Equitable Vaccine Access in Light of COVID-19 Vaccine Procurement Strategies in Africa
Abstract
1. Introduction
1.1. Equity, Evidence, and the (Re)Negotiation of International Pandemic Law and Finance
1.2. Future Systems for Addressing Equity in Vaccine Access Must Address Donation, Capacity to Procure Bilaterally, Regional Procurement, and International Facilities like COVAX
2. Materials and Methods
- (1)
- Burkina Faso;
- (2)
- Burundi;
- (3)
- Central African Republic;
- (4)
- Chad;
- (5)
- Democratic Republic of the Congo;
- (6)
- Eritrea;
- (7)
- Ethiopia;
- (8)
- The Gambia;
- (9)
- Guinea;
- (10)
- Guinea-Bissau;
- (11)
- Liberia;
- (12)
- Madagascar;
- (13)
- Malawi;
- (14)
- Mali;
- (15)
- Mozambique;
- (16)
- Niger;
- (17)
- Rwanda;
- (18)
- Sierra Leone;
- (19)
- Somalia;
- (20)
- South Sudan;
- (21)
- Sudan;
- (22)
- Togo;
- (23)
- Uganda;
- (24)
- Zambia.
- (1)
- Angola;
- (2)
- Algeria;
- (3)
- Benin;
- (4)
- Cabo Verde;
- (5)
- Cameroon;
- (6)
- Comoros;
- (7)
- Republic of the Congo;
- (8)
- Côte d’Ivoire;
- (9)
- Djibouti;
- (10)
- Egypt;
- (11)
