Long-Term Myocardial Involvement and Outcome in the Post-COVID-19 Condition
Abstract
1. Introduction
2. Materials and Methods
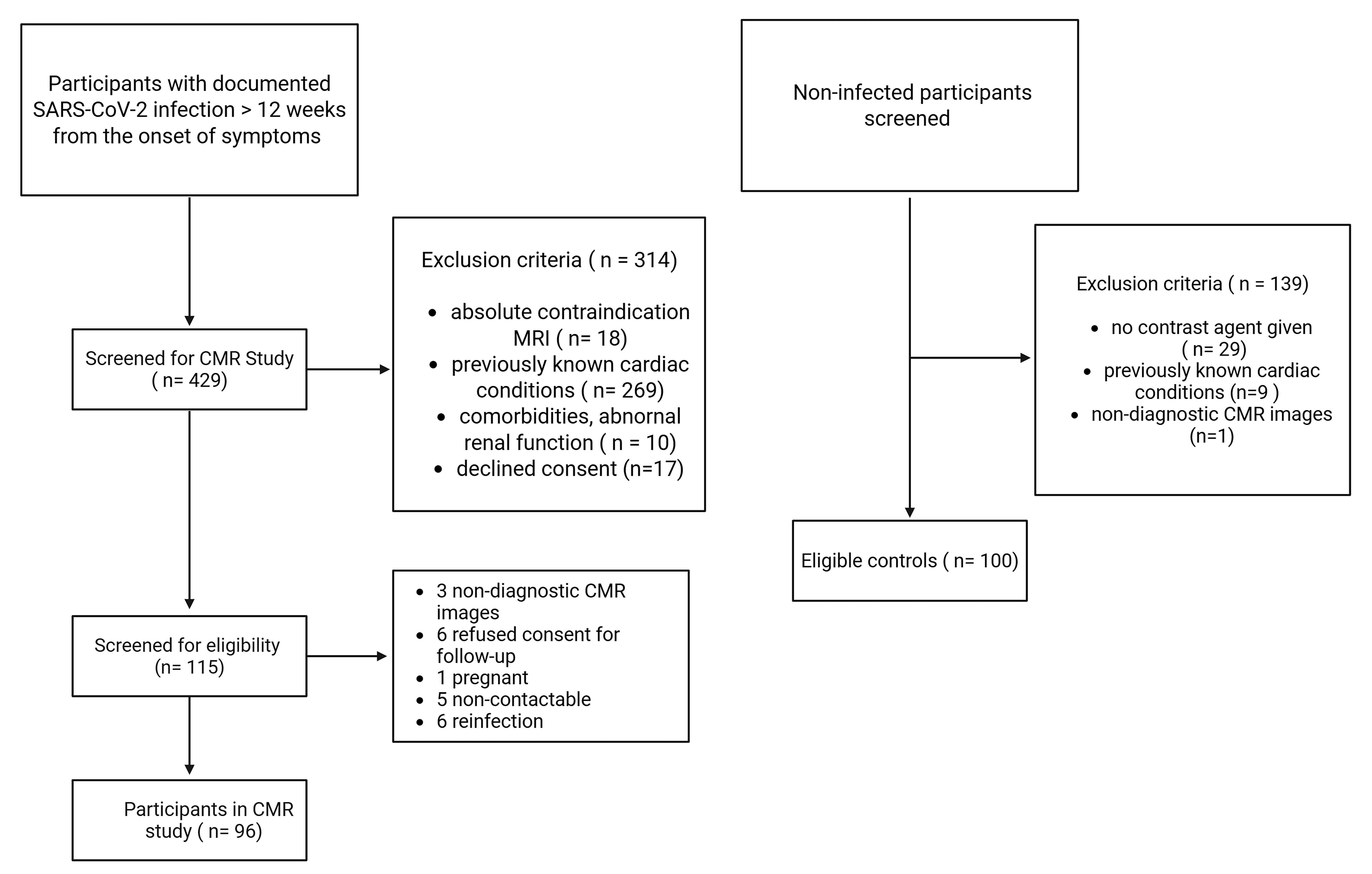
2.1. Study Population and Design
- Confirmed prior SARS-CoV-2 infection by reverse transcription polymerase chain reaction (RT-PCR), not requiring hospitalization;
- Persistent cardiac symptoms (e.g., chest pain, dyspnea, palpitations, or exercise intolerance) for at least three months after COVID-19 diagnosis, with negative RT-PCR at the time of CMR assessment;
- No history of cardiac symptoms prior to the COVID-19 infection.
