Yearly Spatiotemporal Patterns of COVID-19 During the Pandemic Period: An In-Depth Analysis of Regional Trends and Risk Factors in the Republic of Korea
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Collection
2.2. Data Analysis
2.2.1. Spatiotemporal Pattern Analysis
2.2.2. Risk Factor Analysis
2.2.3. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korea Centers for Disease Control and Prevention Agency. 2024. Available online: https://www.kdca.go.kr/ (accessed on 1 December 2023).
- Kang, D.; Choi, J.; Kim, Y.; Kwon, D. An analysis of the dynamic spatial spread of COVID-19 across South Korea. Sci. Rep. 2022, 12, 9364. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Castro, M.C. Spatiotemporal pattern of COVID-19 and government response in South Korea (as of 31 May 2020). Int. J. Infect. Dis. 2020, 98, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Hwang, S.-S.; Song, I.; Park, C.; Kim, H.; Song, I.-K.; Choi, H.M.; Prifti, K.; Kwon, Y.; Kim, J.; et al. COVID-19 in South Korea: Epidemiological and spatiotemporal patterns of the spread and the role of aggressive diagnostic tests in the early phase. Int. J. Epidemiol. 2020, 49, 1106–1116. [Google Scholar] [CrossRef]
- Lym, Y.; Lym, H.; Kim, K.J. Local-level spatiotemporal dynamics of COVID-19 transmission in the Greater Seoul Area, Korea: A view from a Bayesian perspective. Epidemiol. Health 2020, 44, e2022016. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, M.; Lee, S.; Lee, Y.J. Discovering spatiotemporal patterns of COVID-19 pandemic in South Korea. Sci. Rep. 2021, 11, 24470. [Google Scholar] [CrossRef]
- Sutton, J.; Shahtahmassebi, G.; Ribeiro, H.V.; Hanley, Q.S. Population density and spreading of COVID-19 in England and Wales. PLoS ONE 2022, 17, e0261725. [Google Scholar] [CrossRef]
- Watts, M.J. Macro-level drivers of SARS-CoV-2 transmission: A data-driven analysis of factors contributing to epidemic growth during the first wave of outbreaks in the United States. Spat. Spatiotemporal Epidemiol. 2022, 43, 100539. [Google Scholar] [CrossRef]
- Yin, H.; Sun, T.; Yao, L.; Jiao, Y.; Ma, L.; Lin, L.; Graff, J.C.; Aleya, L.; Postlethwaite, A.; Gu, W.; et al. Association between population density and infection rate suggests the importance of social distancing and travel restriction in reducing the COVID-19 pandemic. Environ. Sci. Pollut. Res. Int. 2021, 28, 40424–40430. [Google Scholar]
- Zhang, A.; Shi, W.; Tong, C.; Zhu, X.; Liu, Y.; Liu, Z.; Yao, Y.; Shi, Z. The fine-scale associations between socioeconomic status, density, functionality, and spread of COVID-19 within a high-density city. BMC Infect. Dis. 2022, 22, 274. [Google Scholar] [CrossRef]