- Eswatini;
- (12)
- Ghana;
- (13)
- Kenya;
- (14)
- Lesotho;
- (15)
- Mauritania;
- (16)
- Morocco;
- (17)
- Nigeria;
- (18)
- São Tomé and Principe;
- (19)
- Senegal;
- (20)
- Tanzania;
- (21)
- Tunisia;
- (22)
- Zimbabwe.
- (1)
- Bilateral/multilateral purchase agreements;
- (2)
- Donations (non-purchased doses delivered through non-COVAX bilateral and/or multilateral donations);
- (3)
- COVID-19 Vaccines Global Access (COVAX) initiative;
- (4)
- African Vaccine Acquisition Trust (AVAT);
- (5)
- Unknown.
3. Results
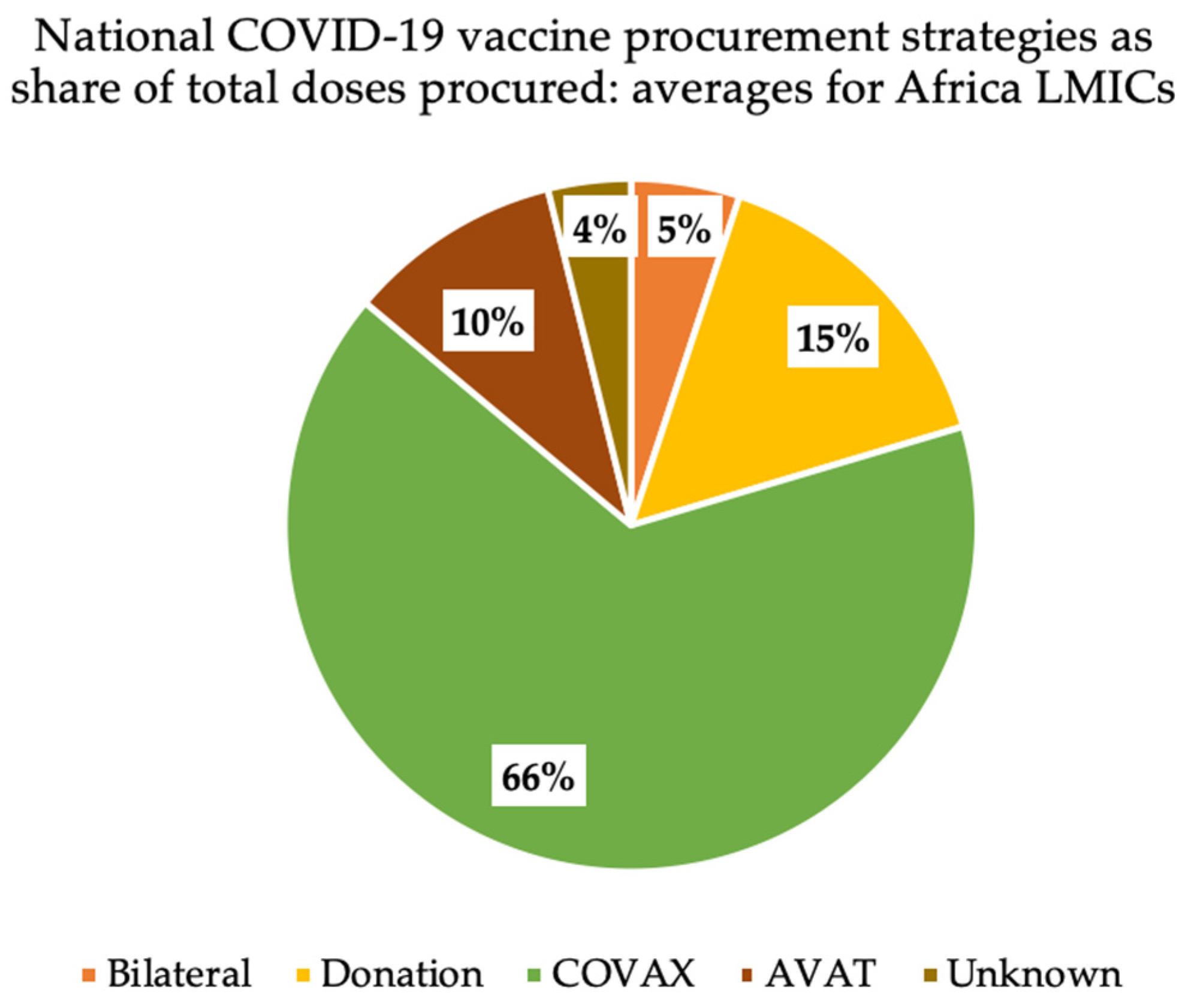
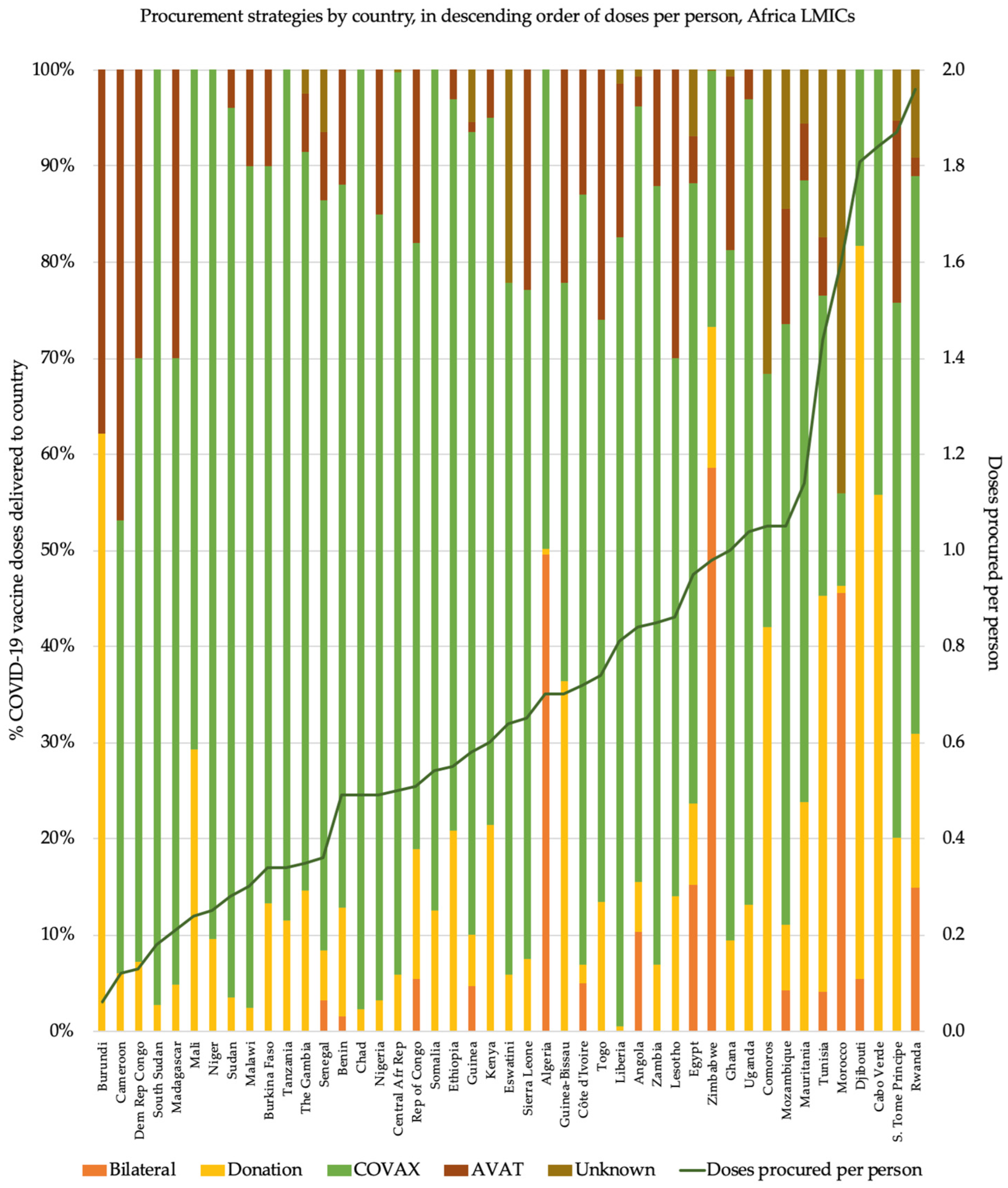
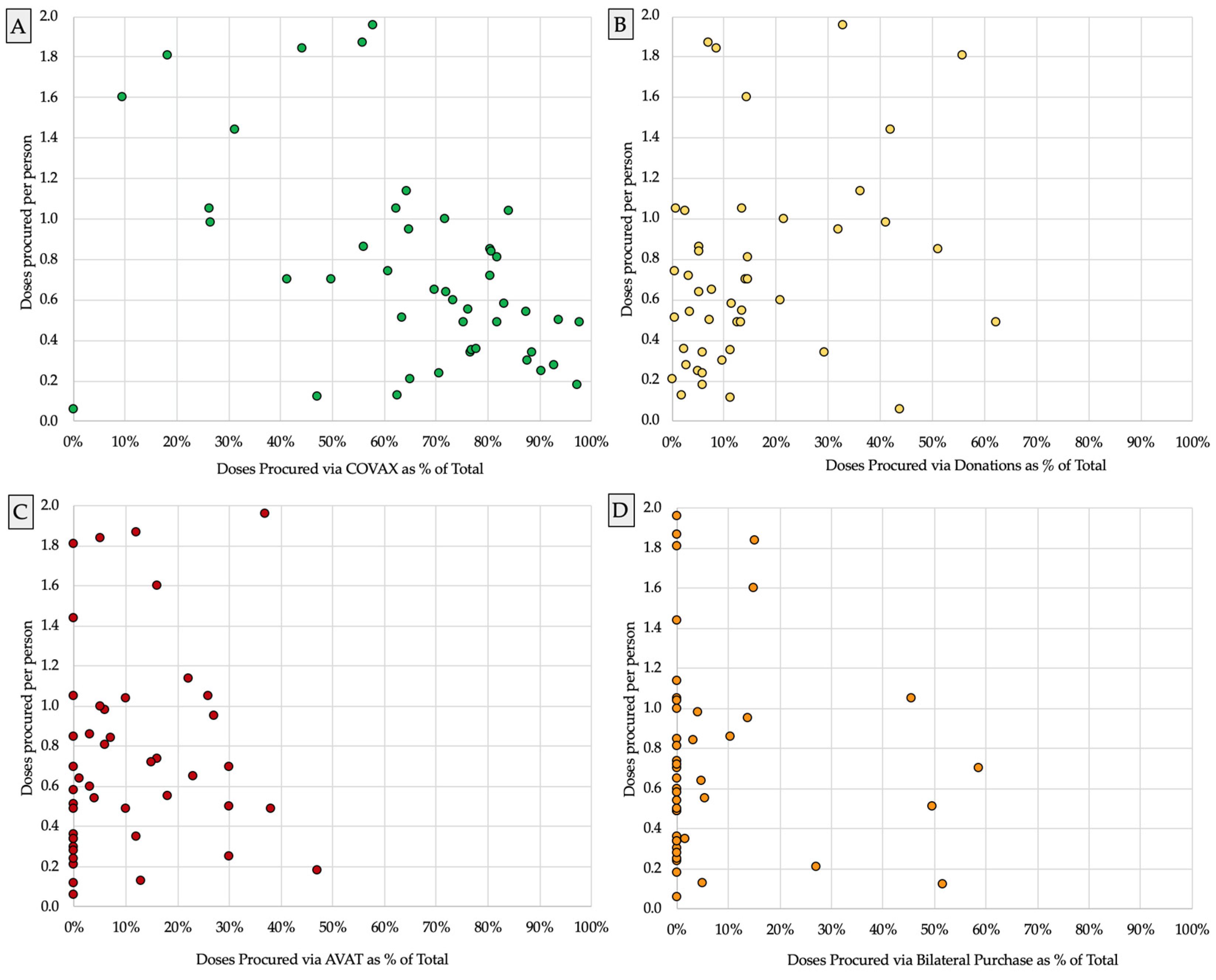
3.1. Frequent Reliance on COVAX Ahead of Other Procurement Routes
3.2. Amid Greater Reliance on COVAX, Vaccine Supply Often Fell Further behind Population Need
4. Discussion
4.1. Ex-Post Pooled Procurement (e.g., COVAX) Must Be Complemented by Dedicated Legal and Logistical Pathways for Donation, Support for Bilateral Procurement, and Regional Procurement
4.2. Bilateral Procurement Is the Best-Known Path for Technology Transfer
