COVID-19 Therapeutics Use by Social Deprivation Index in England, July 2020–April 2023
Abstract
1. Introduction
2. Methods
2.1. Data and Data Sources
2.2. Data Processing and Statistical Analyses
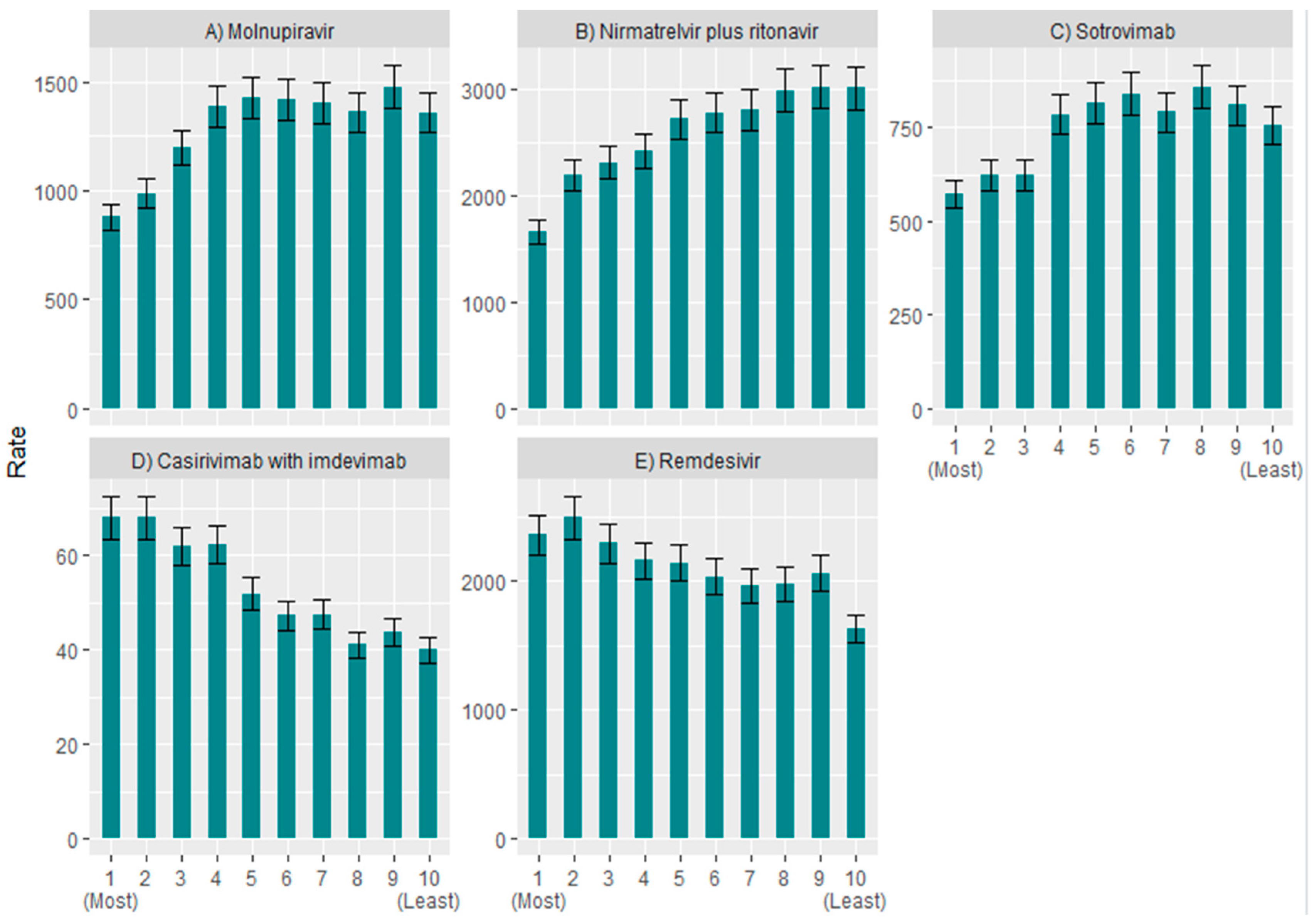
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Løchen, A.; Squire, H.; Ashiru-Oredope, D.; Hand, K.S.; Hartman, H.; Triggs-Hodge, C.; Fountain, H.; Bou-Antoun, S.; Demirjian, A.; Gerver, S.M. Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report). Med. Sci. Forum 2022, 15, 2. [Google Scholar]
- Falola, A.; Demirjian, A.; Thompson, W.; Brown, C.S.; Gerver, S.; Bou-Antoun, S. The impact of COVID-19 national restrictions on dental antibiotic dispensing trends and treatment activity in England: January 2016 to July 2021. JAC-Antimicrob. Resist. 2023, 5, dlad081. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care. Government Launches COVID-19 Antivirals Taskforce to Roll Out Innovative Home Treatments this Autumn. Available online: https://www.gov.uk/government/news/government-launches-covid-19-antivirals-taskforce-to-roll-out-innovative-home-treatments-this-autumn (accessed on 14 September 2023).