- Kim, J.; Heo, N.; Kang, H. Sex-Based Differences in Outcomes of Coronavirus Disease 2019 (COVID-19) in Korea. Asian Nurs. Res. 2022, 16, 224–230. [Google Scholar] [CrossRef]
- Ramírez-Soto, M.C.; Arroyo-Hernández, H.; Ortega-Cáceres, G. Sex differences in the incidence, mortality, and fatality of COVID-19 in Peru. PLoS ONE 2021, 16, e0253193. [Google Scholar] [CrossRef]
- Abate, B.B.; Kassie, A.M.; Kassaw, M.W.; Aragie, T.G.; Masresha, S.A. Sex difference in coronavirus disease (COVID-19): A systematic review and meta-analysis. BMJ Open 2020, 10, e040129. [Google Scholar] [CrossRef]
- Falandry, C.; Malapert, A.; Roche, M.; Subtil, F.; Berthiller, J.; Boin, C.; Dubreuil, J.; Ravot, C.; Bitker, L.; Abraham, P.; et al. Risk factors associated with day-30 mortality in patients over 60 years old admitted in ICU for severe COVID-19: The Senior-COVID-Rea Multicentre Survey protocol. BMJ Open 2021, 11, e044449. [Google Scholar] [CrossRef]
- Oster, A.M.; Caruso, E.; DeVies, J.; Hartnett, K.P.; Boehmer, T.K. Transmission Dynamics by Age Group in COVID-19 Hotspot Counties-United States, April-September 2020. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1494–1496. [Google Scholar] [CrossRef]
- Hwang, M.J.; Park, S.Y.; Yoon, T.H.; Jang, J.; Lee, S.Y.; Yoo, M.; Kim, Y.Y.; Cheong, H.K.; Kwon, D.; Kim, J.H.; et al. Effect of socioeconomic disparities on the risk of COVID-19 in 8 metropolitan cities in the Korea: A community-based study. Epidemiol. Health 2022, 44, e2022107. [Google Scholar] [CrossRef]
- Abou Ghayda, R.; Lee, K.H.; Han, Y.J.; Ryu, S.; Hong, S.H.; Yoon, S.; Jeong, G.H.; Yang, J.W.; Lee, H.J.; Lee, J.; et al. The global case fatality rate of coronavirus disease 2019 by continents and national income: A meta-analysis. J. Med. Virol. 2022, 94, 2402–2413. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Min, C.; Choi, H.G. The Effects of Income Level on Susceptibility to COVID-19 and COVID-19 Morbidity/Mortality: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021, 10, 4733. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M.; et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Jung, C.Y.; Park, H.; Kim, D.W.; Lim, H.; Chang, J.H.; Choi, Y.J.; Kim, S.W.; Chang, T.I. Association between Body Mass Index and Risk of Coronavirus Disease 2019 (COVID-19): A Nationwide Case-control Study in South Korea. Clin. Infect. Dis. 2021, 73, E1855–E1862. [Google Scholar] [CrossRef]
- Lee, S.C.; Son, K.J.; Kim, D.W.; Han, C.H.; Choi, Y.J.; Kim, S.W.; Park, S.C. Smoking and the Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Nicotine Tob. Res. 2021, 23, 1787–1792. [Google Scholar] [CrossRef]
