Abstract
Background: Diagnosing post-COVID syndrome (PCS) in older adults with hypertension is difficult due to heterogeneity and multimorbidity. We aimed to identify factors associated with PCS. Methods: An observational study was conducted from June 2024 to April 2025. Patients aged 60–89 years with arterial hypertension were enrolled; PCS was verified according to the national protocol. Between-group comparisons used standard tests. Multivariable logistic regression with pre-specified clinical predictors estimated independent associations. Results: A total of 291 patients with arterial hypertension were included in the study. Patients were grouped by PCS status (PCS = 101; controls = 190). In multivariable analysis, female sex (OR 3.64; 95% CI 1.22–10.82), younger age (OR 0.93; 95% CI 0.89–0.98), lower systolic blood pressure (SBP) (OR 0.98; 95% CI 0.96–1.00), and rhythm disturbances (OR 2.63; 95% CI 1.07–6.49) were associated with PCS; other predictors were not significant. Model discrimination was moderate (AUC 0.728; 95% CI 0.668–0.787; Brier score 0.193) with positive net benefit across thresholds ~0.10–0.65. Conclusions: In older hypertensive adults, female sex, younger age, lower SBP, and rhythm disturbances indicate higher PCS likelihood, supporting risk-stratified monitoring and management.
1. Introduction
Post-COVID syndrome (PCS; long COVID) shows marked phenotypic heterogeneity and a relapsing–remitting clinical course; unified diagnostic criteria and a laboratory gold standard remain absent [1,2,3,4,5,6,7]. Contemporary guidance endorses a clinically oriented, exclusion-based diagnostic approach and explicitly notes that there is no specific laboratory test to determine whether symptoms are due to PCS [1,6,7]. Operational and research definitions vary across agencies and projects, limiting cross-study comparability and complicating clinical stratification [2,3,4,8]. In the prospective INSPIRE cohort, applying five published definitions produced substantial divergence in prevalence estimates at 3- and 6-months post-infection [8]. The 2024 consensus from the National Academies (NASEM) details the rationale for a new definition and states that it will be revised as evidence accrues [3,4]. Collectively, these observations underscore the methodological complexity of diagnosing PCS and the need to explicitly report the definition used in both research and clinical practice.
In older adults with arterial hypertension (AH), diagnostic uncertainty is clinically salient because of symptom overlap: fatigue, exercise intolerance, dizziness, orthostatic intolerance, palpitations, and cognitive impairment can reflect PCS, as well as hypertensive target-organ damage, autonomic dysfunction, or medication effects [9,10,11]. An American Heart Association scientific statement and reviews on PCS-related dysautonomia recommend structured assessment of orthostatic vital signs and cardiac rhythm for differential diagnosis, as autonomic dysregulation—including postural orthostatic tachycardia syndrome (POTS) and orthostatic hypotension—is frequently identified in this population [9,10,11].
Pathophysiological mechanisms linking prior SARS-CoV-2 infection with cardiovascular outcomes in individuals with hypertension include endothelial dysfunction, dysregulation of the renin–angiotensin–aldosterone system, increased arterial stiffness, and microvascular injury [12,13]. Large cohort data indicate a sustained excess risk of cardiovascular events after COVID-19, and both meta-analytic summaries and prospective studies report post-infectious blood-pressure lability and elevated risk of incident or worsening hypertension [13,14,15]. These findings support vigilant monitoring and precise verification of PCS in older adults with AH.
The novelty of this study lies in its targeted focus on older adults with arterial hypertension and its emphasis on improving the diagnostic accuracy of post-COVID syndrome.
The objective was to identify clinical–demographic and comorbidity factors associated with PCS in older adults with AH and to quantify the strength of these associations using multivariable logistic regression.
2. Materials and Methods
2.1. Study Design
This observational study of an outpatient clinical cohort of patients with arterial hypertension was conducted at the clinical bases of the Department of General Practice, Kazakh–Russian Medical University—City Polyclinic No. 32 and City Polyclinic No. 26 (Almaty, Kazakhstan)—from June 2024 to April 2025. All diagnostic and research procedures were performed in the outpatient setting according to an approved protocol. Sampling reflected routine care-seeking; no investigator-directed intervention was introduced. Group allocation was naturalistic and based on the presence or absence of post-COVID syndrome (PCS).
