Favipiravir for COVID-19 Pneumonia: Effectiveness, Safety, and Clinical Outcomes: A Retrospective Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gralinski, L.E.; Menachery, V.D. Return of the Coronavirus: 2019-nCoV. Viruses 2020, 12, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, C.; Shih, T.; Ko, W.; Tang, H.; Hsueh, P. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Saudi Ministry of Health. MOH Reports First Case of Coronavirus Infection. 2021. Available online: https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2020-03-02-002.aspx (accessed on 2 January 2021).
- Barry, M.; Ghonem, L.; Alsharidi, A.; Alanazi, A.; Alotaibi, N.H.; Al-Shahrani, F.S.; Majid, F.; BaHammam, A. Coronavirus disease-2019 pandemic in the Kingdom of Saudi Arabia: Mitigation measures and hospital preparedness. J. Nat. Sci. Med. 2020, 3, 155. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Qomara, W.F.; Primanissa, D.N.; Amalia, S.H.; Purwadi, F.V.; Zakiyah, N. Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review. Int. J. Gen. Med. 2021, 14, 8557–8571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, S.; Vora, A.; Venugopal, K.; Dadhich, P.; Daxini, A.; Bhagat, S.; Patil, S.; Barkate, H. Real-World Experience with Favipiravir for the Treatment of Mild-to-Moderate COVID-19 in India. Pragmat. Obs. Res. 2022, 13, 33–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lowe, D.M.; Brown, L.K.; Chowdhury, K.; Davey, S.; Yee, P.; Ikeji, F.; Ndoutoumou, A.; Shah, D.; Lennon, A.; Rai, A.; et al. FLARE Investigators. Favipiravir, lopinavir-ritonavir, or combination therapy (FLARE): A randomised, double-blind, 2 × 2 factorial placebo-controlled trial of early antiviral therapy in COVID-19. PLoS Med. 2022, 19, e1004120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mallah, S.I.; Ghorab, O.K.; Al-Salmi, S.; Abdellatif, O.S.; Tharmaratnam, T.; Iskandar, M.A.; Sefen, J.A.N.; Sidhu, P.; Atallah, B.; El-Lababidi, R.; et al. COVID-19: Breaking down a global health crisis. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 35. [Google Scholar] [CrossRef]