4.3. Regional Procurement Channels Are Better Tailored for Resource Constrained Countries
4.4. Despite Stigma, Donations Are a Credible and Important Part of Pandemic Preparedness
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gostin, L.O. Global Health Security: A Blueprint for the Future; Harvard University Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Das, J.K.; Chee, H.Y.; Lakhani, S.; Khan, M.H.; Islam, M.; Muhammad, S.; Bhutta, Z.A. COVID-19 Vaccines: How Efficient and Equitable Was the Initial Vaccination Process? Vaccines 2022, 11, 11. [Google Scholar] [CrossRef]
- Sheikh, A.B.; Pal, S.; Javed, N.; Shekhar, R. COVID-19 Vaccination in Developing Nations: Challenges and Opportunities for Innovation. Infect. Dis. Rep. 2021, 13, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K. The Global Vaccine Imbalance. 2021. Available online: https://www.statista.com/chart/26055/share-of-vaccine-doses-covid-19-share-of-world-population/ (accessed on 19 January 2024).
- Erondu, N.A.; Mofokeng, T.; Kavanagh, M.M.; Matache, M.; Bosha, S.L. Towards anti-racist policies and strategies to reduce poor health outcomes in racialised communities: Introducing the O’Neill–Lancet Commission on Racism, Structural Discrimination, and Global Health. Lancet 2023, 401, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Zero Draft of the WHO CA+ for the Consideration of the Intergovernmental Negotiating Body at Its Fourth Meeting; WHO: Geneva, Switzerland, 1 February 2023.
- World Health Organization. International Health Regulations (2005), 3rd ed.; WHO: Geneva, Switzerland, 2016.
- Boyce, M.R.; Sorrell, E.M.; Standley, C.J. An early analysis of the World Bank’s Pandemic Fund: A new fund for pandemic prevention, preparedness and response. BMJ Glob. Health 2023, 8, e011172. [Google Scholar] [CrossRef]
- Fan, V.; Smitham, E.; Regan, L. The Pandemic Fund Should Invest in Proven Surveillance Approaches 2023. Available online: https://www.cgdev.org/blog/pandemic-fund-should-invest-proven-surveillance-approaches#:~:text=Similarly%2C%20the%20Pandemic%20Fund%20would,surveillance%20as%20the%20broader%20concept (accessed on 30 July 2022).
