skincare routine starts with a spoon, not a serum.
Author Contributions
D.G.: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—original draft, Writing—review & editing. E.G.: Resources, Validation, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Dalla Vecchia, I.; Auerbach, N.; Morra, M.; Górska, A.; Sciammarella, C.; Lambertenghi, L.; Gentilotti, E.; Mirandola, M.; Tacconelli, E.; et al. Gut Microbiome Disruption Following SARS-CoV-2: A Review. Microorganisms 2024, 12, 131. [Google Scholar] [CrossRef]
- Zhang, F.; Lau, R.I.; Liu, Q.; Su, Q.; Chan, F.K.L.; Ng, S.C. Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Hazan, S.; Stollman, N.; Bozkurt, H.S.; Dave, S.; Papoutsis, A.J.; Daniels, J.; Barrows, B.D.; Quigley, E.M.; Borody, T.J. Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 2022, 9, e000871. [Google Scholar] [CrossRef]
- Taufer, C.R.; Rampelotto, P.H. The Role of Bifidobacterium in COVID-19: A Systematic Review. Life 2023, 13, 1847. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, D.K. Role of Gut Microbiota in Long COVID: Impact on Immune Function and Organ System Health. Arch. Microbiol. Immunol. 2025, 9, 38–53. [Google Scholar]
- Raj, S.T.; Bruce, A.W.; Anbalagan, M.; Srinivasan, H.; Chinnappan, S.; Rajagopal, M.; Khanna, K.; Chandramoorthy, H.C.; Mani, R.R. COVID-19 influenced gut dysbiosis, post-acute sequelae, immune regulation, and therapeutic regimens. Front. Cell Infect. Microbiol. 2024, 14, 1384939. [Google Scholar] [CrossRef]
- Fekete, R.; Simats, A.; Bíró, E.; Pósfai, B.; Cserép, C.; Schwarcz, A.D.; Szabadits, E.; Környei, Z.; Tóth, K.; Fichó, E.; et al. Microglia dysfunction, neurovascular inflammation and focal neuropathologies are linked to IL-1- and IL-6-related systemic inflammation in COVID-19. Nature Neuroscience 2025, 28, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Zollner, A.; Meyer, M.; Jukic, A.; Adolph, T.; Tilg, H. The Intestine in Acute and Long COVID: Pathophysiological Insights and Key Lessons. Yale, J. Biol. Med. 2024, 97, 447–462. [Google Scholar] [CrossRef]
- Qiu, Y.; Mo, C.; Chen, L.; Ye, W.; Chen, G.; Zhu, T. Alterations in microbiota of patients with COVID-19: Implications for therapeutic interventions. MedComm 2024, 5, e513. [Google Scholar] [CrossRef]
- Bernard-Raichon, L.; Venzon, M.; Klein, J.; Axelrad, J.E.; Zhang, C.; Sullivan, A.P.; Hussey, G.A.; Casanovas-Massana, A.; Noval, M.G.; Valero-Jimenez, A.M.; et al. Gut microbiome dysbiosis in antibiotic-treated COVID-19 patients is associated with microbial translocation and bacteremia. Nat. Commun. 2022, 13, 5926. [Google Scholar] [PubMed]
- Li, Z.; Denning, D.W. The Impact of Corticosteroids on the Outcome of Fungal Disease: A Systematic Review and Meta-analysis. Curr. Fungal Infect. Rep. 2023, 17, 54–70. [Google Scholar] [PubMed]
- Wallace, V.J.; Sakowski, E.G.; Preheim, S.P.; Prasse, C. Bacteria exposed to antiviral drugs develop antibiotic cross-resistance and unique resistance profiles. Commun. Biol. 2023, 6, 837. [Google Scholar]
- Mendes de Almeida, V.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, Í.S.; Alves, D.A.; d’ Auriol, M.; Magalhães, J.; Machado, E.C.; Rocha, V.M.; et al. Gut microbiota from patients with COVID-19 cause alterations in mice that resemble post-COVID symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar]
- Wang, B.; Zhang, L.; Wang, Y.; Dai, T.; Qin, Z.; Zhou, F.; Zhang, L. Alterations in microbiota of patients with COVID-19: Potential mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 143. [Google Scholar]
- Zhang, T.; Liu, X.; Yang, F.; Xu, Y.; Jiang, X. Effect of COVID-19 and Face Masks on the Condition of Rosacea—A Retrospective Analysis of 87 Patients. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2855–2862. [Google Scholar]