2.2. Cardiovascular Magnetic Resonance Protocol
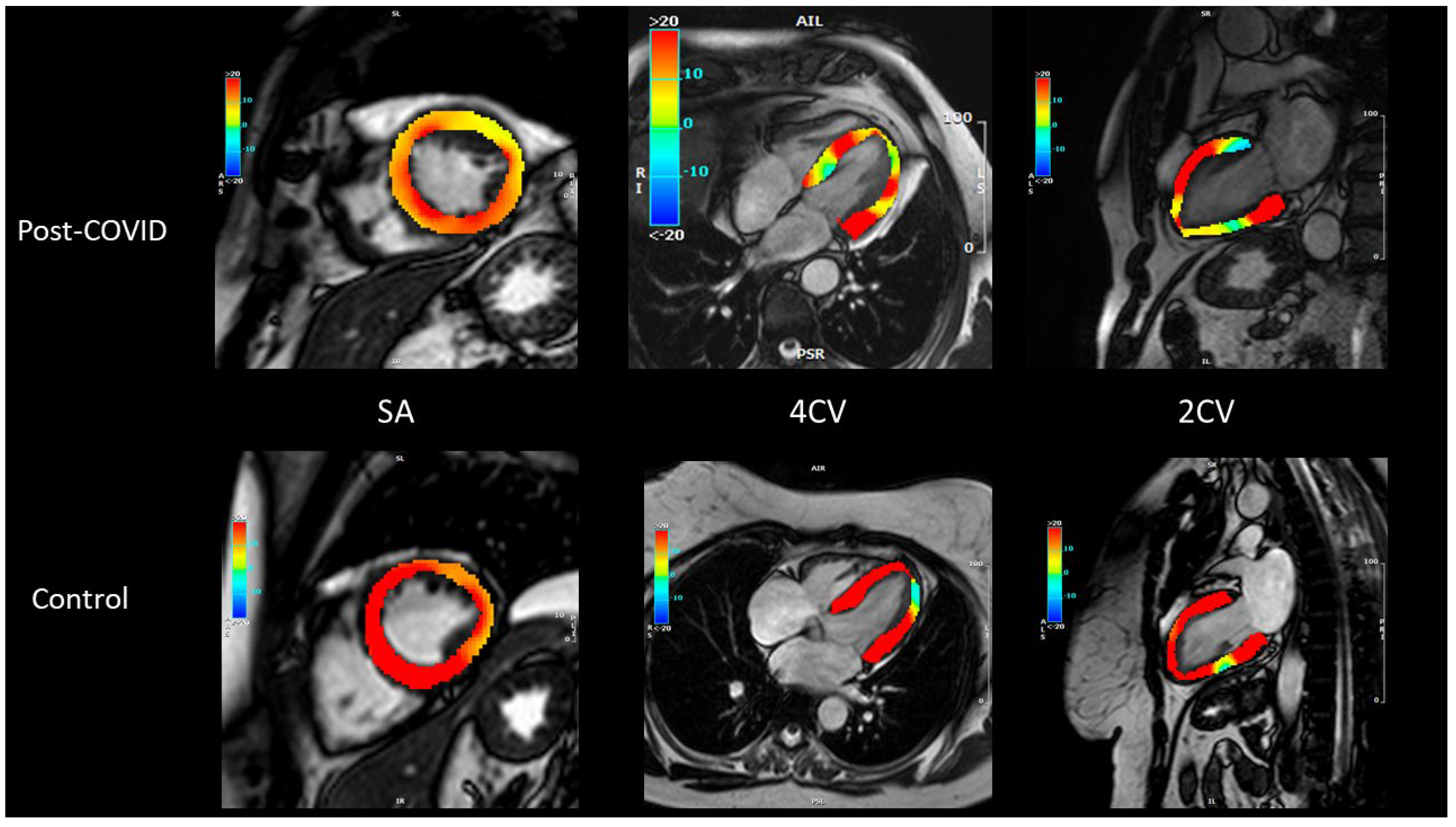
2.3. Image Analysis
2.4. Statistical Analysis
2.5. Ethics Approval and Study Registration
3. Results
3.1. Baseline Characteristics and Initial Symptoms
3.2. Functional and Myocardial Tissue Parameters
3.3. Follow-Up Scan and Clinical Course
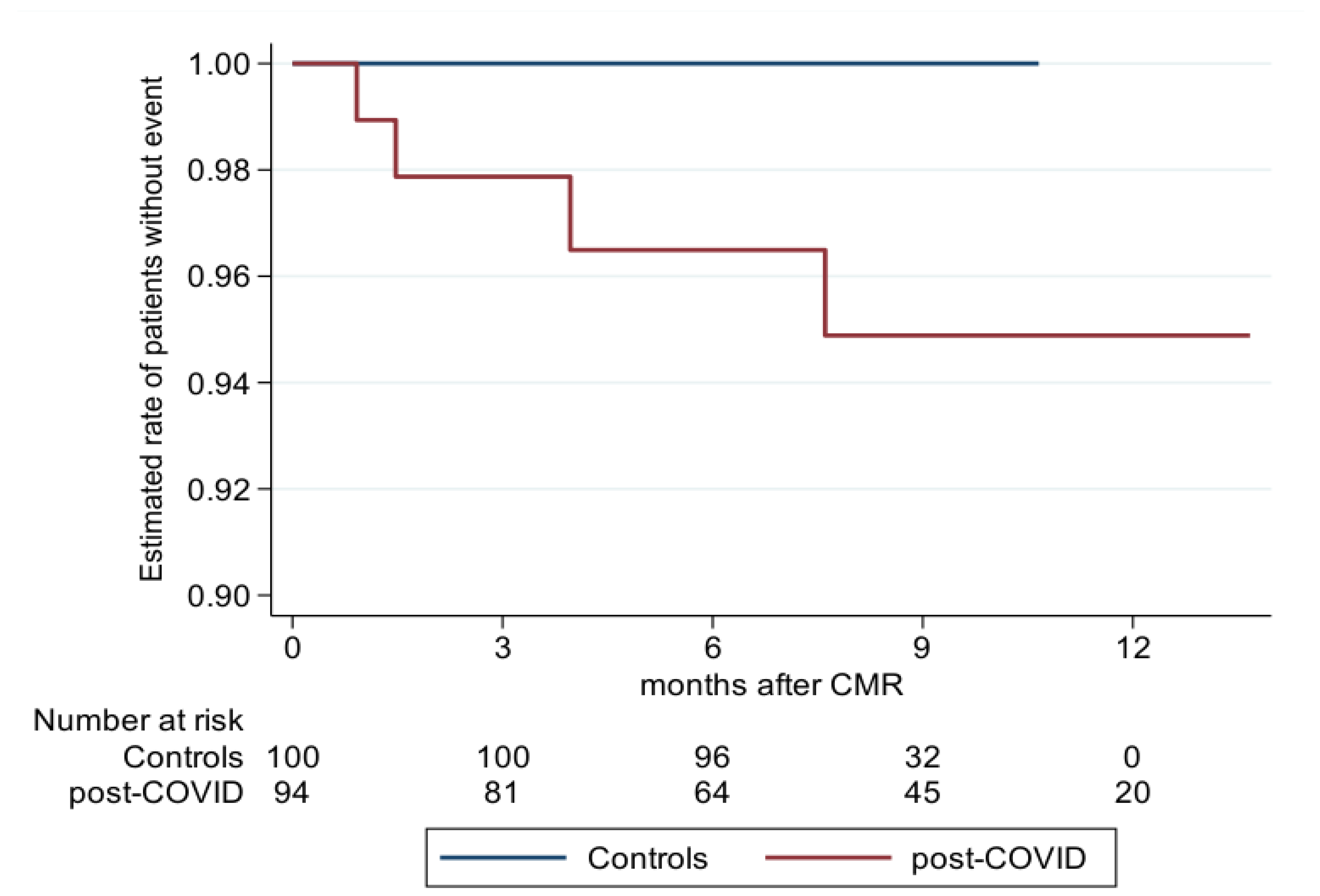
3.4. Prognosis
4. Discussion
4.1. First Post-COVID-19 Assessment
4.2. Follow-Up Assessment
4.3. Prognostic Considerations
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| COVID-19 | Coronavirus Disease 2019 |
| MR | Magnetic resonance |
| CMR | Cardiovascular magnetic resonance |
| PC | Post-COVID-19 |
| RT-PCR | Reverse transcription polymerase |
| MRI | Magnetic resonance imaging |
| LGE | Late gadolinium enhancement |
| LV-EF | Left ventricular ejection fraction |
| GLS | Global longitudinal strain |
| GCS | Global circumferential strain |
| GRS | Global radial strain |
| MACE | Major adverse cardiac event |
| NYHA Class | New York Heart Association classification |
| CCS Class | Canadian Cardiovascular Society classification |
| LVEDV | Left ventricular end-diastolic volume |
| LVESV | Left ventricular end-systolic volume |
| LVSV | Left ventricular stroke volume |
References
- Available online: https://covid19.who.int/ (accessed on 1 August 2025).
