Evaluating Opioid Dosing in COVID-19 and Non-COVID-19 ICU Patients Using Nociception Level Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. The NOL Monitor
2.3. Trial Procedures
2.4. Data Collection
2.5. Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
3.3. Questionnaire
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASV | Adaptive Support Ventilation |
| AUC | Area Under the Curve |
| BIS | Bispectral Index |
| BMI | Body Mass Index |
| CCMO | Central Committee on Research Involving Human Subjects |
| CoCo | Covid Committee |
| CPOT | Critical Care Pain Observation Tool |
| eCRF | Electronic Case Report Form |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LUMC | Leiden University Medical Centre |
| NOL | Nociception Level |
| PCMV | Pressure-Controlled Continuous Mandatory Ventilation |
| PSV | Pressure Support Ventilation |
| RASS | Richmond Agitation Sedation Score |
| SD | Standard Deviation |
| TWA | Time-Weighted Average |
| VV-ECMO | Veno-Venous Extracorporeal Membrane Oxygenation |
| VA-ECMO | Veno-Arterial Extracorporeal Membrane Oxygenation |
Appendix A. Evaluation Questionnaire
- What is your general impression of the pain medication the patient has received today?
- Sufficient
- Reasonable
- Insufficient
- Too much
- When did the patient give the impression they were experiencing pain? (multiple answers possible):
- During interventions (e.g.,: oral care, change of position)
- Throughout the whole day
- In intermittent episodes throughout the day
- Other, please specify: _______________________________________________
- The patient was comfortable and did not experience any pain
- Only answer the next questions if answers a through d were provided in the previous question.
- What signals gave you the impression that the patient was in pain?
- Facial grimaces
- Higher blood pressure/heart rate
- Motor restlessness
- Other, please specify: _______________________________________________
- Was any action taken when the patient gave the impression of being in pain?
- Yes
- No
- Only answer the next question if the answer was “yes” on the previous question.
- What actions were taken? Multiple answers are possible:
- A bolus of pain medication was administered
- The maintenance dose was increased
- Initiated new pain medication
- Other, please specify: ________________________________________________
- Did you communicate your concerns regarding the pain with the attending physician?
- Yes
- No
- If “yes”, did this lead to a change in the treatment plan?
- Yes
- No
Appendix B. Additional Sedative and Analgesic Medication
| COVID-19 | Non-COVID-19 | p-Value | |
|---|---|---|---|
| Remifentanil (ug/kg/min) (mean (SD)) | NA | 4.17 | - |
| Number of patients (N) | 0 | 1 | |
| Fentanyl ug/h (mean (SD)) | 9.33 (NA) * | NA | - |
| Number of patients (N) | 1 | 0 | |