- Choi, J.W. Association between smoking status and death from COVID-19 in South Korea: A nationwide cohort study. Tob. Induc. Dis. 2023, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Rhee, E.-J.; Jung, J.-H.; Han, K.-D.; Kim, S.-R.; Lee, W.-Y.; Yoon, K.-H. Independent Impact of Diabetes on the Severity of Coronavirus Disease 2019 in 5,307 Patients in South Korea: A Nationwide Cohort Study. Diabetes Metab. J. 2020, 44, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Huh, K.; Ko, K.-P.; Im, J.-S.; Jung, J.; Kang, M.; Hong, J.; Bae, G.H.; Lee, R.; Na, Y.; et al. Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: A Nationwide Case-Control Study. J. Korean Med. Sci. 2020, 35, e237. [Google Scholar] [CrossRef]
- Chun, S.Y.; Kim, D.W.; Lee, S.A.; Lee, S.J.; Chang, J.H.; Choi, Y.J.; Kim, S.W.; Song, S.O. Does Diabetes Increase the Risk of Contracting COVID-19? A Population-Based Study in Korea. Diabetes Metab. J. 2020, 44, 897–907. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chin, Y.; Yu, S.; Huang, J.; Zhang, C.J.P.; Zhu, K.; Azarakhsh, N.; Sheng, J.; He, Y.; Jayavanth, P.; et al. The Influence of Average Temperature and Relative Humidity on New Cases of COVID-19: Time-Series Analysis. JMIR Public. Health Surveill. 2021, 7, e20495. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, H.J.; Hong, M.; Kwon, S.K.; Kim, J.H.; Lee, S.J.; Lee, S.W.; Han, H.W. Effects of meteorological factors and air pollutants on the incidence of COVID-19 in South Korea. Environ. Res. 2022, 212, 113392. [Google Scholar] [CrossRef]
- Lym, Y.; Kim, K.J. Exploring the effects of PM2.5 and temperature on COVID-19 transmission in Seoul, South Korea. Environ. Res. 2022, 203, 111810. [Google Scholar] [CrossRef]
- Lim, Y.K.; Kweon, O.J.; Kim, H.R.; Kim, T.H.; Lee, M.K. The impact of environmental variables on the spread of COVID-19 in the Republic of Korea. Sci. Rep. 2021, 11, 5977. [Google Scholar] [CrossRef]
- Menebo, M.M. Temperature and precipitation associate with Covid-19 new daily cases: A correlation study between weather and Covid-19 pandemic in Oslo, Norway. Sci. Total Environ. 2020, 737, 139659. [Google Scholar] [CrossRef]
- Majumder, P.; Ray, P.P. A systematic review and meta-analysis on correlation of weather with COVID-19. Sci. Rep. 2021, 11, 10746. [Google Scholar] [CrossRef]
- Korean Statistical Information Service. 2024. Available online: https://kosis.kr/eng/ (accessed on 1 December 2023).
- Statistics Korea. 2024. Available online: https://kostat.go.kr/anse/ (accessed on 1 December 2023).
- Korean Metrological Agency. 2024. Available online: https://www.weather.go.kr/w/index.do (accessed on 1 December 2023).
- Kim, Y.J.; Seo, M.H.; Yeom, H.E. Estimating a breakpoint in the pattern of spread of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 97, 360–364. [Google Scholar] [CrossRef]