- The Pandemic Fund. The Pandemic Fund: First Call for Proposals (CfP); The Pandemic Fund: Washington, DC, USA, 2023. [Google Scholar]
- The New Pandemic Fund: Overview and Key Issues for the U.S.; KFF Health News: Washington, DC, USA, 2023; press release.
- Merelli, A. The WHO Pandemic Accord is Shaping Up to Be All but Meaningless. 2023. Available online: https://qz.com/un-pandemic-accord-equity-1850548356 (accessed on 20 January 2024).
- Agarwal, R.; Gopinath, M.G. A Proposal to End the COVID-19 Pandemic; International Monetary Fund: Washington, DC, USA, 2021. [Google Scholar]
- WHO: Developing Countries Call for Legal Obligations on Equity in Pandemic Instrument; TWN Info Service on Health Issues; WHO: Geneva, Switzerland, 2023.
- Global Alliance for Vaccines and Immunization. COVAX Facility 2020. Available online: https://www.gavi.org/covax-facility (accessed on 30 August 2022).
- Global Alliance for Vaccines and Immunization. Co-Financing Policy 2023. 2023. Available online: https://www.gavi.org/programmes-impact/programmatic-policies/co-financing-policy (accessed on 30 August 2022).
- Global Alliance for Vaccines and Immunization. Evaluation of the Gavi Supply and Procurement Strategy, 2016–2020; Global Alliance for Vaccines and Immunization: Geneva, Switzerland, 2022. [Google Scholar]
- UNICEF. The African Union’s African Vaccine Acquisition Trust (AVAT) Initiative. 2021. Available online: https://www.unicef.org/supply/african-unions-african-vaccine-acquisition-trust-avat-initiative (accessed on 30 August 2022).
- AVAT to Partner with UNICEF in Scaling Up Vaccine Deliveries to African Union Member States; UNICEF: New York, NY, USA, 2021; press release.
- Massinga Loembé, M.; Nkengasong, J.N. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity 2021, 54, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- ElAteek, A.; Heikal, S.A.; Rozanova, L.; Flahault, A. Between Ambitious Strategies and Reality: The African Union Strategy on COVID-19 Vaccine. Epidemiologia 2021, 2, 621–638. [Google Scholar] [CrossRef]
- Lawal, L.; Bello, M.A.; Murwira, T.; Avoka, C.; Ma’Aruf, S.Y.; Omonhinmin, I.H.; Maluleke, P.; Tsagkaris, C.; Onyeaka, H. Low coverage of COVID-19 vaccines in Africa: Current evidence and the way forward. Hum. Vaccines Immunother. 2022, 18, 2034457. [Google Scholar] [CrossRef]
- World Health Organization. Africa Faces 470 Million COVID-19 Vaccine Shortfall in 2021; WHO: Geneva, Switzerland, 2021.
- Goldhill, O. We Have Enough COVID Vaccines for Most of the World. But Rich Countries Are Stockpiling More Than They Need for Boosters. STAT: Reporting from the Frontiers of Health and Medicine. 2021. Available online: https://www.statnews.com/2021/12/13/we-have-enough-covid-vaccines-for-most-of-world-but-rich-countries-stockpiling-more-than-they-need/ (accessed on 20 January 2024).
- The Economic Times. India Pulled Back Global Supply of COVAX Due to Surge of Cases Inside the Country: USAID Administrator; The Economic Times. 2021. Available online: https://economictimes.indiatimes.com/news/international/world-news/india-pulled-back-global-supply-of-covax-due-to-surge-of-cases-inside-the-country-usaid-administrator/articleshow/84453939.cms?from=mdr (accessed on 15 January 2024).
- The World Bank. New World Bank Country Classifications by Income Level: 2022–2023; The World Bank: Washington, DC, USA, 2022. [Google Scholar]
- Yoo, K.; Mehta, A.; Mak, J.; Bishai, D.; Chansa, C.; Patenaude, B. COVAX and equitable access to COVID-19 vaccines. Bull World Health Organ. 2022, 100, 315–328. [Google Scholar] [CrossRef]
- Achieving 70% COVID-19 Immunization Coverage by Mid-2022; WHO: Geneva, Switzerland, 2021; press release.