| Esketamine mg/kg/hour (mean (SD)) | 0.20 (NA) * | 0.10 (NA) * | - |
| Number of patients (N) | 1 | 1 | |
| Midazolam mg/hour (mean (SD)) | 8.97 (5.51) | 10.12 (10.83) | 0.791 |
| Number of patients (N) | 10 | 4 | |
| Clonidine ug/kg/hour (mean (SD)) | 0.95 (0.35) | 0.70 (0.14) | 0.451 |
| Number of patients (N) | 2 | 2 | |
| Dexmedetomidine ug/kg/hour (mean (SD)) | 0.50 (NA) * | 0.50 (NA) * | - |
| Number of patients (N) | 1 | 1 |
References
- Lindenbaum, L.; Milia, D.J. Pain Management in the ICU. Surg. Clin. N. Am. 2012, 92, 1621–1636. [Google Scholar] [CrossRef] [PubMed]
- Rittner, H.L.; Machelska, H.; Stein, C. Leukocytes in the regulation of pain and analgesia. J. Leukoc. Biol. 2005, 78, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, M.; Von Dossow, V.; Seeling, M.; Ahlborn, R.; Tamarkin, A.; Conroy, P.; Boemke, W.; Wernecke, K.-D.; Spies, C. Key Performance Indicators in Intensive Care Medicine. A Retrospective Matched Cohort Study. J. Int. Med. Res. 2009, 37, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Payen, J.F.; Bosson, J.L.; Chanques, G.; Mantz, J.; Labarere, J.; Investigators, D. Pain assessment is associated with decreased duration of mechanical ventilation in the intensive care unit: A post Hoc analysis of the DOLOREA study. Anesthesiology 2009, 111, 1308–1316. [Google Scholar] [CrossRef]
- Temesgen, N.; Chekol, B.; Tamirie, T.; Eshetie, D.; Simeneh, N.; Feleke, A. Adult sedation and analgesia in a resource limited intensive care unit—A Systematic Review and evidence based guideline. Ann. Med. Surg. 2021, 66, 102356. [Google Scholar] [CrossRef]
- Hanidziar, D.; Bittner, E.A. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth. Analg. 2020, 131, e40–e41. [Google Scholar] [CrossRef]
- Kapp, C.M.; Zaeh, S.; Niedermeyer, S.; Punjabi, N.M.; Siddharthan, T.; Damarla, M. The Use of Analgesia and Sedation in Mechanically Ventilated Patients with COVID-19 Acute Respiratory Distress Syndrome. Anesth. Analg. 2020, 131, e198–e200. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gélinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef]
- Sigakis, M.J.; Bittner, E.A. Ten Myths and Misconceptions Regarding Pain Management in the ICU. Crit. Care Med. 2015, 43, 2468–2478. [Google Scholar] [CrossRef]
- Chanques, G.; Pohlman, A.; Kress, J.P.; Molinari, N.; De Jong, A.; Jaber, S.; Hall, J.B. Psychometric comparison of three behavioural scales for the assessment of pain in critically ill patients unable to self-report. Crit. Care 2014, 18, R160. [Google Scholar] [CrossRef]