- Petersen, S.E.; Friedrich, M.G.; Leiner, T.; Elias, M.D.; Ferreira, V.M.; Fenski, M.; Flamm, S.D.; Fogel, M.; Garg, R.; Halushka, M.K.; et al. Cardiovascular Magnetic Resonance for Patients With COVID-19. JACC Cardiovasc. Imaging 2022, 15, 685–699. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19, a longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, R.; Zhou, Z.; Jiang, H.; Yan, Z.; Tao, X.; Li, H.; Xu, L. Cardiac involvement in COVID-19 patients: Mid-term follow up by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhao, P.; Tang, D.; Zhu, T.; Han, R.; Zhan, C.; Liu, W.; Zeng, H.; Tao, Q.; Xia, L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2330–2339. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef]
- Wright, J.; Adriaenssens, T.; Dymarkowski, S.; Desmet, W.; Bogaert, J. Quantification of Myocardial Area at Risk With T2-Weighted CMR. JACC Cardiovasc. Imaging 2009, 2, 825–831. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef]
- Goto, Y.; Ishida, M.; Takase, S.; Sigfridsson, A.; Uno, M.; Nagata, M.; Ichikawa, Y.; Kitagawa, K.; Sakuma, H. Comparison of Displacement Encoding With Stimulated Echoes to Magnetic Resonance Feature Tracking for the Assessment of Myocardial Strain in Patients With Acute Myocardial Infarction. Am. J. Cardiol. 2017, 119, 1542–1547. [Google Scholar] [CrossRef]
- Liu, B.; Dardeer, A.M.; Moody, W.E.; Hayer, M.K.; Baig, S.; Price, A.M.; Leyva, F.; Edwards, N.C.; Steeds, R.P. Reference ranges for three-dimensional feature tracking cardiac magnetic resonance: Comparison with two-dimensional methodology and relevance of age and gender. Int. J. Cardiovasc. Imaging 2017, 34, 761–775. [Google Scholar] [CrossRef]
- Bavishi, C.; Bonow, R.O.; Trivedi, V.; Abbott, J.D.; Messerli, F.H.; Bhatt, D.L. Special Article—Acute myocardial injury in patients hospitalized with COVID-19 infection: A review. Prog. Cardiovasc. Dis. 2020, 63, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef] [PubMed]
- Buss, S.J.; Breuninger, K.; Lehrke, S.; Voss, A.; Galuschky, C.; Lossnitzer, D.; Andre, F.; Ehlermann, P.; Franke, J.; Taeger, T.; et al. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 307–315. [Google Scholar] [CrossRef]