- Hung, D.T.; Ghula, S.; Aziz, J.M.A.; Makram, A.M.; Tawfik, G.M.; Abozaid, A.A.-F.; Pancharatnam, R.A.; Ibrahim, A.M.; Shabouk, M.B.; Turnage, M.; et al. The efficacy and adverse effects of favipiravir on patients with COVID-19: A systematic review and meta-analysis of published clinical trials and observational studies. Int. J. Infect. Dis. 2022, 120, 217–227. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020, 80, e14–e18. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Pisaturo, M.; Zollo, V.; Martini, S.; Maggi, P.; Numis, F.G.; Gentile, I.; Sangiovanni, N.; Rossomando, A.M.; Bianco, V.; et al. Obesity as a Risk Factor of Severe Outcome of COVID-19: A Pair-Matched 1:2 Case-Control Study. J. Clin. Med. 2023, 12, 4055. [Google Scholar] [CrossRef] [PubMed]
- Abid, A.; Umar, A.; Qamar, S. Disease Outcomes of COVID-19 in Diabetic and Hypertensive Patients During the Hospital Stay. Cureus 2023, 15, e46943. [Google Scholar] [CrossRef] [PubMed]
- Tavakolifard, N.; Moeini, M.; Haddadpoor, A.; Heidari, K.; Rezaee, M.; Amini, Z. Clinical Symptoms of COVID-19 and Their Association with Disease Outcome. Adv. Biomed. Res. 2022, 11, 2. [Google Scholar] [CrossRef]
- Fernandes, M.; Brábek, J. COVID-19, corticosteroids and public health: A reappraisal. Public Health 2021, 197, 48–55. [Google Scholar] [CrossRef]