- Mahmud, M.R.; Akter, S.; Tamanna, S.K.; Mazumder, L.; Esti, I.Z.; Banerjee, S.; Akter, S.; Hasan, M.R.; Acharjee, M.; Hossain, M.S.; et al. Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 2022, 14, 2096995. [Google Scholar]
- Zhang, J.; Zhang, Y.; Xia, Y.; Sun, J. Microbiome and intestinal pathophysiology in post-acute sequelae of COVID-19. Genes. Dis. 2023, 11, 100978. [Google Scholar] [PubMed]
- Martín, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermúdez-Humarán, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar]
- De Pessemier, B.; Grine, L.; Debaere, M.; Maes, A.; Paetzold, B.; Callewaert, C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms 2021, 9, 353. [Google Scholar] [CrossRef]
- Pan, Y.; Du, D.; Wang, L.; Wang, X.; He, G.; Jiang, X. The Role of T Helper 22 Cells in Dermatological Disorders. Front. Immunol. 2022, 13, 911546. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, H.S. Microbiome of the Skin and Gut in Atopic Dermatitis (AD): Understanding the Pathophysiology and Finding Novel Management Strategies. J. Clin. Med. 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Widhiati, S.; Purnomosari, D.; Wibawa, T.; Soebono, H. The role of gut microbiome in inflammatory skin disorders: A systematic review. Dermatol. Rep. 2022, 14, 9188. [Google Scholar] [CrossRef] [PubMed]
- Oparaugo, N.C.; Ouyang, K.; Nguyen, N.P.N.; Nelson, A.M.; Agak, G.W. Human Regulatory T Cells: Understanding the Role of Tregs in Select Autoimmune Skin Diseases and Post-Transplant Nonmelanoma Skin Cancers. Int. J. Mol. Sci. 2023, 24, 1527. [Google Scholar] [CrossRef] [PubMed]
- Kalekar, L.A.; Cohen, J.N.; Prevel, N.; Sandoval, P.M.; Mathur, A.N.; Moreau, J.M.; Lowe, M.M.; Nosbaum, A.; Wolters, P.J.; Haemel, A.; et al. Regulatory T cells in skin are uniquely poised to suppress profibrotic immune responses. Sci. Immunol. 2019, 4, eaaw2910. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Min, B. Tissue Resident Foxp3(+) Regulatory T Cells: Sentinels and Saboteurs in Health and Disease. Front. Immunol. 2022, 13, 865593. [Google Scholar] [CrossRef]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 Inflammation Contributes to Skin Barrier Dysfunction in Atopic Dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishida, A.; Yamano, M.; Kimura, I. Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol. Ther. 2022, 239, 108273. [Google Scholar] [CrossRef]
- Ică, O.M.; Mitroi, G.; Ianoşi, S.L.; Tutunaru, C.V.; Leru, P.M.; Matei, D.; Avramescu, E.T.; Tănasie, C.A.; Mitroi, I.B.; Neagoe, C.D.; et al. Defining the short-term and long-term skin manifestations of COVID-19: Insights after more than three years of the pandemic. Rom. J. Morphol. Embryol. 2023, 64, 291–304. [Google Scholar] [CrossRef]
- Panda, M.; Dash, S.; Behera, B.; Sil, A. Dermatological Manifestations Associated with COVID-19 Infection. Indian, J. Dermatol. 2021, 66, 237–245. [Google Scholar]
- Yang, S.; Bin Han, S.; Kang, S.; Lee, J.; Kim, D.; Kozlova, A.; Song, M.; Park, S.-H.; Lee, J. The relationship of skin disorders, COVID-19, and the therapeutic potential of ginseng: A review. J. Ginseng Res. 2023, 47, 33–43. [Google Scholar] [PubMed]
- Zhu, W.; Hamblin, M.R.; Wen, X. Role of the skin microbiota and intestinal microbiome in rosacea. Front. Microbiol. 2023, 14, 1108661. [Google Scholar]
- Zhao, Q.; Yu, J.; Zhou, H.; Wang, X.; Zhang, C.; Hu, J.; Hu, Y.; Zheng, H.; Zeng, F.; Yue, C.; et al. Intestinal dysbiosis exacerbates the pathogenesis of psoriasis-like phenotype through changes in fatty acid metabolism. Signal Transduct. Target. Ther. 2023, 8, 40. [Google Scholar] [PubMed]
- Bakhshandi, A.K.; Minasazi, A.; Yeganeh, O.; Behi, M. Therapeutic potential of microbiota modulation in psoriasis: Current evidence and future directions. Arch. Dermatol. Res. 2025, 317, 561. [Google Scholar]
- Buhaș, M.C.; Gavrilaș, L.I.; Candrea, R.; Cătinean, A.; Mocan, A.; Miere, D.; Tătaru, A. Gut Microbiota in Psoriasis. Nutrients 2022, 14, 2970. [Google Scholar] [CrossRef]