- UNICEF. COVID-19 Market Dashboard; UNICEF. 2022. Available online: https://www.unicef.org/supply/covid-19-market-dashboard (accessed on 30 August 2022).
- Medicines Patent Pool. WHO and MPP Announce Names of 15 Manufactures to Receive Training from mRNA Technology Transfer Hub; Medicines Patent Pool: Geneva, Switzerland, 2022; press release. [Google Scholar]
- BioNTech Starts Construction of First mRNA Vaccine Manufacturing Facility in Africa; BioNTech SE: Mainz, Germany, 2022; press release.
- Wadvalla, B.-A. How Africa has tackled COVID-19. BMJ 2020, 370, m2830. [Google Scholar] [CrossRef]
- Donnenfeld, Z. Africa Medical Supplies Platform: A Model for the World? Institute for Security Studies: Pretoria, South Africa, 13 May 2021. [Google Scholar]
- Gomez, P.L.; Robinson, J.M.; Rogalewicz, J.A. Vaccine manufacturing. Vaccines 2013, 44–57. [Google Scholar]
- Shah-Neville, W. Is Africa Ready to Become the Next Big Biopharma and Vaccine Manufacturing Destination? Available online: https://www.labiotech.eu/in-depth/africa-pharma-manufacturing/ (accessed on 10 January 2024).
- Petesch, C. BioNTech to Work with Senegal, Rwanda to Make mRNA Vaccines; The Associated Press: Dakar, Senegal, 2021. [Google Scholar]
- BioNTech. Update on First BioNTainer for African-Based mRNA Manufacturing Facility; BioNTech: Mainz, Germany, 2022. [Google Scholar]
- Louden, E.M. Scaling Up the Global COVID-19 Vaccination Program: Production, Allocation, and Distribution with an Emphasis on Equity. Yale J. Biol. Med. 2022, 95, 379–387. [Google Scholar] [PubMed]
- Pascolo, S. Messenger RNA: The inexpensive biopharmaceutical. J. Multidiscip. Eng. Sci. Technol. 2017, 4, 6937–6941. [Google Scholar]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Fisher, W.; Okediji, R.L.; Sampath, P.G. Fostering Production of Pharmaceutical Products in Developing Countries. Mich. J. Int. Law 2022, 43, 1. [Google Scholar] [CrossRef]
- Ekström, A.M.; Tomson, G.; Wanyenze, R.; Bhutta, Z.; Kyobutungi, C.; Binagwaho, A.; Ottersen, O.P. Addressing production gaps for vaccines in African countries. Bull. World Health Organ. 2021, 99, 910–912. [Google Scholar] [CrossRef] [PubMed]
- Rigby, J. New $1 Billion Plan for African Vaccine Manufacturing -GAVI Alliance; Reuters: London, UK, 2023. [Google Scholar]
- Reuters Staff. Morocco’s Sothema to Produce China’s Sinopharm Vaccine; Reuters: Rabat, Morocco, 2021. [Google Scholar]
- Reuters Staff. Morocco Gets Half Million Doses of Sinopharm COVID-19 Vaccine; Reuters: Rabat, Morocco, 2021. [Google Scholar]
- Reuters Staff. Morocco Gets 2 Million AstraZeneca Vaccine Doses, First Big Shipment to Africa; Reuters: Rabat, Morocco, 2021. [Google Scholar]
- Reuters Staff. Egypt to Purchase 20 Million Doses of Sinopharm Vaccine; Reuters: Cairo, Egypt, 2021. [Google Scholar]
- Aspen Welcomes President Ramaphosa and His Delegation to Aspen’s World Class Sterile Manufacturing Facility; Aspen Pharmacare: Gqeberha, South Africa, 2021; press release.