- Gélinas, C.; Puntillo, K.; Joffe, A.; Barr, J. A Validated Approach to Evaluating Psychometric Properties of Pain Assessment Tools for Use in Nonverbal Critically Ill Adults. Semin. Respir. Crit. Care Med. 2013, 34, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Gelinas, C.; Tousignant-Laflamme, Y.; Tanguay, A.; Bourgault, P. Exploring the validity of the bispectral index, the Critical-Care Pain Observation Tool and vital signs for the detection of pain in sedated and mechanically ventilated critically ill adults: A pilot study. Intensive Crit. Care Nurs. 2011, 27, 46–52. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Ben-Israel, N.; Kliger, M.; Zuckerman, G.; Katz, Y.; Edry, R. Monitoring the nociception level: A multi-parameter approach. J. Clin. Monit. Comput. 2013, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Meijer, F.S.; Martini, C.H.; Broens, S.; Boon, M.; Niesters, M.; Aarts, L.; Olofsen, E.; van Velzen, M.; Dahan, A. Nociception-guided versus Standard Care during Remifentanil-Propofol Anesthesia: A Randomized Controlled Trial. Anesthesiology 2019, 130, 745–755. [Google Scholar] [CrossRef]
- Meijer, F.; Honing, M.; Roor, T.; Toet, S.; Calis, P.; Olofsen, E.; Martini, C.; van Velzen, M.; Aarts, L.; Niesters, M.; et al. Reduced postoperative pain using Nociception Level-guided fentanyl dosing during sevoflurane anaesthesia: A randomised controlled trial. Br. J. Anaesth. 2020, 125, 1070–1078. [Google Scholar] [CrossRef]
- Martini, C.H.; Boon, M.; Broens, S.J.; Hekkelman, E.F.; Oudhoff, L.A.; Buddeke, A.W.; Dahan, A. Ability of the nociception level, a multiparameter composite of autonomic signals, to detect noxious stimuli during propofol-remifentanil anesthesia. Anesthesiology 2015, 123, 524–534. [Google Scholar] [CrossRef]
- Edry, R.; Recea, V.; Dikust, Y.; Sessler, D.I. Preliminary Intraoperative Validation of the Nociception Level Index: A Noninvasive Nociception Monitor. Anesthesiology 2016, 125, 193–203. [Google Scholar] [CrossRef]
- Stockle, P.A.; Julien, M.; Issa, R.; Decary, E.; Brulotte, V.; Drolet, P.; Henri, M.; Poirier, M.; Latulippe, J.-F.; Dorais, M.; et al. Validation of the PMD100 and its NOL Index to detect nociception at different infusion regimen of remifentanil in patients under general anesthesia. Minerva Anestesiol. 2018, 84, 1160–1168. [Google Scholar] [CrossRef]
- Coeckelenbergh, S.; Doria, S.; Patricio, D.; Perrin, L.; Engelman, E.; Rodriguez, A.; Di Marco, L.; Van Obbergh, L.; Estebe, J.-P.; Barvais, L.; et al. Effect of dexmedetomidine on Nociception Level Index-guided remifentanil antinociception: A randomised controlled trial. Eur. J. Anaesthesiol. 2021, 38, 524–533. [Google Scholar] [CrossRef]
- Fuica, R.; Krochek, C.; Weissbrod, R.; Greenman, D.; Freundlich, A.; Gozal, Y. Reduced postoperative pain in patients receiving nociception monitor guided analgesia during elective major abdominal surgery: A randomized, controlled trial. J. Clin. Monit. Comput. 2023, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Shahiri, T.S.; Richard-Lalonde, M.; Richebé, P.; Gélinas, C. Exploration of the Nociception Level (NOL™) Index for Pain Assessment during Endotracheal Suctioning in Mechanically Ventilated Patients in the Intensive Care Unit: An Observational and Feasibility Study. Pain Manag. Nurs. 2020, 21, 428–434. [Google Scholar] [CrossRef]