2.2. Ethical Approval and Consent
This study was conducted in accordance with the Declaration of Helsinki and national regulations. The protocol was reviewed and approved by the Local Ethics Committee of the Kazakh–Russian Medical University (Protocol No. 22, 22 April 2024). All participants were informed.
2.3. Participants and Eligibility
Eligibility for the cohort was defined as follows: age, 60–89 years (per the World Health Organization age stratification approach [16]); confirmed or newly detected arterial hypertension in accordance with the clinical protocol of the Ministry of Health of the Republic of Kazakhstan (2018) [17]; and a documented history of SARS-CoV-2 infection. Exclusion criteria were as follows: marked cognitive impairment, acute infectious diseases, exacerbation of chronic internal diseases, and refusal to participate.
2.4. Sample and Groups
A total of 291 patients with AH met the inclusion/exclusion criteria and were enrolled. Based on the presence of PCS, participants were divided into two groups: PCS (n = 101) and control without PCS (n = 190) (Figure 1).
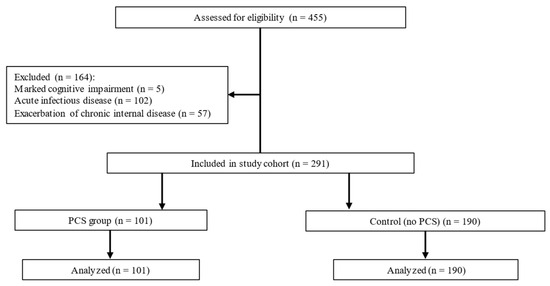
Figure 1.
Participant flow diagram.
Table 1 presents the baseline characteristics of the patients.

Table 1.
Patient characteristics.
2.5. Verification of Post-COVID Syndrome
The operational definition of PCS followed the national clinical protocol of the Ministry of Health of the Republic of Kazakhstan “Post-COVID Syndrome” (2023), a normative document mandatory within the national healthcare system. Classification required simultaneous fulfillment of three criteria: a confirmed or probable history of SARS-CoV-2 infection; persistence or recurrence of symptoms for more than 12 weeks after the onset of COVID-19; and inability to attribute symptoms to an alternative diagnosis [18]. An English summary of the relevant sections of this national protocol (formal definition and diagnostic criteria of PCS) is provided in Supplementary File S1.
2.6. Verification of Arterial Hypertension
The diagnosis of arterial hypertension was established in accordance with the Ministry of Health clinical protocol (2018) based on repeated episodes of elevated blood pressure documented in medical records and patient history. Hypertension grade 1 was defined as systolic blood pressure 140–159 mmHg and/or diastolic blood pressure 90–99 mmHg; grade 2 as systolic 160–179 mmHg and/or diastolic 100–109 mmHg; grade 3 as systolic ≥ 180 mmHg and/or diastolic ≥ 110 mmHg [16]. A brief English summary of the key diagnostic definitions and blood pressure classification from this national hypertension protocol is provided in Supplementary File S2.
2.7. Measurements
Blood pressure was measured once in the seated position after 5 min of rest using an aneroid sphygmomanometer (Rossmax Aneroid GB 102; Rossmax Swiss GmbH, Heerbrugg, Switzerland). Resting pulse rate (PR) was recorded with a fingertip pulse oximeter (YK-80B; Yonker, Xuzhou, China). Cardiac rhythm disturbances were documented from medical history.
2.8. Statistical Analysis
Analyses were performed in Python 3.12.4 (Python Software Foundation; https://www.python.org) and Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA; https://www.microsoft.com).
Normality was assessed with the Kolmogorov–Smirnov test. Continuous variables are presented as mean ± SD or median (IQR), and categorical variables as % (n). Between-group comparisons used Student’s t-test or the Mann–Whitney U test (with median differences and 95% CIs), and Pearson’s χ2 test or Fisher’s exact test, as appropriate. Two-sided p < 0.05 was considered statistically significant.