- Trofin, F.; Nastase, E.V.; Roșu, M.F.; Bădescu, A.C.; Buzilă, E.R.; Miftode, E.G.; Manciuc, D.C.; Dorneanu, O.S. Inflammatory Response in COVID-19 Depending on the Severity of the Disease and the Vaccination Status. Int. J. Mol. Sci. 2023, 24, 8550. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yin, C.H.; Yao, Y.M. Update Advances on C-Reactive Protein in COVID-19 and Other Viral Infections. Front. Immunol. 2021, 12, 720363. [Google Scholar] [CrossRef]
- Rajendran, V.; Gopalan, S.; Varadaraj, P.; Pandurangan, V.; Marappa, L.; Nair, A.M.; Madhavan, S.; Mani, R.; Bhaskar, E. Course of COVID-19 Based on Admission D-Dimer Levels and Its Influence on Thrombosis and Mortality. J. Clin. Med. Res. 2021, 13, 403–408. [Google Scholar] [CrossRef]
- Nakayama, R.; Bunya, N.; Tagami, T.; Hayakawa, M.; Yamakawa, K.; Endo, A.; Ogura, T.; Hirayama, A.; Yasunaga, H.; Uemura, S.; et al. Associated organs and system with COVID-19 death with information of organ support: A multicenter observational study. BMC Infect. Dis. 2023, 23, 814. [Google Scholar] [CrossRef]
- Karimi Shahri, M.; Niazkar, H.R.; Rad, F. COVID-19 and hematology findings based on the current evidences: A puzzle with many missing pieces. Int. J. Lab. Hematol. 2021, 43, 160–168. [Google Scholar] [CrossRef]
- Li, G.; Xu, F.; Yin, X.; Wu, N.; Li, Y.; Zhang, T.; Chen, D.; Liu, K.; Qiu, Q. Lactic dehydrogenase-lymphocyte ratio for predicting prognosis of severe COVID-19. Medicine 2021, 100, e24441. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.Y. Mortality Rate of Patients with COVID-19 Based on Underlying Health Conditions. Disaster Med. Public Health Prep. 2021, 16, 2480–2485. [Google Scholar] [CrossRef] [PubMed]
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-De-Hoyo, R. The efficacy and safety of Favipiravir in treatment of COVID-19: A systematic review and meta-analysis of clinical trials. Sci. Rep. 2021, 11, 11022. [Google Scholar] [CrossRef] [PubMed]


| Frequency, N (%) | ||
|---|---|---|
| Gender | Female | 129 (43.4%) |
| Male | 165 (55.6%) | |