- Houard, L.; Benaets, M.B.; de Meester de Ravenstein, C.; Rousseau, M.F.; Ahn, S.A.; Amzulescu, M.S.; Roy, C.; Slimani, A.; Vancraeynest, D.; Pasquet, A.; et al. Additional Prognostic Value of 2D Right Ventricular Speckle-Tracking Strain for Prediction of Survival in Heart Failure and Reduced Ejection Fraction. JACC Cardiovasc. Imaging 2019, 12, 2373–2385. [Google Scholar] [CrossRef]
- Wong, D.T.; Leong, D.P.; Weightman, M.J.; Richardson, J.D.; Dundon, B.K.; Psaltis, P.J.; Leung, M.C.; Meredith, I.T.; Worthley, M.I.; Worthley, S.G. Magnetic resonance-derived circumferential strain provides a superior and incremental assessment of improvement in contractile function in patients early after ST-segment elevation myocardial infarction. Eur. Radiol. 2014, 24, 1219–1228. [Google Scholar] [CrossRef]
- Ulloa, J.U.; de Vega, V.M.; Montañés, O.S.; Vázquez, A.Á.; Sánchez-Enrique, C.; Jiménez, S.H.; García, F.D.S.; Ruiz, L.L.; Rodríguez, M.R.; Pizarro, G.; et al. Cardiac magnetic resonance in recovering COVID-19 patients. Feature tracking and mapping analysis to detect persistent myocardial involvement. IJC Heart Vasc. 2021, 36, 100854. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Zhao, R.; Wang, T.; Zhu, Y.; Qian, Y.; Liu, B.; Yu, Y.; Han, Y. Elevated Extracellular Volume Fraction and Reduced Global Longitudinal Strains in Participants Recovered from COVID-19 without Clinical Cardiac Findings. Radiology 2021, 299, E230–E240. [Google Scholar] [CrossRef] [PubMed]
- Salatzki, J.; Ochs, A.; Weberling, L.D.; Heins, J.; Zahlten, M.; Whayne, J.G.; Stehning, C.; Giannitsis, E.; Denkinger, C.M.; Merle, U.; et al. Absence of cardiac impairment in patients after severe acute respiratory syndrome coronavirus type 2 infection: A long-term follow-up study. J. Cardiovasc. Magn. Reson. 2024, 26, 101124. [Google Scholar] [CrossRef]
- Kotanidis, C.P.; Bazmpani, M.-A.; Haidich, A.-B.; Karvounis, C.; Antoniades, C.; Karamitsos, T.D. Diagnostic Accuracy of Cardiovascular Magnetic Resonance in Acute Myocarditis. JACC Cardiovasc. Imaging 2018, 11, 1583–1590. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Natale, L.; Ligabue, G.; Peretto, G.; Lovato, L.; Vignale, D.; Fiocchi, F.; Marano, R.; Russo, V. Cardiac Magnetic Resonance Characterization of Myocarditis-Like Acute Cardiac Syndrome in COVID-19. JACC Cardiovasc. Imaging 2020, 13, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Ferreira, V.M.; Leung, S.T.; Yin Lee, J.C.; Ho-Tung Fong, A.; To Liu, R.W.; Man Chan, J.W.; Wu, A.K.L.; Lung, K.C.; Crean, A.M.; et al. Patients Recovered From COVID-19 Show Ongoing Subclinical Myocarditis as Revealed by Cardiac Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2020, 13, 2476–2478. [Google Scholar] [CrossRef]