- Reali, E.; Caliceti, C.; Lorenzini, A.; Rizzo, P. The Use of Microbial Modifying Therapies to Prevent Psoriasis Exacerbation and Associated Cardiovascular Comorbidity. Inflammation 2024, 47, 13–29. [Google Scholar]
- Kim, M.H. Epidemiological insights into chronic urticaria, vitiligo, alopecia areata, and herpes zoster following COVID-19 infection: A nationwide population-based study. J. Dermatol. 2025, 52, 499–504. [Google Scholar] [PubMed]
- Afshar, Z.M.; Babazadeh, A.; Hasanpour, A.; Barary, M.; Sayad, B.; Janbakhsh, A.; Aryanian, Z.; Ebrahimpour, S. Dermatological manifestations associated with COVID-19: A comprehensive review of the current knowledge. J. Med. Virol. 2021, 93, 5756–5767. [Google Scholar]
- Giannos, P.; Prokopidis, K. Gut dysbiosis and long COVID-19: Feeling gutted. J. Med. Virol. 2022, 94, 2917–2918. [Google Scholar]
- Scher, J.U.; Ubeda, C.; Artacho, A.; M, A.; Isaac, S.; Reddy, S.M.; Marmon, S.; Neimann, A.; Brusca, S.; Patel, T.; et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015, 67, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Santacruz, C.; Tyrkalska, S.D.; Candel, S. The Microbiota in Long COVID. Int. J. Mol. Sci. 2024, 25, 1330. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Deng, F.; Li, Y.; Wei, X.; Zhao, J. Editorial: The gut-skin axis: Interaction of gut microbiome and skin diseases. Front. Microbiol. 2024, 15, 1427770. [Google Scholar] [CrossRef]
- Canale, M.P.; Menghini, R.; Martelli, E.; Federici, M. COVID-19-Associated Endothelial Dysfunction and Microvascular Injury: From Pathophysiology to Clinical Manifestations. Card. Electrophysiol. Clin. 2022, 14, 21–28. [Google Scholar] [CrossRef]
- Gu, S.X.; Tyagi, T.; Jain, K.; Gu, V.W.; Lee, S.H.; Hwa, J.M.; Kwan, J.M.; Krause, D.S.; Lee, A.I.; Halene, S.; et al. Thrombocytopathy and endotheliopathy: Crucial contributors to COVID-19 thromboinflammation. Nat. Rev. Cardiol. 2021, 18, 194–209. [Google Scholar] [CrossRef]
- Genovese, G.; Moltrasio, C.; Berti, E.; Marzano, A.V. Skin Manifestations Associated with COVID-19: Current Knowledge and Future Perspectives. Dermatology 2021, 237, 1–12. [Google Scholar] [CrossRef]
- Sadeghzadeh-Bazargan, A.; Rezai, M.; Nobari, N.N.; Mozafarpoor, S.; Goodarzi, A. Skin manifestations as potential symptoms of diffuse vascular injury in critical COVID-19 patients. J. Cutan. Pathol. 2021, 48, 1266–1276. [Google Scholar] [CrossRef]
- Valencia, I.; Lumpuy-Castillo, J.; Magalhaes, G.; Sánchez-Ferrer, C.F.; Lorenzo, Ó.; Peiró, C. Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: A link with diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 75. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H.; Sako, A. The Significance of Endothelial Dysfunction in Long COVID-19 for the Possible Future Pandemic of Chronic Kidney Disease and Cardiovascular Disease. Biomolecules 2024, 14, 965. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar]
- Sahle, Z.; Engidaye, G.; Gebreyes, D.S.; Adenew, B.; Abebe, T.A. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024, 12, 20503121241257486. [Google Scholar]
- Díez-Madueño, K.; Dobao, P.d.l.C.; Torres-Rojas, I.; Fernández-Gosende, M.; Hidalgo-Cantabrana, C.; Coto-Segura, P. Gut Dysbiosis and Adult Atopic Dermatitis: A Systematic Review. J. Clin. Med. 2024, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Borzyszkowska, D.; Niedzielska, M.; Kozłowski, M.; Brodowska, A.; Przepiera, A.; Malczyk-Matysiak, K.; Cymbaluk-Płoska, A.; Sowińska-Przepiera, E. Evaluation of Hormonal Factors in Acne Vulgaris and the Course of Acne Vulgaris Treatment with Contraceptive-Based Therapies in Young Adult Women. Cells 2022, 11, 4078. [Google Scholar] [CrossRef]