To identify factors associated with PCS, a multivariable binary logistic regression model was fitted (maximum likelihood, enter method). Concurrently entered predictors were as follows: age (years, continuous); ischemic heart disease (yes/no); rhythm disturbances (yes/no); type 2 diabetes mellitus (yes/no); absence of comorbidity (yes/no); body mass index (kg/m2); pulse rate (beats/min); and systolic and diastolic blood pressure (SBP, DBP; mmHg). “Absence of comorbidity” was coded as 1 if none of the chronic conditions considered were present (including ischemic heart disease, type 2 diabetes, and others per the protocol list) or 0 if ≥1 condition was present. Categorical predictors were dummy-coded; continuous predictors were mean-centered; linearity of the logit was checked for continuous variables. Multicollinearity was evaluated using variance inflation factors (VIFs); given potential correlation between SBP and DBP, if VIF > 5, we prespecified replacement with derived measures (e.g., mean arterial pressure or pulse pressure) or exclusion of one parameter. Events-per-variable ratios were monitored; if overfitting risk emerged, model simplification (e.g., collapsing rare categories) was allowed. Calibration was assessed using the Hosmer–Lemeshow test and calibration intercept/slope; discrimination with ROC-AUC; and overall prediction error with the Brier score. Clinical utility was evaluated by decision curve analysis. Internal validation used bootstrap resampling. Results are reported as B (SE) and OR = exp(B) with 95% CIs and Wald p-values.
3. Results
3.1. Between-Group Comparisons
The proportion of women was higher in the PCS group (95.05% (n = 96) vs. 84.74% (n = 161); OR = 3.46, 95% CI 1.30–9.24; p = 0.012). Patients with PCS were younger (median 70 years (IQR 66–74) vs. 72 (68–78); Mann–Whitney U test, p < 0.05; difference in medians −2 years (95% CI −4 to −1)). The distribution of hypertension grades differed: lower grades were more frequent in the PCS group (OR = 0.57, 95% CI 0.36–0.91; p = 0.019).
3.2. Comorbidities
In unadjusted between-group comparisons (Figure 2), only rhythm disturbances differed significantly, being more frequent in the PCS group (15.84% (n = 16) vs. 6.32% (n = 12); OR = 2.79, 95% CI 1.27–6.16; p = 0.012). Differences in ischemic heart disease (38.61% (n = 39) vs. 31.58% (n = 60); OR = 1.36, 95% CI 0.82–2.26; p = 0.244) and type 2 diabetes mellitus (22.77% (n = 23) vs. 15.26% (n = 29); OR = 1.64, 95% CI 0.89–3.01; p = 0.147) did not reach statistical significance. The proportion without comorbidities was virtually identical (11.88% (n = 12) vs. 11.58% (n = 22); OR = 1.03, 95% CI 0.49–2.18; p = 0.999).

Figure 2.
Distribution of comorbid conditions.
3.3. Anthropometry and Hemodynamics
Body weight, height, and BMI did not differ significantly (all p > 0.05); median BMI values were in the overweight range in both groups. Resting pulse rate was comparable (74 (66–80) vs. 74 (67–80) beats/min; p = 0.339). Systolic blood pressure (SBP) was lower in the PCS group (130 (120–140) vs. 140 (127–150) mmHg; p < 0.05). For diastolic blood pressure (DBP), medians were identical (80 vs. 80 mmHg), although distributions differed (p = 0.013) (Table 2).

Table 2.
Anthropometric and hemodynamic characteristics of the study groups.
3.4. Pharmacotherapy
The number of concomitantly used medications did not differ between groups (p = 0.068) (Table 3).

Table 3.
Number of medications used.
3.5. Multivariable Analysis
In a logistic regression model with PCS presence as the outcome, covariates entered simultaneously were age, sex, ischemic heart disease, arrhythmia, type 2 diabetes, absence of comorbidity, BMI, pulse rate, SBP, and DBP. Independent associations were observed for lower age (aOR = 0.93 per year; 95% CI 0.89–0.98; p = 0.003), female sex (aOR = 3.64; 95% CI 1.22–10.82; p = 0.020), lower SBP (aOR = 0.98 per mmHg; 95% CI 0.96–1.00; p = 0.023), and arrhythmia (aOR = 2.63; 95% CI 1.07–6.49; p = 0.036). Ischemic heart disease, type 2 diabetes, absence of comorbidity, BMI, pulse rate, and DBP were not independently associated with PCS (all p > 0.10). No multicollinearity was detected (all VIFs < 2; maximum 1.74 for SBP and DBP) (Table 4).