- Gélinas, C.; Shahiri, T.S.; Richard-Lalonde, M.; Laporta, D.; Morin, J.-F.; Boitor, M.; Ferland, C.E.; Bourgault, P.; Richebé, P. Exploration of a Multi-Parameter Technology for Pain Assessment in Postoperative Patients After Cardiac Surgery in the Intensive Care Unit: The Nociception Level Index (NOL)TM. J. Pain Res. 2021, 14, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Ledowski, T.; Schlueter, P.; Hall, N. Nociception level index: Do intra-operative values allow the prediction of acute postoperative pain? J. Clin. Monit. Comput. 2022, 36, 349–354. [Google Scholar] [CrossRef]
- Castor EDC. Castor Electronic Data Capture 2019. Available online: https://castoredc.com (accessed on 27 August 2019).
- Dahan, A.; Niesters, M.; Smith, T.; Overdyk, F. Opioids, Clinical Anesthesia; Wolters Kluwer, Lippincot Wlliams & Wilkens: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ego, A.; Peluso, L.; Gorham, J.; Diosdado, A.; Restuccia, G.; Creteur, J.; Taccone, F.S. Use of Sedatives and Neuromuscular-Blocking Agents in Mechanically Ventilated Patients with COVID-19 ARDS. Microorganisms 2021, 9, 2393. [Google Scholar] [CrossRef] [PubMed]
- Flinspach, A.N.; Booke, H.; Zacharowski, K.; Balaban, Ü.; Herrmann, E.; Adam, E.H. High sedation needs of critically ill COVID-19 ARDS patients—A monocentric observational study. PLoS ONE 2021, 16, e0253778. [Google Scholar] [CrossRef]
- Rose, L.; Smith, O.; Gelinas, C.; Haslam, L.; Dale, C.; Luk, E.; Burry, L.; McGillion, M.; Mehta, S.; Watt-Watson, J. Critical care nurses’ pain assessment and management practices: A survey in Canada. Am. J. Crit. Care 2012, 21, 251–259. [Google Scholar] [CrossRef]
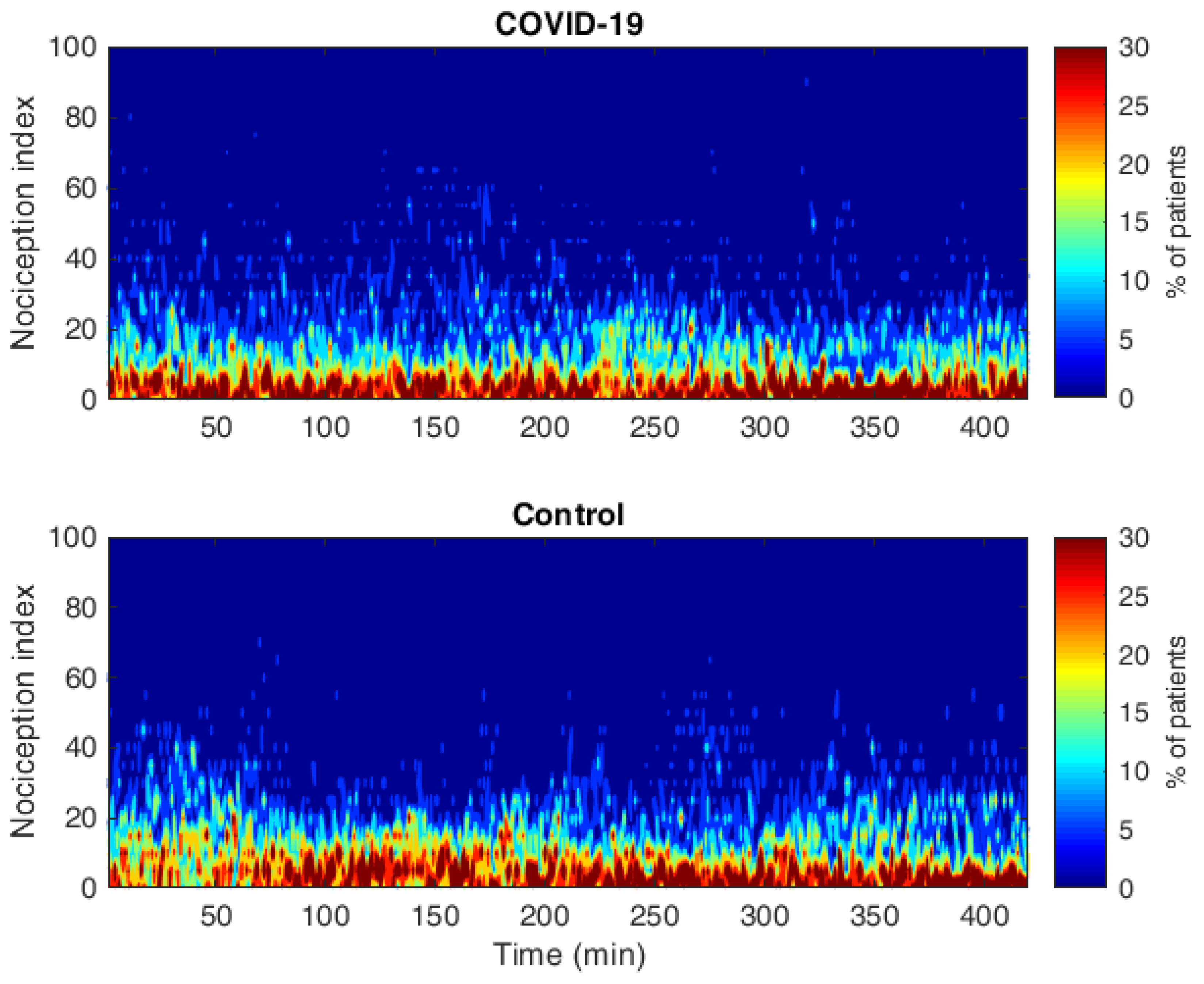
| Variables | COVID-19 ICU (N = 20) | Control (N = 20) |
|---|---|---|
| Age (median [IQR]) | 67 [61, 71] | 65 [51, 71] |
| Sex = female (%) | 16 (80) | 13 (65) |
| BMI (mean (SD)) | 29 (5) | 28 (5) |
| Day the ICU admission measurement took place (median [IQR]) | 3.5 [2, 7.25] | 3.5 [2, 6.75] |
| Factors potentially influencing the NOL measurements | ||
| Vasopressive/inotropic medication | 14 (70) | 15 (75) |
| Arrythmia | 6 (30) | 6 (30) |
| Hypertension | 2 (10) | 1 (5) |
| Hypotension | 1 (5) | 1 (5) |
| Hypothermia | 4 (20) | 1 (5) |
| Bradycardia | 6 (30) | 1 (5) |
| Tachycardia | 2 (10) | 8 (40) |
| Peripheral edema | 3 (15) | 9 (45) |
| VV-ECMO * | 2 (10) | 0 (0) |
| No influential circumstances | 2 (10) | 0 (0) |
| RASS (%) | ||
| −5 | 9 (45) | 8 (40) |
| −4 | 10 (50) | 8 (40) |
| −3 | 1 (5) | 4 (20) |
| BPS (%) | ||
| 3 | 16 (80) | 15 (75) |
| 4 | 3 (15) | 4 (20) |
| 5 | 1 (5) | 1 (5) |
| Ventilation mode (%) | ||
| PCMV | 14 (70) | 9 (45) |