- Cannata’, A.; Artico, J.; Gentile, P.; Merlo, M.; Sinagra, G. Myocarditis evolving in cardiomyopathy: When genetics and offending causes work together. Eur. Heart J. Suppl. 2019, 21, B90–B95. [Google Scholar] [CrossRef]
- Joy, G.; Artico, J.; Kurdi, H.; Seraphim, A.; Lau, C.; Thornton, G.D.; Oliveira, M.F.; Adam, R.D.; Aziminia, N.; Menacho, K.; et al. Prospective Case-Control Study of Cardiovascular Abnormalities 6 Months Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc. Imaging 2021, 14, 2155–2166. [Google Scholar] [CrossRef]
- Wu, Q.I.; Zhou, L.; Sun, X.; Yan, Z.; Hu, C.; Wu, J.; Xu, L.; Li, X.; Liu, H.; Yin, P.; et al. Altered Lipid Metabolism in Recovered SARS Patients Twelve Years after Infection. Sci. Rep. 2017, 7, 9110. [Google Scholar] [CrossRef]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819. [Google Scholar] [CrossRef]
- Gravinay, P.; Issa, N.; Girard, D.; Camou, F.; Cochet, H. CMR and serology to diagnose COVID-19 infection with primary cardiac involvement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 133. [Google Scholar] [CrossRef]
- Luetkens, J.A.; Isaak, A.; Zimmer, S.; Nattermann, J.; Sprinkart, A.M.; Boesecke, C.; Rieke, G.J.; Zachoval, C.; Heine, A.; Velten, M.; et al. Diffuse Myocardial Inflammation in COVID-19 Associated Myocarditis Detected by Multiparametric Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2020, 13, e010897. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Weberling, L.D.; Hillier, E.; Friedrich, M.G.; Zahlten, M.; Frey, N.; André, F.; Steen, H. Abnormal coronary vascular response in patients with long COVID syndrome—A case-control study using oxygenation-sensitive cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2025, 27, 101890. [Google Scholar] [CrossRef] [PubMed]
| PC n = 94 | C n = 100 | p-Value * | ||
|---|---|---|---|---|
| Gender | male | 35 (37.2%) | 40 (40.0%) | |
| female | 59 (62.8%) | 60 (60.0%) | 0.768 | |
| Age | 44.9 ± 16.0 | 48.7 ± 16.5 | 0.093 | |
| BMI | 26.3 ± 4.9 | 26.0 ± 4.2 | 0.801 | |
| Positive COVID-19 Test | no | 0 (0%) | 100 (100%) | |
| yes | 94 (100%) | 0 (0%) | <0.001 | |
| Hypertension | no | 60 (73.2%) | 66 (66.0%) | |