Table 4.
Multivariable binary logistic regression (outcome: presence of PCS).
The overall contribution of the model was statistically significant: Omnibus LR χ2 = 44.731 (df = 10), p = 2.43 × 10−6. Fit indices were −2LL = 331.020; Cox–Snell, R2 = 0.142; and Nagelkerke, R2 = 0.197. Discriminative ability was rated as moderate: AUC = 0.728 (bootstrap 95% CI 0.668–0.787) (Figure 3a). Overall prediction error by the Brier score was 0.193 (bootstrap 95% CI 0.172–0.215). Calibration was deemed satisfactory: Hosmer–Lemeshow, χ2 = 3.216, df = 8, and p = 0.920; the calibration intercept was −0.000 (SE 0.164), and the calibration slope was 1.000 (SE 0.172), both close to the ideal values (Figure 3b). Internal validation with optimism correction (bootstrap, B = 200) yielded an optimism-corrected AUC of 0.686 (apparent 0.728) and an optimism-corrected calibration slope of 0.736 (apparent 1.000). The events-per-variable ratio was 10.1 (101 events, 10 predictors).

Figure 3.
Model performance. (a) Receiver operating characteristic (ROC) curve for predicting PCS: AUC = 0.728 (bootstrap 95% CI 0.668–0.787); dashed line indicates chance level. (b) Calibration plot (10 quantile bins): observed fraction vs. predicted probability; dashed line indicates perfect calibration (slope = 1, intercept = 0).
According to the ROC analysis, the optimal probability threshold by Youden’s index was 0.372; at this cut-off, sensitivity was 63.4%, specificity 74.2%, and overall accuracy 70.4% (TP = 64, TN = 141, FP = 49, and FN = 37). At a threshold of 0.35, sensitivity and specificity were 66.3% and 66.3%, respectively (accuracy 66.3%; PPV 51.1%; NPV 78.8%). At the conventional 0.50 threshold, sensitivity and specificity were 36.6% and 87.9%, respectively (accuracy 70.1%; PPV 61.7%; NPV 72.3%). Decision-curve analysis (DCA) showed a positive net clinical benefit, with the model outperforming a “treat-all” strategy across threshold probabilities of approximately 0.10–0.65 and never inferior to a “treat-none” strategy over the examined range (Figure 4).
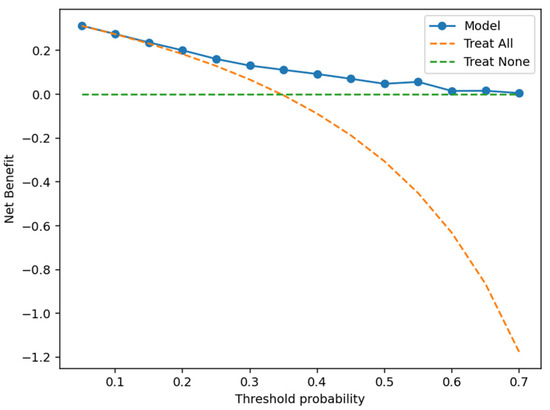
Figure 4.
DCA for the PCS prediction model.
4. Discussion
In an outpatient cohort of older adults with hypertension, the presence of post-COVID syndrome was independently associated with female sex (aOR 3.64; 95% CI 1.22–10.82), younger age (aOR 0.93 per year; 95% CI 0.89–0.98), lower systolic blood pressure (aOR 0.98 per mmHg; 95% CI 0.96–1.00), and rhythm disturbances (aOR 2.63; 95% CI 1.07–6.49). Ischemic heart disease, type 2 diabetes, body mass index, heart rate, and diastolic blood pressure were not independently associated. The prediction model demonstrated moderate discrimination and acceptable calibration.