| ASV | 2 (10) | 11 (55) |
| PSV | 4 (20) | 0 (0) |
| Rocuronium | 3 (15) | 0 (0) |
| Variables | COVID-19 ICU | Control | Total |
|---|---|---|---|
| NOL and BIS * | N = 20 | N = 18 | N = 38 |
| TWANOL>25 (median [IQR]) | 0.33 [0.12–0.83] | 0.46 [0.06–0.81] | 0.39 [0.09, 0.82] |
| NOL < 10 (%) | 63 | 57 | 60 |
| NOL 10–25 (%) | 22 | 33 | 28 |
| BIS (mean ± SD) | 34 ± 15 | 47 ± 17 | 40 ± 17 |
| Medication * | N = 20 | N = 18 | N = 38 |
| Propofol (mg/h) (mean ± SD) | 307 ± 127 | 178 ± 137 | 245 ± 147 |
| Sufentanil (µg/h) (mean ± SD) | 18 ± 9 | 9 ± 6 | 13 ± 9 |
| Quality of NOL signal | N = 20 | N = 20 | N = 40 |
| Very good, No. (%) | 11 (55) | 8 (40) | 19 (48) |
| Good, No. (%) | 6 (30) | 5 (25) | 11 (28) |
| Moderate, No. (%) | 3 (15) | 4 (20) | 7 (18) |
| Poor, No. (%) | 0 (0) | 0 (0) | 0 (0) |
| Very poor, No. (%) | 0 (0) | 1 (5) | 1 (3) |
| Unusable, No. (%) | 0 (0) | 2 (1) | 2 (5) |
| Annotations clinical intervention * | |||
| All annotations # | N = 39 | N = 39 | N = 78 |
| Before (median [IQR]) | 3 [2–7] | 4 [2–7] | 4 [3–16] |
| After (median [IQR]) | 23 [16–32] | 25 [14–34] | 25 [13–41] |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
| Airway management | N = 20 | N = 21 | N = 41 |
| Before (median [IQR]) | 4 [3–16] | 3 [2–7] | 4 [3–9] |
| After (median [IQR]) | 39 [23–47] | 26 [17–34] | 32 [17–39] |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
| Change of position | N = 15 | N = 13 | N = 28 |
| Before (median [IQR]) | 7 [4–15] | 3 [2–5] | 7 [4–15] |
| After (median [IQR]) | 22 [13–28] | 18 [12–26] | 22 [13–28] |
| p-value | p = 0.02 | p < 0.001 | p < 0.001 |
| Patient care | N = 3 | N = 4 | N = 7 |
| NOL Before (median [IQR]) | 1 [1–8] | 8 [4–14] | 7 [1–19] |
| NOL After (median [IQR]) | 13 [13–18] | 28 [22–31] | 19 [15–29] |
| p-value | p = 0.1 | p = 0.05 | p = 0.007 |
| Variables | COVID-19 | Control | p-Value |
|---|---|---|---|
| NOL < 10 | |||
| BPS (median [IQR]) | 3 [3, 3.5] | 3 [3, 4] | 0.5 |
| BIS (median [IQR]) | 33 [18, 41] | 46 [40, 52] | 0.1 |
| RASS (median [IQR]) | −4.5 [−5, −4] | −4 [−4, −3.5] | 0.2 |
| Sufentanil µg/h (mean (SD)) | 16.4 (10) | 10.5 (6) | 0.08 |
| Propofol mg/h (mean (SD)) | 334 (94) | 233 (143) | 0.08 |
| NOL 10–25 | |||
| BPS (median [IQR]) | 3 [3, 3] | 3.3 [3, 4] | 0.08 |
| BIS (median [IQR]) | 42 [36, 44] | 48 [39, 62] | 0.6 |
| RASS (median [IQR]) | −4.8 [−5, −4.5] | −3.8 [−4.6, −3] | 0.03 |
| Sufentanil µg/h (mean (SD)) | 20 (6.3) | 8.8 (3.3) | 0.005 |
| Propofol mg/h (mean (SD)) | 311 (139) | 233 (61) | 0.3 |
| NOL > 25 | |||
| BPS (median [IQR]) | 3.5 [3.5, 3.5] | NA | NA |
| BIS (median [IQR]) * | NA | NA | NA |
| RASS (median [IQR]) | −4 [−4, −4] | NA | NA |
| Sufentanil µg/h (mean (SD)) | 20 (NA) † | NA | NA |
| Questions | Control (N = 10) | COVID-19 (N = 10) | Total (N = 20) |
|---|---|---|---|