| yes | 22 (26.8%) | 34 (34.0%) | 0.335 | |
| Hyperlipidemia | no | 58 (70.7%) | 93 (93.0%) | |
| yes | 24 (29.3%) | 7 (7.0%) | <0.001 | |
| Diabetes | no | 77 (93.9%) | 96 (96.0%) | |
| yes | 5 (6.1%) | 4 (4.0%) | 0.733 | |
| Smoking | no | 71 (86.6%) | 90 (90.9%) | |
| yes | 11 (13.4%) | 9 (9.1%) | 0.476 | |
| Family History of Cardiovascular Disease | no | 47 (58.0%) | 87 (87.0%) | |
| yes | 34 (42.0%) | 13 (13.0%) | <0.001 | |
| Obesity | no | 61 (74.4%) | 94 (94.0%) | |
| yes | 21 (25.6%) | 6 (6.0%) | <0.001 | |
| Clinical Symptoms in The Acute Phase | no yes | 3 (3.2%) 91 (96.8%) | - - | |
| Dyspnea | no yes | 40 (44.9%) 49 (55.1%) | - - | |
| NYHA Class | 0 1 2 3 4 | 40 (44.9%) 9 (10.1%) 16 (18.0%) 14 (15.7%) 10 (11.2%) | - - - - - | |
| Angina CCS Class | 0 1 2 3 4 | 49 (55.1%) 12 (13.5%) 13 (14.6%) 3 (3.4%) 12 (13.5%) | - - - - - | |
| Fever | no yes | 25 (28.1%) 64 (71.9%) | - - | |
| Diarrhea | no yes | 64 (71.9%) 25(28.1%) | - - | |
| Loss of Smell/Taste | no yes | 29 (32.6%) 60 (67.4%) | - - |
| PC n = 94 | C n = 100 | p-Value * | |
|---|---|---|---|
| LVEF [%] | 63.4 ± 4.9 | 63.4 ± 5.1 | 0.850 |
| LVEDV [mL] | 152.9 ± 38.8 | 149.3 ± 36.2 | 0.558 |
| LVESV [mL] | 56.7 ± 17.6 | 55.5 ± 16.9 | 0.627 |
| LVSV [mL] | 96.2 ± 23.7 | 94.8 ± 23.4 | 0.732 |
| LGE-Volume [mL] | 0.4 ± 1.3 | 0 | 0.168 |
| T2-Volume [mL] | 0.1 ± 1.3 | 0 | 0.485 |
| Post-COVID-19 n = 94 | Control n = 100 | p-Value * | 95% CI, p-Value ** | |
|---|---|---|---|---|
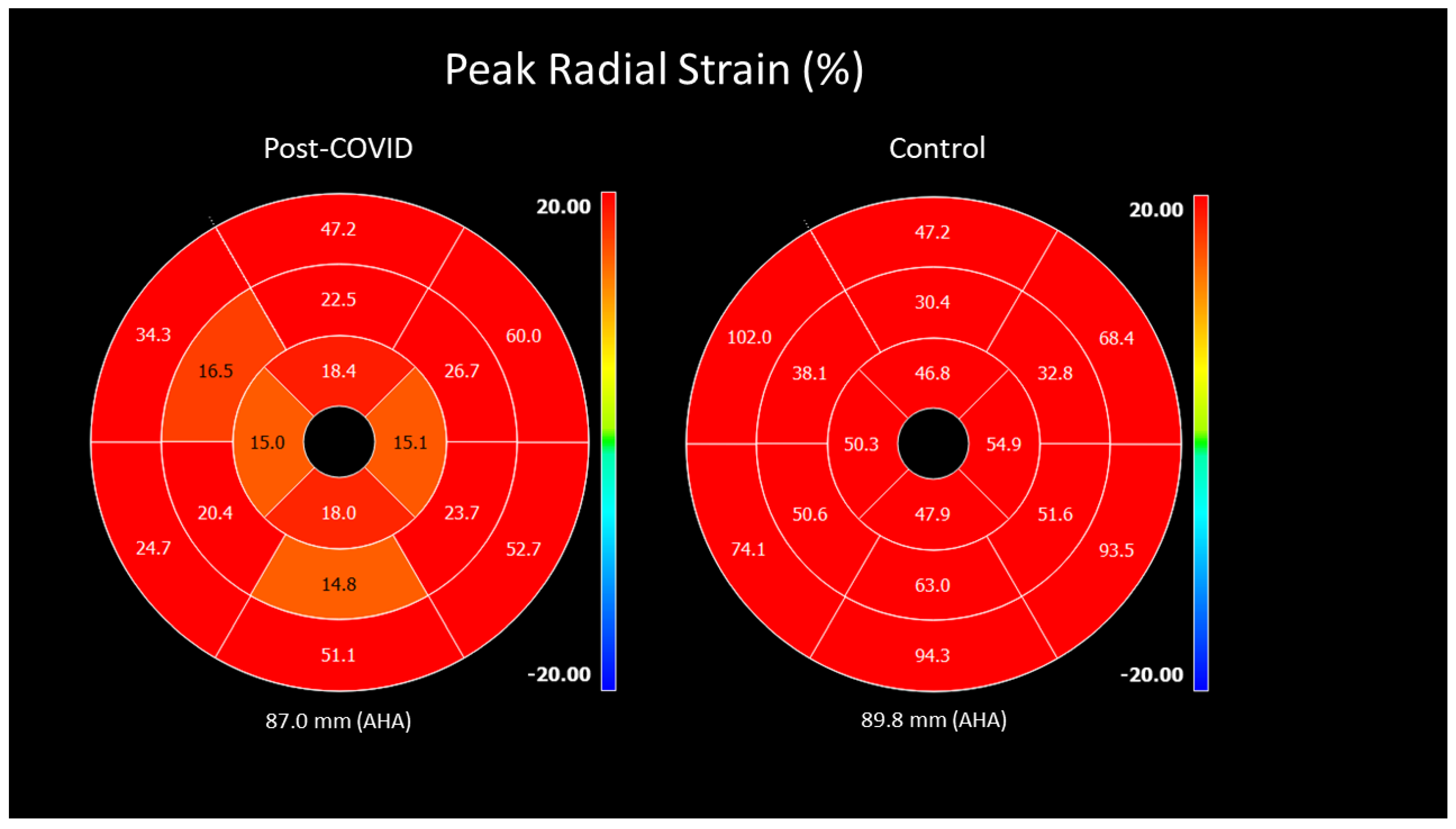
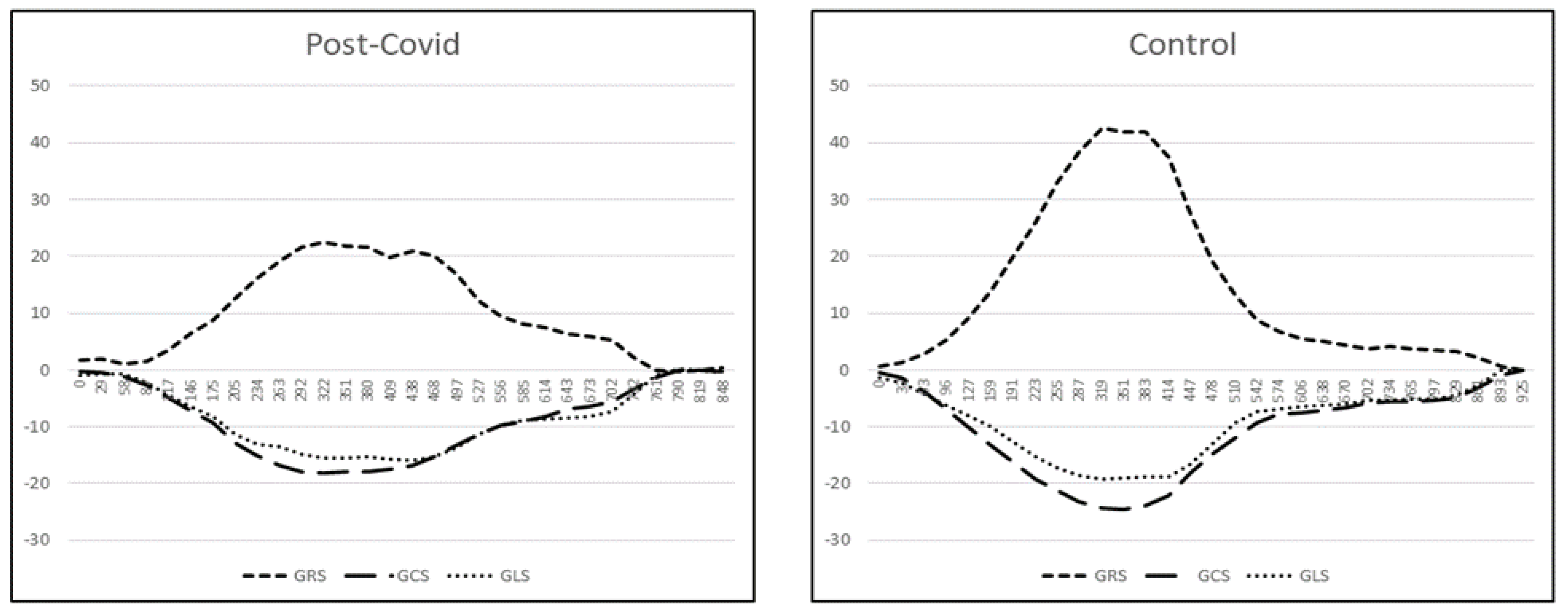
| Peak strain% radial (GRS) | 32.6 ± 8.9 [18.1–72.3] | 34.9 ± 8.4 [20.5–58.0] | 0.049 | −2.9 (−5.4; −0.4), p = 0.025 |
| Peak strain% circumferential | −20.5 ± 2.8 | −21.5 ± 2.6 | 0.015 | 1.2 (0.4; 2.0), p = 0.003 |
| (GCS) | [−26.4–11.8] | [−27.2–15.5] | ||
| Peak strain% longitudinal | −14.7 ± 2.3 | −15.1 ± 2.2 | 0.379 | 0.4 (−0.3; 1.1), p = 0.292 |
| (GLS) | [−19.6–9.2] | [−20.1–9.0] | ||
| Time to peak strain (ms) | 326.1 ± 45.9 | 315.4 ± 44. | 0.068 | 11.8 (−1.3; 25.0), p = 0.078 |
| radial | [239.2–423.2] | [213.2–442.4] | ||
| Time to peak strain (ms) | 317.7 ± 44.5 | 308.2 ± 38.4 | 0.153 | 6.8 (−5.5; 19.1), p = 0.277 |
| circumferential | [232.8–445.2] | [222.0–401.8] | ||
| Time to peak strain (ms) | 355.7 ± 59.8 | 340.1 ± 54.9 | 0.106 | 6.5 (−9.9; 22.9), p = 0.437 |