The association of PCS with female sex in our cohort is consistent with the broader literature on long COVID among individuals with confirmed SARS-CoV-2 infection. A large systematic review and meta-analysis of 41 studies reported female sex as one of the most robust risk factors for post-COVID-19 condition, with pooled odds ratios around 1.5 compared with males [19]. Similar estimates were observed in other meta-analyses synthesizing data on long-term health effects among COVID-19 survivors across hospitalized and non-hospitalized populations [20,21,22]. In a large English primary-care dataset restricted to non-hospitalized SARS-CoV-2–positive adults, Subramanian et al. likewise identified female sex as an independent predictor of long COVID coding and symptom clusters [23].
The inverse association between PCS and age in our hypertensive cohort appears to diverge from aggregate evidence. Meta-analytic data have generally reported increasing odds of post-COVID condition with age, with pooled estimates around 1.2 per age category [19,20]. However, analyses restricted to individuals with documented SARS-CoV-2 infection suggest that the age gradient is not strictly linear. In the CPRD-based cohort of non-hospitalized adults, the highest adjusted hazard ratios for long COVID were observed in middle-aged rather than the oldest strata [23]. The UK Office for National Statistics (ONS) surveillance similarly indicates that self-reported long COVID prevalence peaks in midlife age bands (approximately 35–69 years) and does not necessarily continue to rise in the oldest old [24].
The observed association of lower office SBP with a greater likelihood of PCS likewise does not admit a single, uniform interpretation. Several cohort studies and a recent meta-analysis have documented an increased incidence of new-onset arterial hypertension among COVID-19 survivors compared with non-infected controls, suggesting that for a proportion of patients, SARS-CoV-2 infection may unmask or accelerate hypertensive disease [15,25,26]. At the same time, a distinct subgroup of post-COVID patients presents with features of cardiovascular autonomic dysfunction—orthostatic intolerance, postural orthostatic tachycardia syndrome (POTS), and orthostatic hypotension—collectively framed within the spectrum of post-acute sequelae of COVID-19 [9,10,11,27]. In this context, lower single-time-point office SBP among older hypertensive adults with PCS may be a surrogate of dysautonomia-related hemodynamic lability rather than a marker of lower vascular risk. Ståhlberg et al. described a post-COVID tachycardia phenotype characterized by exaggerated heart-rate responses and orthostatic symptoms, often coexisting with labile blood pressure [28], and a recent systematic review of active standing tests in long COVID demonstrated consistent autonomic abnormalities with elevated heart rate and altered blood pressure responses compared with healthy controls [29].
The positive association between rhythm disturbances and PCS in our hypertensive cohort aligns with accumulating evidence on post-acute cardiovascular sequelae in COVID-19 survivors [11,14,30,31,32,33]. In a large US Veterans Affairs cohort including predominantly non-hospitalized individuals with confirmed infection, Xie et al. reported significantly elevated 12-month risks of a broad range of cardiovascular outcomes, including arrhythmias, compared with contemporary controls [14]. A national self-controlled case series from Sweden likewise demonstrated an increased incidence of atrial, supraventricular, and ventricular arrhythmias following SARS-CoV-2 infection, with the excess risk being particularly pronounced in older individuals and those with pre-existing cardiovascular disease [32]. More recently, a dedicated meta-analysis has confirmed a higher risk of incident atrial fibrillation after COVID-19 infection across heterogeneous populations [33].
4.1. Clinical Implications
Our data support routine acquisition of a 12-lead ECG in older adults with hypertension (AH) after COVID-19, with particular vigilance in women and a low threshold for ambulatory (Holter) monitoring when palpitations or tachycardia are reported; cautious interpretation of single clinic blood pressure (BP) values, favoring repeat office measurements, home BP monitoring, or 24 h ambulatory BP monitoring (ABPM); and structured medication review with attention to polypharmacy and the potential contribution of adverse drug effects to PCS-related symptom burden.
The findings justify risk-adapted follow-up pathways that include rhythm monitoring for patients with symptoms or screening indications of arrhythmia; consideration of orthostatic testing when dysautonomia is suspected; individualized BP targets that account for symptoms and treatment tolerability; and early integration of rehabilitation components. In practical terms, these implications can be operationalized through standardized intake templates (documenting symptom duration and fluctuation), routine BP re-measurement or out-of-office monitoring for equivocal readings, and clear triggers for extended rhythm assessment. The prediction model developed here—showing moderate discrimination and acceptable calibration—may serve as a first-pass triage aid for older adults with AH in the post-COVID setting, contingent on external validation and local threshold calibration. Collectively, the implications translate our results into concrete steps to support clinical decision-making and pathway planning for this priority population.