- Wang, D.; Li, D.; Wang, X.; Sun, Z. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 23, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Han, C.; Kim, D.; Tsang, T.K.; Cowling, B.J.; Lee, S. Association Between the Relaxation of Public Health and Social Measures and Transmission of the SARS-CoV-2 Omicron Variant in South Korea. JAMA Netw. Open 2022, 5, e2225665. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.D.; Sarkar, A.; Chouhan, D.P. COVID-19 second wave: District level study of concentration of confirmed cases and fatality in India. Environ. Chall. 2021, 5, 100221. [Google Scholar] [CrossRef]
- Hazbavi, Z.; Mostfazadeh, R.; Alaei, N.; Azizi, E. Spatial and temporal analysis of the COVID-19 incidence pattern in Iran. Environ. Sci. Pollut. Res. 2021, 28, 13605–13615. [Google Scholar] [CrossRef]
- Hashim, M.J.; Alsuwaidi, A.R.; Khan, G. Population risk factors for COVID-19 mortality in 93 countries. J. Epidemiol. Glob. Health 2020, 10, 204–208. [Google Scholar] [CrossRef]
- Ahmed, J.; Jaman, M.H.; Saha, G.; Ghosh, P. Effect of environmental and socio-economic factors on the spreading of COVID-19 at 70 cities/provinces. Heliyon 2021, 7, e06979. [Google Scholar] [CrossRef]
- Yan, W.; Nawaz, M.Z.; Xu, W.; Jiang, Z.; Sun, W.; Lai, J.; Shao, Y.; Zhang, W.; Zhang, R. Atmospheric pressure and population density as super-factors influencing the transmission of coronavirus disease 2019. COVID-19 2020. [Google Scholar] [CrossRef]
- Bahl, P.; Doolan, C.; De Silva, C.; Chughtai, A.A.; Bourouiba, L.; MacIntyre, C.R. Airborne or Droplet Precautions for Health Workers Treating Coronavirus Disease 2019? J. Infect. Dis. 2022, 225, 1561–1568. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, L.; Yang, X.; Huang, Z.; Yang, X.; Zhao, N.; He, M.; Shi, Y.; Kang, Y.; Yue, J.; et al. The association between frailty and severe disease among COVID-19 patients aged over 60 years in China: A prospective cohort study. BMC Med. 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Petermann-Rocha, F.; Gray, S.R.; Jani, B.D.; Katikireddi, S.V.; Niedzwiedz, C.L.; Foster, H.; Hastie, C.E.; Mackay, D.F.; Gill, J.M.R.; et al. Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS ONE 2020, 15, e0241824. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E. Data from the COVID-19 epidemic in Florida suggest that younger cohorts have been transmitting their infections to less socially mobile older adults. Rev. Econ. Househ. 2020, 18, 1019–1037. [Google Scholar] [CrossRef]
- Osama, T.; Pankhania, B.; Majeed, A. Protecting older people from COVID-19: Should the United Kingdom start at age 60? J. R. Soc. Med. 2020, 113, 169–170. [Google Scholar] [CrossRef]