- Lundquist, P.; Hagforsen, E.; Wagner, M.; Alimohammadi, M.; Melo, F.R.; Pejler, G.; Artursson, P.; Carlson, M.; Rollman, O.; Lampinen, M. Mild-to-moderate psoriasis is associated with subclinical inflammation in the duodenum and a tendency of disturbed intestinal barrier. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2025, 1871, 167634. [Google Scholar]
- Wang, F.Y.; Chi, C.C. Rosacea, Germs, and Bowels: A Review on Gastrointestinal Comorbidities and Gut-Skin Axis of Rosacea. Adv. Ther. 2021, 38, 1415–1424. [Google Scholar]
- Cai, R.; Zhou, C.; Tang, R.; Meng, Y.; Zeng, J.; Li, Y.; Wen, X. Current insights on gut microbiome and chronic urticaria: Progress in the pathogenesis and opportunities for novel therapeutic approaches. Gut Microbes 2024, 16, 2382774. [Google Scholar]
- Bain, K.A.; McDonald, E.; Moffat, F.; Tutino, M.; Castelino, M.; Barton, A.; Cavanagh, J.; Ijaz, U.Z.; Siebert, S.; McInnes, I.; et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. Br. J. Dermatol. 2020, 182, 130–137. [Google Scholar]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, X.; Yao, J.; Cao, W.; Zou, Z.; Wang, L.; Qin, H.; Zhong, D.; Li, Y.; Xue, P.; et al. The role of short-chain fatty acids in inflammatory skin diseases. Front. Microbiol. 2022, 13, 1083432. [Google Scholar]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The Role of Probiotics in Skin Health and Related Gut-Skin Axis: A Review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Xie, A.; Chen, A.; Chen, Y.; Luo, Z.; Jiang, S.; Chen, D.; Yu, R. Lactobacillus for the treatment and prevention of atopic dermatitis: Clinical and experimental evidence. Front. Cell Infect. Microbiol. 2023, 13, 1137275. [Google Scholar]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Alenazy, M.F.; Aljohar, H.I.; Alruwaili, A.R.; Daghestani, M.H.; Alonazi, M.A.; Labban, R.S.; El-Ansary, A.K.; Balto, H.A. Gut Microbiota Dynamics in Relation to Long-COVID-19 Syndrome: Role of Probiotics to Combat Psychiatric Complications. Metabolites 2022, 12, 912. [Google Scholar] [CrossRef]
- Karimi, M.; Shirsalimi, N.; Hashempour, Z.; Omran, H.S.; Sedighi, E.; Beigi, F.; Mortezazadeh, M. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: A comprehensive literature review. Front. Immunol. 2024, 15, 1439176. [Google Scholar]
- Kim, J.-H.; Kim, K.; Kim, W. Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 2021, 53, 907–916. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Cai, J. Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 2023, 141, 103038. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front. Immunol. 2022, 13, 1063543. [Google Scholar]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Greuter, T.; Navarini, A.; Vavricka, S.R. Skin Manifestations of Inflammatory Bowel Disease. Clin. Rev. Allergy Immunol. 2017, 53, 413–427. [Google Scholar]
- Bénard, M.V.; de Bruijn, C.M.A.; Fenneman, A.C.; Wortelboer, K.; Zeevenhoven, J.; Rethans, B.; Herrema, H.J.; van Gool, T.; Nieuwdorp, M.; Benninga, M.A.; et al. Challenges and costs of donor screening for fecal microbiota transplantations. PLoS ONE 2022, 17, e0276323. [Google Scholar]
- Oh, C.K.; Chung, H.H.; Kim, Y.J.; Kim, J.B. Comparison of Rifaximin Monotherapy and Rifaximin Combined with Probiotics in Patients with Irritable Bowel Syndrome: A Randomized Controlled Trial. Nutrients 2025, 17, 763. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar]
- Wensel, C.R.; Pluznick, J.L.; Salzberg, S.L.; Sears, C.L. Next-generation sequencing: Insights to advance clinical investigations of the microbiome. J. Clin. Investig. 2022, 132, e154944. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Fischer, M. Fecal Microbiota Transplantation. Clin. Colon. Rectal Surg. 2023, 36, 151–156. [Google Scholar] [PubMed]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal barrier permeability: The influence of gut microbiota, nutrition, and exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).