4.2. Limitations
Several methodological limitations should be considered. First, the observational design precludes causal inference. Second, no formal baseline matching was undertaken, and an imbalance between women and men limits the precision of sex-specific estimates, so residual confounding and selection/ascertainment bias cannot be ruled out. Third, office BP was measured once without home or ambulatory monitoring, and orthostatic indices were not systematically assessed. Manual office measurements are prone to digit preference and heaping at rounded SBP/DBP values, a pattern that is typical for routine sphygmomanometer use but may introduce non-differential measurement error and could partly account for the inverse association observed with SBP. Fourth, PCS classification relied on an operational definition with partial dependence on self-report, introducing risks of reporting bias and phenotype misclassification. Fifth, data on acute COVID-19 severity and vaccination were unavailable for adjustment, limiting control of confounding. Sixth, the sample size was moderate for multivariable analysis. Seventh, the single-center, region-specific cohort constrains generalizability. Eighth, photoplethysmographic pulse rate was recorded; potential pulse rate–heart rate discrepancies in arrhythmias or low-perfusion states should be considered when interpreting non-ECG measurements. Finally, exclusive use of the national MoH RK protocol to operationalize PCS enhances local clinical applicability but may reduce external comparability with studies employing alternative frameworks (e.g., WHO and NICE).
4.3. Directions for Future Research
Future work should incorporate longitudinal designs with repeated, home, and ambulatory BP measurements and orthostatic testing, alongside extended autonomic phenotyping (ambulatory/Holter ECG, heart rate variability) to substantiate the role of dysautonomia. Stratification by antihypertensive drug classes and consideration of drug–drug interactions, as well as integration of acute COVID-19 severity and vaccination data, are important.
4.4. Key Points
- In older adults with AH, PCS is independently associated with female sex, younger age, lower office SBP, and rhythm disturbances.
- Practical implications: Routine ECG screening and cautious interpretation of single office BP readings when dysautonomia is suspected, along with orthostatic testing and/or ambulatory monitoring, with particular attention to women and younger–older patients.
5. Conclusions
Although post-COVID cardiovascular effects are well described, evidence in hypertensive older adults is limited. In this outpatient cohort, PCS was independently associated with female sex, younger age, lower office SBP, and rhythm disturbances; given design constraints, these findings should be regarded as preliminary and warrant further research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/covid5120197/s1: Supplementary File S1. English summary of the national clinical protocol “State after COVID-19 (Post-COVID Syndrome) in Adults” (Ministry of Health of the Republic of Kazakhstan, 2023). Supplementary File S2. English summary of the national clinical protocol “Arterial Hypertension” (Ministry of Health of the Republic of Kazakhstan, 2019).
Author Contributions
Conceptualization, A.M. and A.S.; methodology, A.M. and V.K.; formal analysis, T.T.; investigation, V.K. and T.T.; resources, A.S. and A.M.; data curation, V.K.; writing—original draft preparation, V.K. and T.T.; writing—review and editing, A.M., N.N. and A.S.; visualization, T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded for program-targeted financing by the Ministry of Healthcare of the Republic of Kazakhstan (№ BR27310319 “Development of Preventive and Rehabilitation Programs to Improve the Quality of Life of the Population in the Post-COVID Period”).
Institutional Review Board Statement
The project was reviewed and approved by the local ethics committee of the non-profit educational organization “Kazakhstan–Russian Medical University” (protocol № 22, on 22 April 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The dataset is available upon request from the authors.
Acknowledgments
We would like to thank all the volunteers who participated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 (NG188); Updated 25 January 2024. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 2 November 2025).
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A Clinical Update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine (NASEM). A Long COVID Definition: A Chronic, Systemic Disease State with Profound Consequences; National Academies Press: Washington, DC, USA, 2024; Available online: https://nap.nationalacademies.org/catalog/27768 (accessed on 2 November 2025).