- El Karoui, K.; Hourmant, M.; Ayav, C.; Glowacki, F.; Couchoud, C.; Lapidus, N.; REIN Registry. Vaccination and COVID-19 Dynamics in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2022, 17, 395–402. [Google Scholar] [CrossRef]
- Peters, S.A.E.; MacMahon, S.; Woodward, M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: Comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes. Metab. 2021, 23, 258–262. [Google Scholar] [CrossRef]
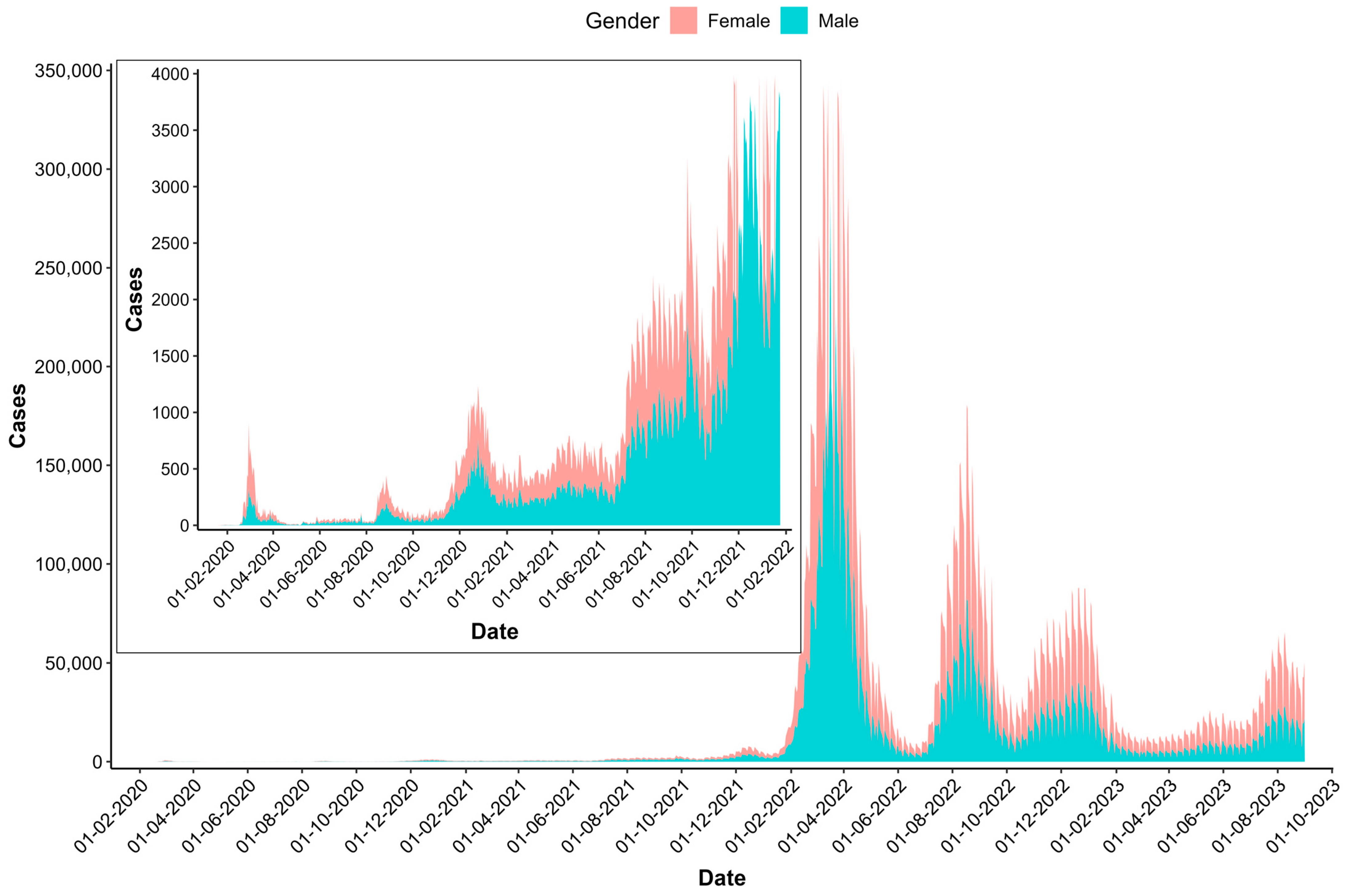
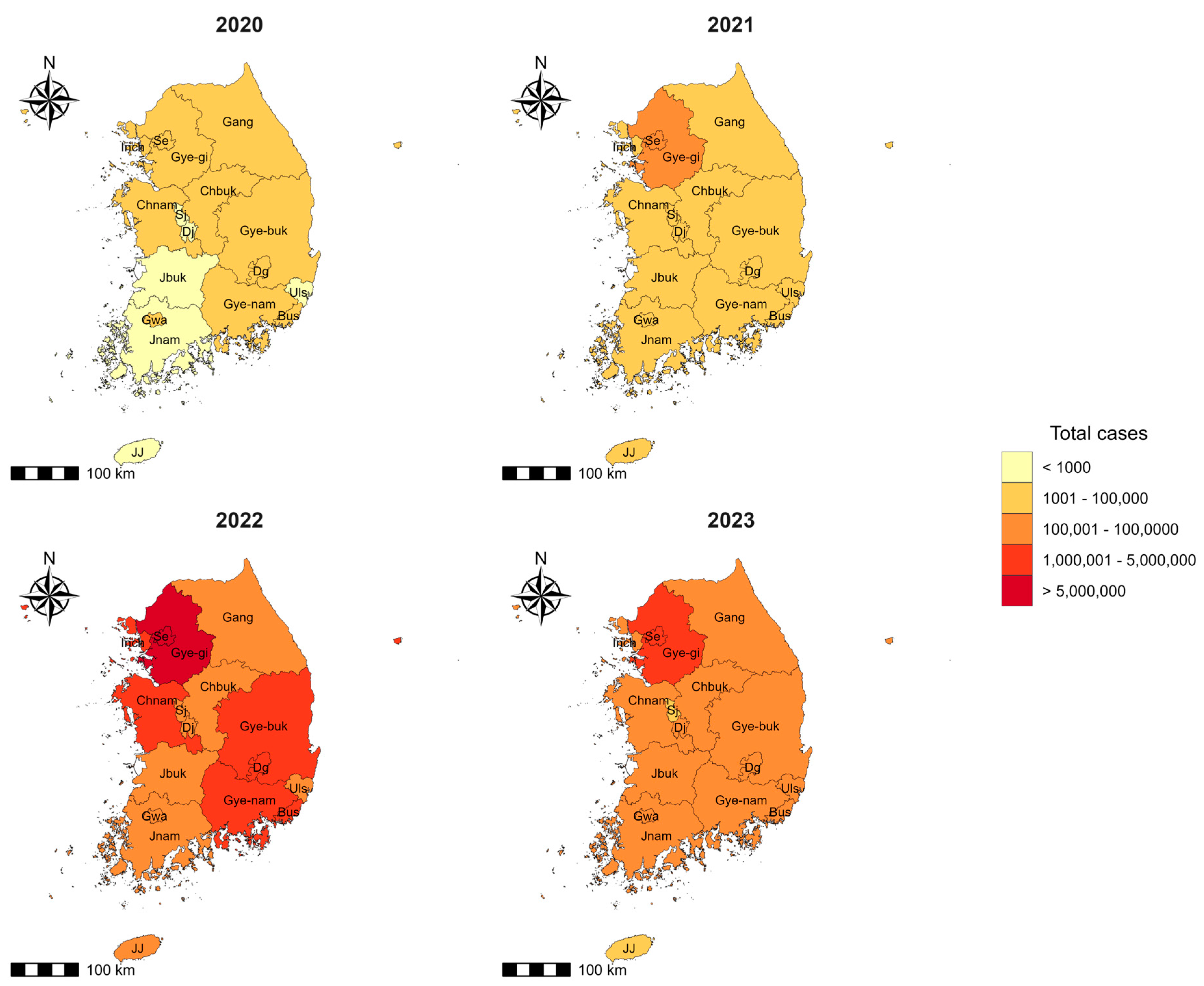
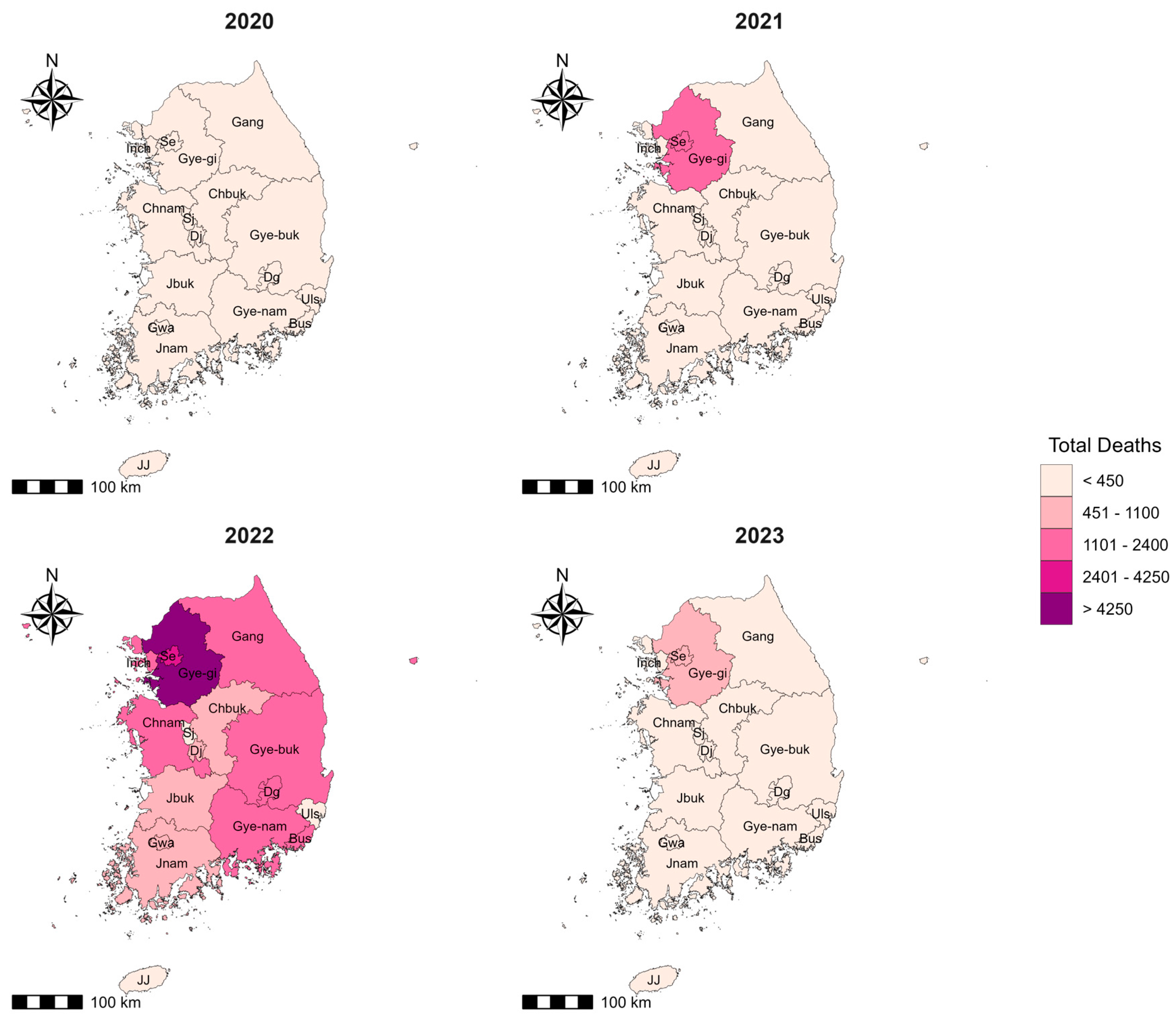

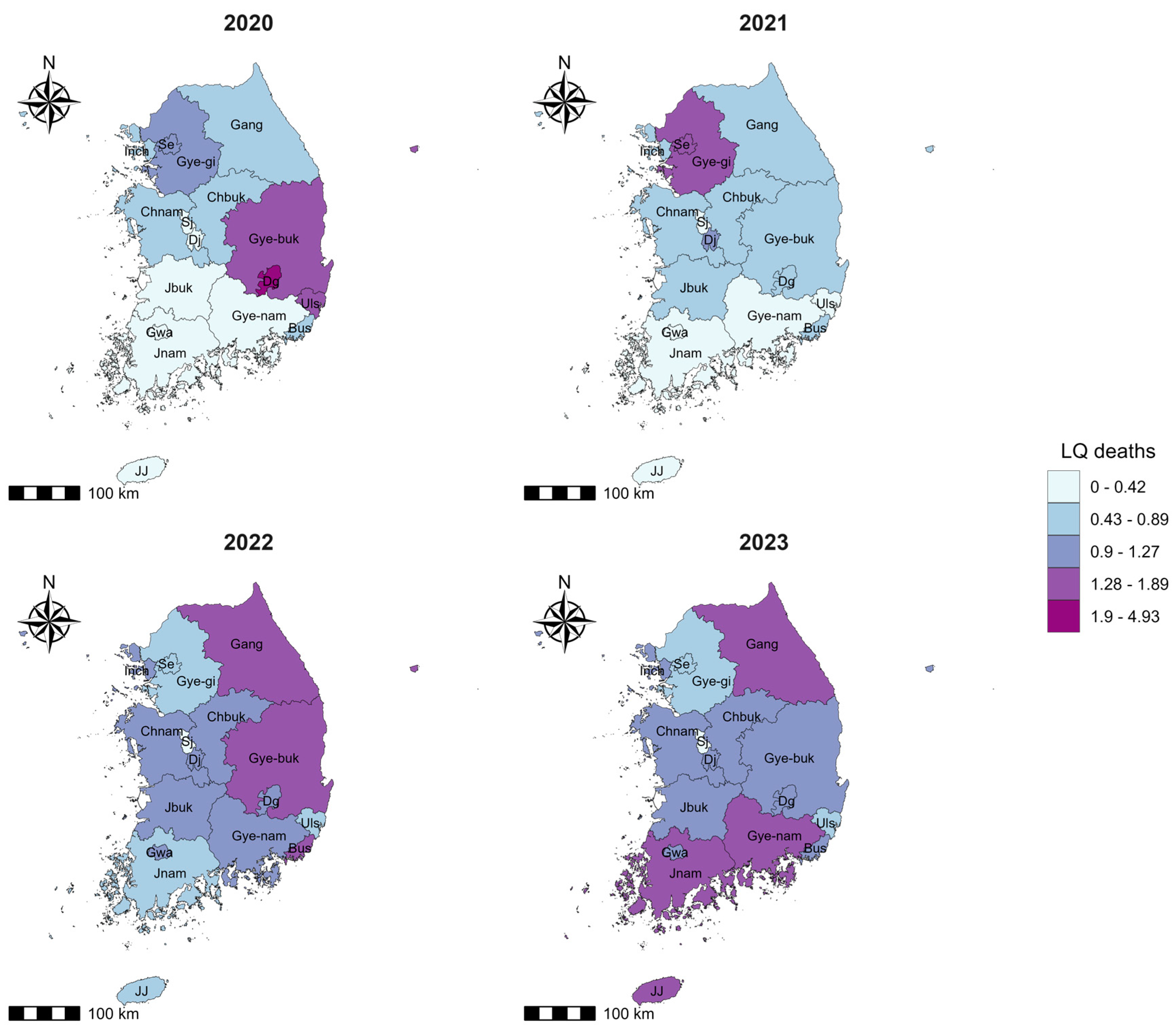
| SN | Region | Abbreviation Used in Spatial Maps | Population (2022) | Cumulative Number of New Cases (2020–2023) | Cumulative Incidence Rate/100,000 Population | Cumulative Number of Deaths (2020–2023) | Cumulative Case-Fatality Rate (2020–2023) |
|---|---|---|---|---|---|---|---|
| 1 | Seoul | Se | 9,428,372 | 6,751,335 | 71,606.58 | 6587 | 0.10 |
| 2 | Busan | Bus | 3,317,812 | 2,092,642 | 63,072.95 | 2911 | 0.14 |
| 3 | Daegu | Dg | 2,363,691 | 1,516,421 | 64,154.79 | 2050 | 0.14 |
| 4 | Incheon | Inch | 2,967,314 | 1,991,892 | 67,127.78 | 1941 | 0.10 |
| 5 | Gwangju | Gwa | 1,431,050 | 1,018,499 | 71,171.45 | 868 | 0.09 |
| 6 | Daejeon | Dj | 1,446,072 | 1,013,275 | 70,070.85 | 1003 | 0.10 |
| 7 | Ulsan | Uls | 1,110,663 | 738,128 | 66,458.32 | 543 | 0.07 |
| 8 | Sejong | Sej | 383,591 | 273,413 | 71,277.22 | 61 | 0.02 |
| 9 | Gyeonggi-do | Gye-gi | 13,589,432 | 9,266,797 | 68,191.20 | 8585 | 0.09 |
| 10 | Gangwon-do | Gang | 1,536,498 | 1,005,836 | 65,462.89 | 1406 | 0.14 |
| 11 | Chungcheongbuk-do | Chbuk | 1,595,058 | 1,075,474 | 67,425.39 | 1093 | 0.10 |
| 12 | Chungcheongnam-do | Chnam | 2,123,037 | 1,390,798 | 65,509.83 | 1638 | 0.12 |
| 13 | Jeollabuk-do | Jbuk | 1,769,607 | 1,167,948 | 66,000.42 | 1261 | 0.11 |
| 14 | Jeollanam-do | Jnam | 1,817,697 | 1,142,483 | 62,853.32 | 1083 | 0.09 |
| 15 | Gyeongsangbuk-do | Gye-buk | 2,600,492 | 1,581,207 | 60,804.15 | 2177 | 0.14 |
| 16 | Gyeongsangnam-do | Gye-nam | 3,280,493 | 2,075,991 | 63,282.90 | 2065 | 0.10 |
| 17 | Jeju | JJ | 678,159 | 451,523 | 66,580.70 | 317 | 0.07 |
| SN | Region | Population (2022) | Incidence Rate 2020/100,000 Population | Incidence Rate 2021/100,000 Population | Incidence Rate 2022/100,000 Population | Incidence Rate 2023/100,000 Population | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Seoul | 9,428,372 | 201.43 | 2186.05 | 57,661.02 | 11,558.08 | <0.001 |
| 2 | Busan | 3,317,812 | 56.27 | 699.47 | 50,983.42 | 11,333.79 | <0.001 |
| 3 | Daegu | 2,363,691 | 330.03 | 648.27 | 52,701.05 | 10,475.44 | <0.001 |
| 4 | Incheon | 2,967,314 | 95.68 | 1141.77 | 55,593.75 | 10,296.58 | <0.001 |
| 5 | Gwangju | 1,431,050 | 75.54 | 485.31 | 58,769.85 | 11,840.75 | <0.001 |