- NHS England. COVID-19 Therapeutics (Antivirals, Neutralising Monoclonal Antibodies and Interleukin 6 Inhibitors). Available online: https://www.england.nhs.uk/statistics/statistical-work-areas/covid-therapeutics-antivirals-and-neutralising-monoclonal-antibodies/ (accessed on 14 September 2023).
- Collaborative, T.O.; Green, A.; Curtis, H.J.; Higgins, R.; Smith, R.; Mehrkar, A.; Inglesby, P.; Mahalingasivam, V.; Drysdale, H.; DeVito, N.J.; et al. Trends, variation and clinical characteristics of recipients of antivirals and neutralising monoclonal antibodies for non-hospitalised COVID-19: A descriptive cohort study of 23.4 million people in OpenSAFELY. medRxiv 2022. [Google Scholar] [CrossRef]
- UKHSA. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20231002172235/https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report (accessed on 31 October 2023).
- NHS England. Commissioning Framework:COVID-19 Therapeutics for Non-Hospitalised Patients. Available online: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2022/12/C1677-commissioning-framework-covid-19-therapeutics-for-non-hospitalised-patients-march-23.pdf.pdf (accessed on 23 August 2023).
- NICE. Casirivimab Plus Imdevimab, Nirmatrelvir Plus Ritonavir, Sotrovimab and Tocilizumab for Treating COVID-19. Available online: https://www.nice.org.uk/guidance/ta878/resources/casirivimab-plus-imdevimab-nirmatrelvir-plus-ritonavir-sotrovimab-and-tocilizumab-for-treating-covid19-pdf-82613679870661 (accessed on 30 January 2024).
- Bou-Antoun, S.; Rokadiya, S.; Ashiru-Oredope, D.; Demirjian, A.; Sherwood, E.; Ellaby, N.; Gerver, S.; Grossi, C.; Harman, K.; Hartman, H.; et al. COVID-19 therapeutics: Stewardship in England and considerations for antimicrobial resistance. J. Antimicrob. Chemother. 2023, 78, ii37–ii42. [Google Scholar] [CrossRef] [PubMed]
- ESPAUR. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1033851/espaur-report-2020-to-2021-16-Nov.pdf (accessed on 31 October 2023).
- England, P.H. Health Profile for England 2021. Available online: https://fingertips.phe.org.uk/static-reports/health-profile-for-england/hpfe_report.html (accessed on 3 November 2023).
- NHS England. Core20PLUS5 an Approach to Reducing Health Inequalities: Supporting Information. Available online: https://www.england.nhs.uk/publication/?filter-keyword=Core20plus&filter-category=&filter-publication=&filter-date-from=&filter-date-to=&filter-order-by=date-desc (accessed on 6 November 2023).
- Ministry of Housing, Communities & Local Government. The English Indices of Deprivation 2019. Available online: https://assets.publishing.service.gov.uk/media/5dfb3d7ce5274a3432700cf3/IoD2019_FAQ_v4.pdf (accessed on 30 January 2024).
- Evandrou, M.; Falkingham, J.; Feng, Z.; Vlachantoni, A. Ethnic inequalities in limiting health and self-reported health in later life revisited. J. Epidemiol. Community Health 2016, 70, 653–662. [Google Scholar] [CrossRef]
- UKHSA. Understanding Health Inequalities in England. Available online: https://ukhsa.blog.gov.uk/2017/07/13/understanding-health-inequalities-in-england/ (accessed on 31 October 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falola, A.; Squire, H.; Bou-Antoun, S.; Løchen, A.; Brown, C.S.; Demirjian, A. COVID-19 Therapeutics Use by Social Deprivation Index in England, July 2020–April 2023. COVID 2024, 4, 645-651. https://doi.org/10.3390/covid4050043
Falola A, Squire H, Bou-Antoun S, Løchen A, Brown CS, Demirjian A. COVID-19 Therapeutics Use by Social Deprivation Index in England, July 2020–April 2023. COVID. 2024; 4(5):645-651. https://doi.org/10.3390/covid4050043
Chicago/Turabian StyleFalola, Angela, Hanna Squire, Sabine Bou-Antoun, Alessandra Løchen, Colin S. Brown, and Alicia Demirjian. 2024. "COVID-19 Therapeutics Use by Social Deprivation Index in England, July 2020–April 2023" COVID 4, no. 5: 645-651. https://doi.org/10.3390/covid4050043
APA StyleFalola, A., Squire, H., Bou-Antoun, S., Løchen, A., Brown, C. S., & Demirjian, A. (2024). COVID-19 Therapeutics Use by Social Deprivation Index in England, July 2020–April 2023. COVID, 4(5), 645-651. https://doi.org/10.3390/covid4050043