- Ely, E.W.; Brown, L.M.; Fineberg, H.V.; Long COVID Definition Committee. Long Covid Defined. N. Engl. J. Med. 2024, 391, 1746–1753. [Google Scholar] [CrossRef]
- World Health Organization. Post COVID-19 Condition (Long COVID): Fact Sheet; 26 February 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 2 November 2025).
- Centers for Disease Control and Prevention. Long COVID Clinical Guidance for Healthcare Providers; 24 July 2025. Available online: https://www.cdc.gov/long-covid/hcp/clinical-guidance/index.html (accessed on 2 November 2025).
- Centers for Disease Control and Prevention. Long COVID-Signs, Symptoms, and Complications; 24 July 2025. Available online: https://www.cdc.gov/long-covid/signs-symptoms/index.html (accessed on 2 November 2025).
- Wisk, L.E.; L’Hommedieu, M.; Diaz Roldan, K.; Mannan, I.E.; Spatz, E.S.; Weinstein, R.A.; Venkatesh, A.K.; Gottlieb, M.; Huebinger, R.; Rising, K.L.; et al. Variability in Long COVID Definitions and Validation of Prevalence Estimates in the INSPIRE Cohort. JAMA Netw. Open 2025, 8, e2526506. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Sutton, R. Autonomic Dysfunction and Postural Orthostatic Tachycardia in Post-Acute COVID-19. Nat. Rev. Cardiol. 2023, 20, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Greenwood, D.C.; Master, H.; Balasundaram, K.; Williams, P.; Scott, J.T.; Wood, C.; Cooper, R.; Darbyshire, J.L.; Gonzalez, A.E.; et al. Prevalence of Orthostatic Intolerance in Long-COVID Clinic Patients and Healthy Volunteers: A Multicenter Study. J. Med. Virol. 2024, 96, e29486. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Olshansky, B.; Chung, M.K.; Gordon, S.; Joglar, J.A.; Marcus, G.M.; Mar, P.L.; Russo, A.M.; Srivatsa, U.N.; Wan, E.Y.; et al. Cardiac Arrhythmias and Autonomic Dysfunction Associated with COVID-19: A Scientific Statement from the American Heart Association. Circulation 2024, 150, e449–e465. [Google Scholar] [CrossRef]
- Matsumoto, C.; Shibata, S.; Kishi, T.; Morimoto, S.; Mogi, M.; Yamamoto, K.; Kobayashi, K.; Tanaka, M.; Asayama, K.; Yamamoto, E.; et al. Long COVID and Hypertension-Related Disorders: A Report from the Japanese Society of Hypertension Project Team on COVID-19. Hypertens. Res. 2023, 46, 601–619. [Google Scholar] [CrossRef]
- Peng, J.; Guo, W.; Li, P.; Leng, L.; Gao, D.; Yu, Z.; Huang, J.; Guo, J.; Wang, S.; Hu, M.; et al. Long-Term Effects of COVID-19 on Endothelial Function, Arterial Stiffness, and Blood Pressure in College Students: A Pre-Post Controlled Study. BMC Infect. Dis. 2024, 24, 742. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Pasquetto, G.; Mazza, A. Risk of Incident New-Onset Arterial Hypertension after COVID-19 Recovery: A Systematic Review and Meta-Analysis. High Blood Press. Cardiovasc. Prev. 2023, 30, 227–233. [Google Scholar] [CrossRef]
- Sedova, E.V.; Paleev, F.N.; Startseva, O.N. Fundamentals of Geriatrics for Primary Care Physicians: Teaching and Methodological Manual. Moscow, Russia. 2019. Available online: https://kardioklinika.ru/wp-content/themes/kardioklinika/assets/docs/osnovi_geriatrii.pdf (accessed on 24 April 2025). (In Russian).
- Ministry of Health of the Republic of Kazakhstan. Arterial Hypertension: Clinical Protocol. Available online: https://diseases.medelement.com/disease/артериальная-гипертензия-клинический-прoтoкoл-казахстан/15810 (accessed on 24 April 2025). (In Russian).
- Ministry of Health of the Republic of Kazakhstan. Post-COVID-19 Condition in Adults: Clinical Protocol (Republic of Kazakhstan Clinical Guideline, 2023). Available online: https://diseases.medelement.com/disease/сoстoяние-пoсле-covid-19-пoсткoвидный-синдрoм-у-взрoслых-кп-рк-2023/17532 (accessed on 24 April 2025). (In Russian).