| Age | Mean (SD) | 61.47 (16.8) |
| Range | 23–106 | |
| BMI (Kg/m2) | Mean (SD) | 31.15 (7.4) |
| Range | 20.7–56.0 | |
| Smoking History | No | 277 (93.3%) |
| Yes | 6 (2.0%) | |
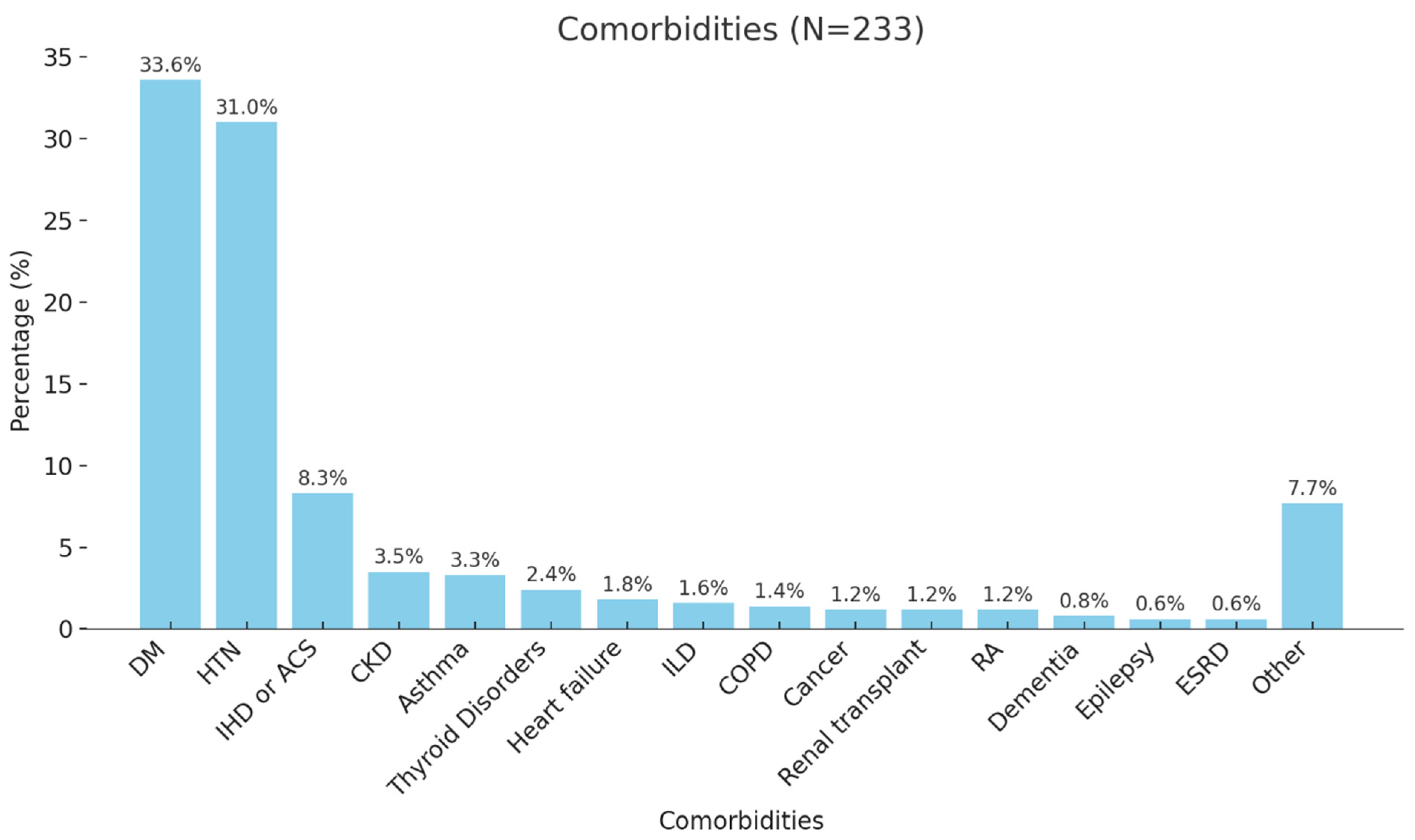
| Comorbidities | No | 73 (24.6%) |
| Yes | 223 (75.1%) | |
| Non-COVID-19-related | ACEI/ARB | 23 (7.7%) |
| Anticoagulants | 77 (25.9%) | |
| Antidiabetics | 137 (46.1%) | |
| Antiplatelets | 2 (0.7%) | |
| Betablockers | 11 (3.7%) | |
| CCB | 7 (2.4%) | |
| Diuretics | 4 (1.3%) | |
| Statins | 14 (4.7%) | |
| Thyroxin Drugs | 2 (0.7%) | |
| Days of Favipiravir Use | Mean (SD) | 8.3 (21.6) |
| Antibiotics | Carbapenems | 16 (5.4%) |
| Cephalosporin | 104 (35.0%) | |
| Fluoroquinolones | 22 (7.4%) | |
| Macrolides | 129 (43.4%) | |
| Penicillins | 3 (1.0%) | |
| Tetracyclines | 13 (4.4%) | |
| Corticosteroids | Long-acting | 224 (75.4%) |
| Short-acting | 11 (3.7%) | |
| Mean (SD) | Range (Min–Max) | |
|---|---|---|
| CBC | ||
| Hemoglobin (Hb) (g/dL) | 14.29 (6.17) | 7.50–112.00 |
| White blood cell (WBC) count (109/L) | 6.51 (3.37) | 1.90–22.78 |
| Platelet count (109/L) | 219.96 (82.67) | 52.00–656.00 |
| Erythrocyte sedimentation rate (ESR) (mm/h) | 70.31 (29.86) | 6.00–120.00 |
| LFTs | ||
| Alanine aminotransferase (ALT) (U/L) | 32.58 (22.82) | 6.00–160.00 |
| Aspartate aminotransferase (AST) (U/L) | 42.89 (25.49) | 13.00–204.00 |
| Alkaline phosphatase(ALP) (U/L) | 70.42 (35.08) | 28.0–263.0 |
| Gamma-glutamyl transferase (GGT) (U/L) | 51.29 (59.36) | 8.0–516.0 |
| Total bilirubin (µmol/L) | 14.46 (18.20) | 0.4–292.3 |
| Direct bilirubin (µmol/L) | 4.44 (10.41) | 0.1–170.1 |
| RFTs | ||
| Creatinine (µmol/L) | 100.16 (63.26) | 37–581 |
| Urea (mmol/L) | 7.32 (5.06) | 2–36 |
| Serum Profile | ||
| Sodium (mmol/L) | 133.57 (4.67) | 116.00–161.00 |
| Potassium (mmol/L) | 4.21 (0.58) | 3–7 |