| 6 | Daejeon | 1,446,072 | 58.43 | 788.69 | 57,834.12 | 11,389.61 | <0.001 |
| 7 | Ulsan | 1,110,663 | 60.50 | 558.23 | 55,040.01 | 10,799.59 | <0.001 |
| 8 | Sejong | 383,591 | 38.84 | 519.56 | 59,452.39 | 11,266.43 | <0.001 |
| 9 | Gyeonggi-do | 13,589,432 | 106.33 | 1264.82 | 56,519.83 | 10,300.22 | <0.001 |
| 10 | Gangwon-do | 1,536,498 | 78.56 | 728.80 | 55,153.28 | 9502.26 | <0.001 |
| 11 | Chungcheongbuk-do | 1,595,058 | 72.91 | 647.94 | 56,475.94 | 10,228.59 | <0.001 |
| 12 | Chungcheongnam-do | 2,123,037 | 77.86 | 774.41 | 54,434.19 | 10,223.37 | <0.001 |
| 13 | Jeollabuk-do | 1,769,607 | 47.30 | 493.39 | 54,432.82 | 11,026.91 | <0.001 |
| 14 | Jeollanam-do | 1,817,697 | 30.81 | 303.57 | 51,792.85 | 10,726.10 | <0.001 |
| 15 | Gyeongsangbuk-do | 2,600,492 | 93.21 | 495.68 | 50,428.57 | 9786.69 | <0.001 |
| 16 | Gyeongsangnam-do | 3,280,493 | 40.39 | 589.88 | 52,196.33 | 10,456.29 | <0.001 |
| 17 | Jeju | 678,159 | 61.34 | 622.57 | 53,278.95 | 12,617.84 | <0.001 |
| Cumulative Number of New Cases (2020–2023) | Incidence Rate 2020/100,000 Population | Incidence Rate 2021/100,000 Population | Incidence Rate 2022/100,000 Population | Incidence Rate 2023/100,000 Population | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age | 0–9 | 3,270,282 | 59.19 | 1331.64 | 84,004.27 | 7178.17 | <0.001 |
| 10–19 | 4,246,977 | 80.14 | 1260.19 | 76,608.37 | 12,351.11 | <0.001 | |
| 20–29 | 5,001,143 | 151.28 | 1320.11 | 64,542.98 | 11,919.27 | <0.001 | |
| 30–39 | 5,077,726 | 116.79 | 1268.93 | 62,727.14 | 12,642.00 | <0.001 | |
| 40–49 | 5,237,546 | 106.79 | 1034.23 | 53,722.32 | 10,013.03 | <0.001 | |
| 50–59 | 4,531,012 | 132.29 | 930.62 | 42,741.18 | 8808.28 | <0.001 | |
| 60–69 | 3,898,836 | 129.99 | 1083.39 | 41,205.28 | 10,243.13 | <0.001 | |
| 70–79 | 2,056,083 | 124.63 | 879.39 | 40,596.89 | 12,142.82 | <0.001 | |
| 80+ | 1,252,949 | 133.95 | 774.75 | 41,684.90 | 12,943.17 | <0.001 | |
| Gender | Male | 18,680,325 | 120.63 | 1051.94 | 59,171.59 | 12,054.34 | <0.001 |
| Female | 15,892,229 | 115.45 | 1164.41 | 51,319.84 | 9389.84 | <0.001 |
| SN | Region | Population (2022) | CFR 2020 | CFR 2021 | CFR 2022 | CFR 2023 | p-Value |
|---|---|---|---|---|---|---|---|
| 1 | Seoul | 9,428,372 | 0.91 | 0.77 | 0.08 | 0.05 | 0.718 |
| 2 | Busan | 3,317,812 | 2.62 | 0.94 | 0.14 | 0.07 | 0.213 |
| 3 | Daegu | 2,363,691 | 2.58 | 1.06 | 0.12 | 0.08 | 0.233 |
| 4 | Incheon | 2,967,314 | 0.92 | 0.68 | 0.09 | 0.07 | 0.744 |
| 5 | Gwangju | 1,431,050 | 0.56 | 0.55 | 0.09 | 0.06 | 0.865 |
| 6 | Daejeon | 1,446,072 | 0.83 | 1.40 | 0.09 | 0.08 | 0.558 |
| 7 | Ulsan | 1,110,663 | 3.87 | 0.56 | 0.07 | 0.04 | 0.030 |
| 8 | Sejong | 383,591 | 0.67 | 0.15 | 0.02 | 0.03 | 0.724 |