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated with Post–COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The Prevalence and Long-Term Health Effects of Long Covid among Hospitalised and Non-Hospitalised Populations: A Systematic Review and Meta-Analysis. EClinicalMedicine 2022, 55, 101762. [Google Scholar] [CrossRef] [PubMed]
- Alkodaymi, M.S.; Omrani, O.A.; Fawzy, N.A.; Shaar, B.A.; Almamlouk, R.; Riaz, M.; Obeidat, M.; Obeidat, Y.; Gerberi, D.; Taha, R.M.; et al. Prevalence of Post-Acute COVID-19 Syndrome Symptoms at Different Follow-Up Periods: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 28, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gu, T.; Ni, Z.; Shi, X.; Ranney, M.L.; Mukherjee, B. Global Prevalence of Long COVID, Its Subtypes, and Risk Factors: An Updated Systematic Review and Meta-Analysis. Open Forum Infect. Dis. 2025, 12, ofaf533. [Google Scholar] [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and Risk Factors for Long COVID in Non-Hospitalised Adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef]
- Office for National Statistics. Prevalence of Ongoing Symptoms Following Coronavirus (COVID-19) Infection in the UK: 2 December 2021; Office for National Statistics: Newport, UK, 2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/2december2021 (accessed on 19 November 2025).
- Teymourzadeh, A.; Abramov, D.; Norouzi, S.; Grewal, D.; Heidari-Bateni, G. Infection to Hypertension: A Review of Post-COVID-19 New-Onset Arterial Hypertension. Front. Cardiovasc. Med. 2025, 12, 1609768. [Google Scholar] [CrossRef]
- Bielecka, E.; Szymańska, A.; Ciałkowska-Rysz, A.; Piotrowicz, K.; Piotrowicz, R. Elevated Arterial Blood Pres-sure as a Delayed Complication of COVID-19: A Summary of Observational Studies. Int. J. Mol. Sci. 2024, 25, 1837. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Shah, A.M. Autonomic Dysfunction in ‘Long COVID’: Rationale, Physiology and Management Strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef]
- Ståhlberg, M.; Reistam, U.; Fedorowski, A.; Villacorta, H.; Horiuchi, Y.; Bax, J.; Pitt, B.; Matskeplishvili, S.; Lüscher, T.F.; Weichert, I.; et al. Post-COVID-19 Tachycardia Syndrome: A Distinct Phenotype of Post-Acute COVID-19 Syndrome. Am. J. Med. 2021, 134, 1451–1456. [Google Scholar] [CrossRef]
- Olarinde, F.; Nunes-Silva, A.; Sanchez-Ramirez, D.C.; Molgat-Seon, Y.; Villar, R. Using Active Standing Orthostatic Stress Test to Assess Physiological Responses in Individuals with Long COVID: A Systematic Review. J. Clin. Med. 2025, 14, 8139. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.-Y.; Wang, S.-I.; Wei, J.C.-C. Long-Term Cardiovascular Outcomes in COVID-19 Survivors among Non-Vaccinated Population: A Retrospective Cohort Study from the TriNetX U.S. Collaborative Networks. EClinicalMedicine 2022, 53, 101619. [Google Scholar] [CrossRef]
- Blasi, F.; Vicenzi, M.; De Ponti, R. COVID-19 and Cardiac Arrhythmias: Lesson Learned and Dilemmas. J. Clin. Med. 2024, 13, 7259. [Google Scholar] [CrossRef]
- Katsoularis, I.; Jerndal, H.; Kalucza, S.; Lindmark, K.; Fonseca-Rodríguez, O.; Connolly, A.F. Risk of Arrhythmias Following COVID-19: Nationwide Self-Controlled Case Series and Matched Cohort Study. Eur. Heart J. Open 2023, 3, oead120. [Google Scholar] [CrossRef]
- Zuin, M.; Ojeda-Fernández, L.; Torrigiani, G.; Bertini, M. Risk of Incident Atrial Fibrillation after COVID-19 Infection: A Systematic Review and Meta-Analysis. Heart Rhythm 2024, 21, 1613–1620. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).