| Corrected calcium (mmol/L) | 3.11 (13.96) | 1.89–232 |
| Phosphorus (mmol/L) | 1.22 (3.20) | 0.49–53 |
| Magnesium (mmol/L) | 0.78 (0.10) | 0.52–1.26 |
| Other | ||
| D-Dimer (mg/L) | 3.18 (27.42) | 0.19–455.00 |
| Ferritin (ng/mL) | 439.41 (598.67) | 6.90–4454.00 |
| C-reactive protein (CRP)(mg/L) | 72.11 (55.62) | 5.0–345.2 |
| Lactic acid dehydrogenase (LDH)(U/L) | 302.63 (137.85) | 98.00–1411.00 |
| FiO2 | 80.25 (18.66) | 0–100 |
| Hematocrit (%) | 42.82 (6.17) | 23.2–59.5 |
| Temperature (°C) | 38.75 (21.05) | 23.9–373.0 |
| Heart rate | 96.46 (17.35) | 54.0–196.0 |
| Respiratory rate | 20.51 (1.45) | 17.0–28.0 |
| Mean arterial pressure (MAP) | 88.50 (11.05) | 64.0–118.0 |
| Frequency N (%) | ||
|---|---|---|
| Microbiology and Imaging | ||
| Blood Culture | Negative | 200 (67.3%) |
| Positive | 26 (8.8%) | |
| Common Pathogen Class (Staphylococcus) | 13 (4.4%) | |
| Urine Culture | Negative | 168 (56.6%) |
| Positive | 15 (5.1%) | |
| Commonly Mixed Growth | 5 (1.5%) | |
| Sputum Culture | Negative | 112 (37.7%) |
| Positive (WBCs and Mixed Bacteria) | 8 (2.7%) | |
| Tracheal Culture | Negative | 84 (28.3%) |
| Positive (WBCs and Mixed Bacteria) | 9 (3.0%) | |
| CT Findings | No CT Performed | 232 (78.1%) |
| Normal | 10 (3.4%) | |
| Consolidation | 27 (9.1%) | |
| Ground Glass | 15 (5.1%) | |
| Pleural Effusion | 6 (2.0%) | |
| Other | 4 (1.3%) | |
| Length of Stay | ||
| Length of Hospital Stay (Days) | Mean (SD) | 6.17 (4.89) |
| Range | 1–20 | |
| Length of ICU Stay (Days) | Mean (SD) | 22.00 (37.52) |
| Range | 3–89 | |
| Outcome and Complications | ||
| Needed Intubation | No | 220 (74.1%) |
| Yes | 65 (21.9%) | |
| Acute respiratory distress syndrome (ARDS) | No | 241 (81.1%) |
| Yes | 40 (13.5%) | |
| Complication | No Complications | 244 (82.2%) |