| 9 | Gyeonggi-do | 13,589,432 | 1.80 | 0.90 | 0.08 | 0.05 | 0.405 |
| 10 | Gangwon-do | 1,536,498 | 1.08 | 0.79 | 0.13 | 0.13 | 0.732 |
| 11 | Chungcheongbuk-do | 1,595,058 | 1.98 | 0.82 | 0.10 | 0.08 | 0.359 |
| 12 | Chungcheongnam-do | 2,123,037 | 1.39 | 0.73 | 0.11 | 0.08 | 0.575 |
| 13 | Jeollabuk-do | 1,769,607 | 1.31 | 1.08 | 0.10 | 0.07 | 0.583 |
| 14 | Jeollanam-do | 1,817,697 | 0.89 | 0.56 | 0.09 | 0.11 | 0.780 |
| 15 | Gyeongsangbuk-do | 2,600,492 | 2.48 | 0.76 | 0.14 | 0.09 | 0.229 |
| 16 | Gyeongsangnam-do | 3,280,493 | 0.30 | 0.44 | 0.09 | 0.10 | 0.948 |
| 17 | Jeju | 678,159 | 0.00 | 0.31 | 0.06 | 0.09 | 0.92633 |
| Region | LQ Cases 2020 | LQ Cases 2021 | LQ Cases 2022 | LQ Cases 2023 | LQ Deaths 2020 | LQ Deaths 2021 | LQ Deaths 2022 | LQ Deaths 2023 |
|---|---|---|---|---|---|---|---|---|
| Busan | 0.49 | 0.63 | 0.92 | 1.05 | 0.85 | 0.73 | 1.40 | 1.05 |
| Chungcheongbuk-do | 0.65 | 0.59 | 1.02 | 0.96 | 0.84 | 0.60 | 1.05 | 1.10 |
| Chungcheongnam-do | 0.69 | 0.71 | 0.99 | 0.96 | 0.63 | 0.63 | 1.22 | 1.12 |
| Daegu | 2.90 | 0.59 | 0.95 | 0.97 | 4.90 | 0.77 | 1.22 | 1.18 |
| Daejeon | 0.52 | 0.72 | 1.05 | 1.06 | 0.28 | 1.23 | 0.96 | 1.18 |
| Gangwon | 0.70 | 0.66 | 1.00 | 0.89 | 0.49 | 0.65 | 1.41 | 1.74 |
| Gwangju | 0.66 | 0.44 | 1.06 | 1.10 | 0.24 | 0.30 | 0.98 | 0.97 |
| Gyeonggi-do | 0.94 | 1.16 | 1.03 | 0.97 | 1.11 | 1.28 | 0.88 | 0.68 |
| Gyeongsangbuk-do | 0.82 | 0.45 | 0.91 | 0.91 | 1.33 | 0.42 | 1.33 | 1.21 |
| Gyeongsangnam-do | 0.35 | 0.53 | 0.94 | 0.97 | 0.07 | 0.29 | 0.96 | 1.46 |
| Incheon | 0.85 | 1.05 | 1.02 | 0.97 | 0.51 | 0.87 | 0.98 | 1.01 |
| Jeju | 0.54 | 0.57 | 0.97 | 1.18 | 0.00 | 0.22 | 0.65 | 1.60 |
| Jeollabuk-do | 0.42 | 0.45 | 0.98 | 1.02 | 0.36 | 0.59 | 1.11 | 1.12 |
| Jeollanam-do | 0.27 | 0.27 | 0.93 | 1.00 | 0.16 | 0.19 | 0.88 | 1.65 |
| Sejong | 0.35 | 0.49 | 1.11 | 1.09 | 0.16 | 0.09 | 0.24 | 0.41 |
| Seoul | 1.77 | 1.98 | 1.04 | 1.07 | 1.06 | 1.88 | 0.87 | 0.87 |
| Ulsan | 0.53 | 0.50 | 0.99 | 1.00 | 1.35 | 0.35 | 0.76 | 0.57 |
| Year | Variable | Moran I | Z Score | p-Value |
|---|---|---|---|---|
| 2020 | Cases | 0.75 | 5.17 | <0.001 |
| Deaths | 0.48 | 3.21 | 0.0006 | |
| 2021 | Cases | 0.89 | 6.26 | <0.001 |
| Deaths | 0.88 | 6.09 | <0.001 | |
| 2022 | Cases | 0.78 | 5.72 | <0.001 |
| Deaths | 0.68 | 4.73 | <0.001 | |
| 2023 | Cases | 0.80 | 5.63 | <0.001 |
| Deaths | 0.71 | 4.57 | <0.001 |
| Risk Factors | Cases | Deaths | ||
|---|---|---|---|---|
| Linear Regression | Negative Binomial Regression | Linear Regression | Negative Binomial Regression | |
| β (95% CI), p-Value | RR (95% CI), p-Value | β (95% CI), p-Value | RR (95% CI), p-Value | |
| Population density | 0.02 (0.00–0.04), 0.03 | 0.02 (0.01–0.04), 0.03 | 0.01 (0.01–0.03), 0.47 | 0.02 (0.01–0.05), 0.99 |