| longitudinal | [222.2–508.8] | [213.6–460.6] | ||
| Systolic strain rate (/s) | 1.9 ± 0.8 | 2.1 ± 0.6 | 0.025 | −0.17 (−0.37; 0.02), p = 0.084 |
| radial | [1.0–5.5] | [1.1–4.1] | ||
| Systolic strain rate (/s) | −1.1 ± 0.3 | −1.2 ± 0.2 | 0.022 | 0.08 (0.01; 0.15), p = 0.020 |
| circumferential | [−2.7–0.7] | [−2.0–0.7] | ||
| Systolic strain rate (/s) | −0.8 ± 0.3 | −0.9 ± 0.2 | 0.099 | 0.08 (0.01; 0.15), p = 0.035 |
| longitudinal | [−2.7–0.6] | [−1.9–0.5] | ||
| Peak diastolic strain rate (/s) | −2.5 ± 1.0 | −2.6 ± 0.8 | 0.237 | 0.16 (−0.11; 0.43), p = 0.242 |
| radial | [−8.2–1.1] | [−5.2–1.0] | ||
| Peak diastolic strain rate (/s) | 1.4 ± 0.5 | 1.4 ± 0.3 | 0.819 | 0.06 (−0.03; 0.14), p = 0.186 |
| circumferential | [−1.8–2.6] | [0.7–2.5] | ||
| Peak diastolic strain rate (/s) | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.588 | −0.02 (−0.10; 0.05), p = 0.535 |
| longitudinal | [0.5–2.3] | [0.6–2.1] |
| PC n = 91 | C n = 100 | p-Value * | |
|---|---|---|---|
| MACE | 4 | 0 | |
| Stroke | 1 | ||
| Myocarditis | 1 | ||
| MI | 2 | ||
| Death | 0 | ||
| Years | 63.7 | 68.3 | |
| Rate per year (95% CI) | 0.063 (0.024; 0.167) | 0 | 0.029 |
| Estimated MACE rate ** after 12 months (95% CI) | 5.2 (2.0; 13.5) | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiadis, M.; Akyol, N.; Kamper, L.; Nadem-Boueini, N.; Ziakos, A.; Haage, P.; Seyfarth, M.; Abanador-Kamper, N. Long-Term Myocardial Involvement and Outcome in the Post-COVID-19 Condition. COVID 2025, 5, 193. https://doi.org/10.3390/covid5110193
Georgiadis M, Akyol N, Kamper L, Nadem-Boueini N, Ziakos A, Haage P, Seyfarth M, Abanador-Kamper N. Long-Term Myocardial Involvement and Outcome in the Post-COVID-19 Condition. COVID. 2025; 5(11):193. https://doi.org/10.3390/covid5110193
Chicago/Turabian StyleGeorgiadis, Miltiadis, Nuriye Akyol, Lars Kamper, Nima Nadem-Boueini, Athanasios Ziakos, Patrick Haage, Melchior Seyfarth, and Nadine Abanador-Kamper. 2025. "Long-Term Myocardial Involvement and Outcome in the Post-COVID-19 Condition" COVID 5, no. 11: 193. https://doi.org/10.3390/covid5110193
APA StyleGeorgiadis, M., Akyol, N., Kamper, L., Nadem-Boueini, N., Ziakos, A., Haage, P., Seyfarth, M., & Abanador-Kamper, N. (2025). Long-Term Myocardial Involvement and Outcome in the Post-COVID-19 Condition. COVID, 5(11), 193. https://doi.org/10.3390/covid5110193