- Fahrni, M.L.; Ismail, I.A.-N.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.-U. Management of COVID-19 vaccines cold chain logistics: A scoping review. J. Pharm. Policy Pract. 2022, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Olofinsao, O.A.; Osmond, E.; Batubo, A.P.; Fayemiro, O.; Sherifdeen, O.; Muraina, L.; Obadawo, B.S.; Ahmad, S.; Fajemisin, E.A. Equal access of COVID-19 vaccine distribution in Africa: Challenges and way forward. J. Med. Virol. 2021, 93, 5212–5215. [Google Scholar] [CrossRef] [PubMed]
- Privor-Dumm, L.; Excler, J.-L.; Gilbert, S.; Karim, S.S.A.; Hotez, P.J.; Thompson, D.; Kim, J.H. Vaccine access, equity and justice: COVID-19 vaccines and vaccination. BMJ Glob. Health 2023, 8, e011881. [Google Scholar] [CrossRef]
- Fang, Y.; Ma, T.; Wu, M.; Tsuei, S.H.-T. Mitigating donor interests in the case of COVID-19 vaccine: The implication of COVAX and DAC membership. BMJ Glob. Health 2023, 8, e010188. [Google Scholar] [CrossRef]
- Blanchet, K.; Mallard, G.; Moret, E.; Sun, J. Sanctioned countries in the global COVID-19 vaccination campaign: The forgotten 70. Confl. Health 2021, 15, 69. [Google Scholar] [CrossRef]
- de Bengy Puyvallée, A.; Storeng, K.T. COVAX, vaccine donations and the politics of global vaccine inequity. Glob. Health 2022, 18, 1–14. [Google Scholar] [CrossRef]
- Zhang, D.; Jamali, A.B. China’s “Weaponized” Vaccine: Intertwining Between International and Domestic Politics. East Asia 2022, 39, 279–296. [Google Scholar] [CrossRef]
- Ekwebelem, O.C.; Tamasiga, P.; Aborode, A.T.; Yunusa, I.; Nwauzoma, U.; Onyeaka, H. COVID-19 vaccination in Africa: A case of unsatisfied expectation and ill-preparedness. Vaccine X 2022, 12, 100234. [Google Scholar] [CrossRef]
- Makinen, M.; Kaddar, M.; Molldrem, V.; Wilson, L. New vaccine adoption in lower-middle-income countries. Health Policy Plan. 2012, 27 (Suppl. S2), ii39–ii49. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.; MacGregor, H.; Akello, G.; Babawo, L.; Baluku, M.; Desclaux, A.; Grant, C.; Kamara, F.; Nyakoi, M.; Parker, M.; et al. Vaccine anxieties, vaccine preparedness: Perspectives from Africa in a COVID-19 era. Soc. Sci. Med. 2022, 298, 114826. [Google Scholar] [CrossRef]
- Abubakari, S.W.; Workneh, F.; Asante, K.P.; Hemler, E.C.; Madzorera, I.; Wang, D.; Ismail, A.; Assefa, N.; Azemraw, T.; Lankoande, B.; et al. Determinants of COVID-19 vaccine readiness and hesitancy among adults in sub-Saharan Africa. PLOS Glob. Public Health 2023, 3, e0000713. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.E.; Dieleman, J.L.; Lim, S.S.; Shearer, J. Determinants of effective vaccine coverage in low and middle-income countries: A systematic review and interpretive synthesis. BMC Health Serv. Res. 2017, 17, 681. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hara, G.L.; Halabi, S.; Egbokhare, O. Equitable Vaccine Access in Light of COVID-19 Vaccine Procurement Strategies in Africa. COVID 2024, 4, 276-288. https://doi.org/10.3390/covid4020019
O’Hara GL, Halabi S, Egbokhare O. Equitable Vaccine Access in Light of COVID-19 Vaccine Procurement Strategies in Africa. COVID. 2024; 4(2):276-288. https://doi.org/10.3390/covid4020019
Chicago/Turabian StyleO’Hara, George L., Sam Halabi, and Olohikhuae Egbokhare. 2024. "Equitable Vaccine Access in Light of COVID-19 Vaccine Procurement Strategies in Africa" COVID 4, no. 2: 276-288. https://doi.org/10.3390/covid4020019
APA StyleO’Hara, G. L., Halabi, S., & Egbokhare, O. (2024). Equitable Vaccine Access in Light of COVID-19 Vaccine Procurement Strategies in Africa. COVID, 4(2), 276-288. https://doi.org/10.3390/covid4020019