| Sex ratio | 5.18 (4.85–11.89), 0.01 | 0.21(0.1–0.30), 0.01 | 0.03 (0.01–0.06), 0.50 | 0.03 (−8.40–8.74), 0.99 |
| Percentage aged 60 years and above | 0.02 (0.01–0.03), 0.03 | 0.03 (0.01–0.05), 0.01 | 0.04 (0.01–0.05), 0.59 | 0.04 (0.03–0.06), 0.97 |
| Obesity prevalence (men) | 8.10 (6.20–12.40), 0.21 | 0.05 (0.02–0.12), 0.18 | 0.01 (−0.03–0.05), 0.44 | 0.08 (−3.22–4.77), 0.96 |
| Obesity prevalence (women) | 15.78 (12.44–39.00), 0.10 | 0.11 (0.09–0.19), 0.89 | −0.02 (−0.06–0.03, 0.20 | −0.23 (−6.01–5.11), 0.90 |
| Smoking prevalence (men) | 29.02 (23.29–0.35),0.05 | 0.11 (0.07–0.12), 0.02 | 0.00 (−0.06–0.05), 0.83 | −0.05 (−4.58–6.47), 0.98 |
| Smoking prevalence (women) | 106.50 (41.67–171.34), 0.02 | 0.79 (0.54–1.04), 0.01 | 0.05 (−0.07–0.18), 0.21 | 0.46 (−11.31–19.09), 0.92 |
| Diabetes prevalence (Men) | 42.13 (36.52–44.74) 0.02 | 0.27 (0.26–0.28), 0.01 | 0.00 (−0.05–0.06), 0.74 | 0.15 (−4.89–6.31), 0.94 |
| Diabetes prevalence (Women) | 74.57 (20.64–128.50), 0.03 | 0.51 (0.32–0.71), 0.04 | 0.04 (−0.06–0.15), 0.22 | 0.40 (−8.07–13.22), 0.91 |
| Per capita income (Won) | 113.79 (106.19–119.40), 0.04 | 0.65 (0.59–0.74), 0.04 | 0.04 (−1.54–1.62), 0.92 | 0.46 (−11.31–19.09), 0.92 |
| Average temperature | 17.45 (15.56–50.47), 0.15 | 0.09 (0.03–0.21), 0.15 | 0.01 (−0.05–0.08), 0.46 | 0.17 (−5.33–7.49), 0.94 |
| Average rainfall | 0.09 (0.05–0.14), 0.10 | 0.10 (0.08–0.12), 0.28 | 0.00 (0.00–0.00), 0.66 | 0.00 (−0.02–0.02), 0.97 |
| Average humidity | 1.54 (0.09–1.89), 0.56 | 0.02 (0.01–0.06), 0.14 | 0.00 (−0.02–0.02), 0.83 | −0.01 (−1.97–1.95), 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achangwa, C.; Park, J.-H.; Lee, M.-S. Yearly Spatiotemporal Patterns of COVID-19 During the Pandemic Period: An In-Depth Analysis of Regional Trends and Risk Factors in the Republic of Korea. COVID 2025, 5, 40. https://doi.org/10.3390/covid5030040
Achangwa C, Park J-H, Lee M-S. Yearly Spatiotemporal Patterns of COVID-19 During the Pandemic Period: An In-Depth Analysis of Regional Trends and Risk Factors in the Republic of Korea. COVID. 2025; 5(3):40. https://doi.org/10.3390/covid5030040
Chicago/Turabian StyleAchangwa, Chiara, Jung-Hee Park, and Moo-Sik Lee. 2025. "Yearly Spatiotemporal Patterns of COVID-19 During the Pandemic Period: An In-Depth Analysis of Regional Trends and Risk Factors in the Republic of Korea" COVID 5, no. 3: 40. https://doi.org/10.3390/covid5030040
APA StyleAchangwa, C., Park, J.-H., & Lee, M.-S. (2025). Yearly Spatiotemporal Patterns of COVID-19 During the Pandemic Period: An In-Depth Analysis of Regional Trends and Risk Factors in the Republic of Korea. COVID, 5(3), 40. https://doi.org/10.3390/covid5030040