| 1. What is your general impression of the pain medication the patient has received today? (%) | |||
| Sufficient | 9 (90) | 9 (90) | 18 (90) |
| Reasonable | 1 (10) | 1 (10) | 2 (10) |
| Insufficient | 0 (0) | 0 (0) | 0 (0) |
| Too much | 0 (0) | 0 (0) | 0 (0) |
| 2. When did the patient give the impression they were experiencing pain? | |||
| During interventions | 4 (40) | 3 (30) | 7 (35) |
| Throughout the whole day | 0 (0) | 0 (0) | 0 (0) |
| In intermittent episodes throughout the day | 0 (0) | 0 (0) | 0 (0) |
| Other | 2 (20) | 2 (20) | 4 (20) |
| The patient was comfortable and did not experience any pain | 5 (50) | 5 (50) | 10 (50) |
| 3. What signals gave you the impression that the patient was in pain? (%) | |||
| Facial grimaces | 1/5 (20) | 0/5 (0) | 1/10 (10) |
| Higher blood pressure/heart rate | 3/5 (60) | 3/5 (60) | 6/10 (60) |
| Motor restlessness | 0/5 (0) | 1/5 (20) | 1/10 (10) |
| Other | 1/5 (20) | 1/5 (10) | 2/10 (20) |
| 4. Was any action taken when the patient gave the impression of being in pain? (%) | |||
| Yes | 3/5 (60) | 3/4 (75) | 6/9 (67) |
| No | 2/5 (40) | 1/4 (25) | 3/9 (33) |
| 5. If yes, what actions were taken? (%) | |||
| Bolus of pain medication | 3/3 (100) | 3/3 (100) | 6/6 (100) |
| Maintenance dose was increased | 0/3 (0) | 1/3 (25) | 1/6 (17) |
| Initiated new pain medication | 0/3 (0) | 0/3 (0) | 0/6 (0) |
| Other | 0/3 (20) | 0/3 (20) | 0/6 (0) |
| 6. Were concerns regarding the patients’ pain communicated with the treating physician? (%) | |||
| Yes | 2/5 (40) | 1/4 (25) | 3/9 (33) |
| No | 3/5 (60) | 3/4 (75) | 6/9 (67) |
| 7. Has this led to any changes in the treatment plan? | |||
| Yes | 2/2 (100) | 1/1 (100) | 3/3 (100) |
| No | 0/2 (0) | 0/1 (0) | 0/3 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Wal, L.I.; Bos, J.v.d.; del Prado, M.; Rotem, O.M.; Helmerhorst, H.; Jonge, E.d.; Dahan, A. Evaluating Opioid Dosing in COVID-19 and Non-COVID-19 ICU Patients Using Nociception Level Monitoring. COVID 2025, 5, 34. https://doi.org/10.3390/covid5030034
van der Wal LI, Bos Jvd, del Prado M, Rotem OM, Helmerhorst H, Jonge Ed, Dahan A. Evaluating Opioid Dosing in COVID-19 and Non-COVID-19 ICU Patients Using Nociception Level Monitoring. COVID. 2025; 5(3):34. https://doi.org/10.3390/covid5030034
Chicago/Turabian Stylevan der Wal, Lea Imeen, Jetske van der Bos, Michael del Prado, Omer Miller Rotem, Hendrik Helmerhorst, Evert de Jonge, and Albert Dahan. 2025. "Evaluating Opioid Dosing in COVID-19 and Non-COVID-19 ICU Patients Using Nociception Level Monitoring" COVID 5, no. 3: 34. https://doi.org/10.3390/covid5030034
APA Stylevan der Wal, L. I., Bos, J. v. d., del Prado, M., Rotem, O. M., Helmerhorst, H., Jonge, E. d., & Dahan, A. (2025). Evaluating Opioid Dosing in COVID-19 and Non-COVID-19 ICU Patients Using Nociception Level Monitoring. COVID, 5(3), 34. https://doi.org/10.3390/covid5030034