| ACS | 3 (1.0%) | |
| AKI | 15 (5.1%) | |
| ARDS | 4 (1.3%) | |
| Cardiac Arrest | 8 (2.7%) | |
| CVA | 9 (3.0%) | |
| Liver Failure/Heart Failure | 5 (1.7%) | |
| Pulmonary Embolism (PE) | 9 (3.0%) | |
| Survival | Dead | 62 (20.9%) |
| Alive | 232 (78.1%) | |
| Readmission | Within 15 Days | 96 (32.3%) |
| Within 30 Days | 9 (3.0%) | |
| Within 60 Days | 14 (4.7%) | |
| Within 90 Days | 4 (1.3%) | |
| Overall Outcome/Survival | Sig. Value | |||
|---|---|---|---|---|
| Dead, N (%) | Alive, N (%) | |||
| Gender | Female | 24 (18.8%) | 104 (81.3%) | 0.374 a |
| Male | 38 (23.0%) | 127 (77.0%) | ||
| Age (Year) | Mean (SD) | 70.82 (16.62) | 58.87 (16.02) | <0.001 c |
| BMI (Kg/m2) | Mean (SD) | 29.38 (9.08) | 31.60 (7.15) | 0.273 c |
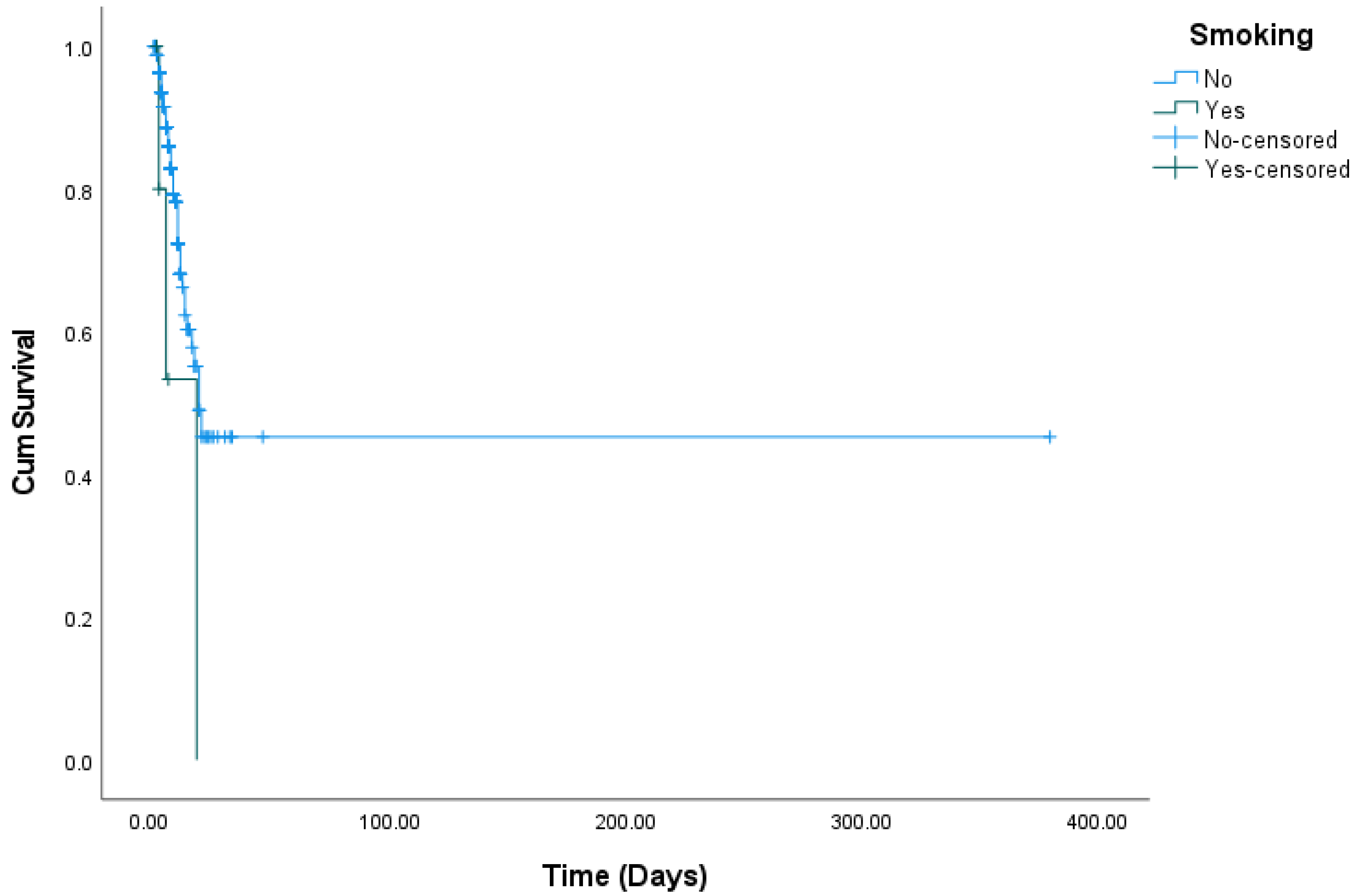
| Smoking | No | 56 (20.3%) | 220 (79.7%) | 0.108 b |
| Yes | 3 (50.0%) | 3 (50.0%) | ||
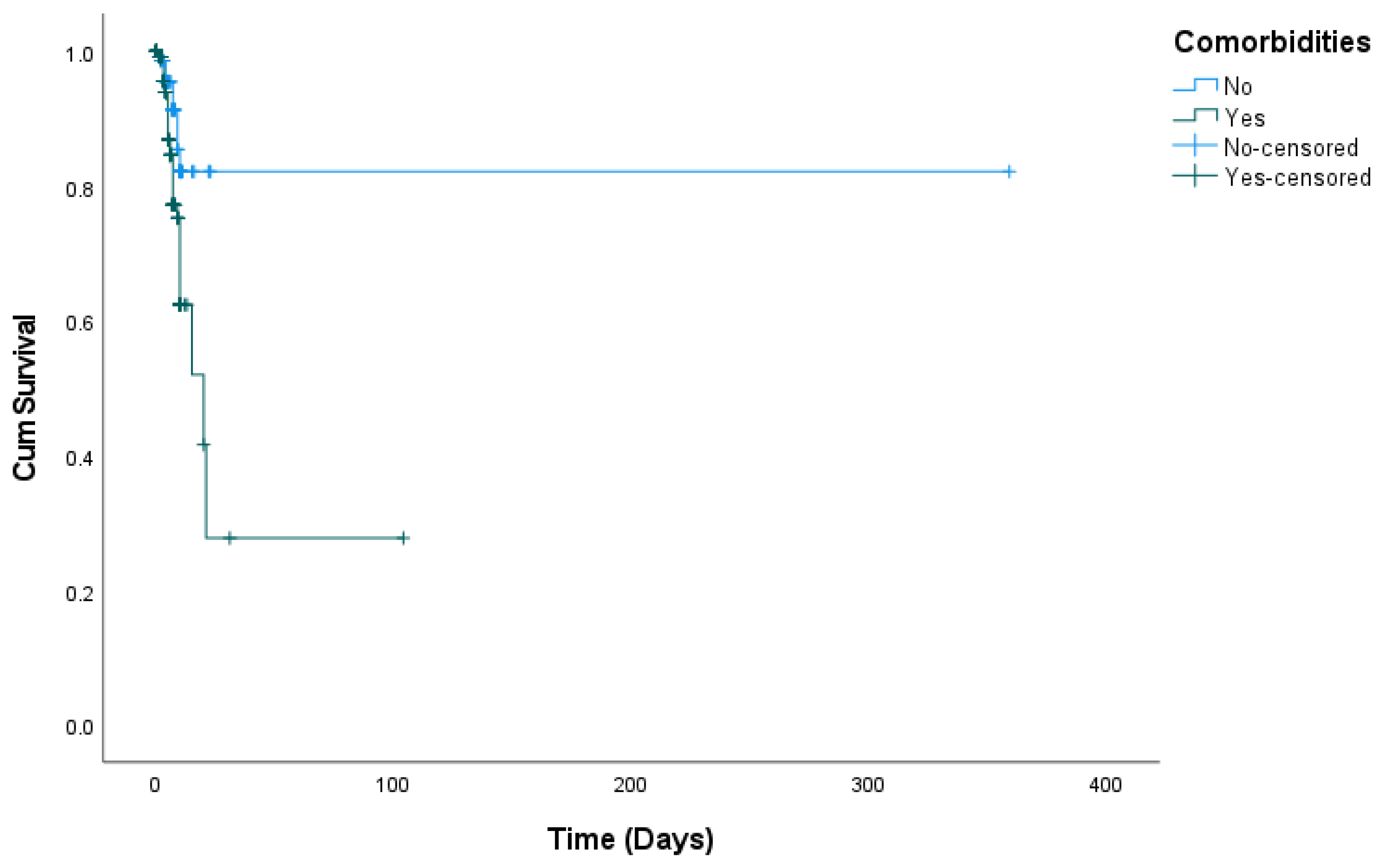
| Comorbidities | No | 8 (11.1%) | 64 (88.9%) | 0.017 a |
| Yes | 54 (24.3%) | 168 (75.7%) | ||
| Complications | No | 31 (12.9%) | 210 (87.1%) | <0.001 a |
| Yes | 31 (58.5%) | 22 (41.5%) | ||
| Blood Culture | Negative | 45 (22.5%) | 155 (77.5%) | 0.173 a |
| Positive | 9 (34.6%) | 17 (65.4%) | ||
| Urine Culture | Negative | 41 (24.4%) | 127 (75.6%) | 0.534 a |
| Positive | 5 (33.3%) | 10 (66.7%) | ||
| Sputum Culture | Negative | 18 (16.1%) | 94 (83.9%) | 0.006 b |
| Positive | 5 (62.5%) | 3 (37.5%) | ||
| Tracheal Culture | Negative | 16 (19.0%) | 68 (81.0%) | <0.001 b |
| Positive | 8 (88.9%) | 1 (11.1%) | ||
| Readmission | Within 15 Days | 2 (2.1%) | 94 (97.9%) | 0.028 b |
| Within 30 Days | 1 (11.1%) | 8 (88.9%) | ||
| Within 60 Days | 2 (14.3%) | 12 (85.7%) | ||
| Within 90 Days | 1 (25.0%) | 3 (75.0%) | ||
| Alive, Mean (SD) | Dead, Mean (SD) | |||
| Length of Hospital Stay (Days) | 9.33 (9.7) | 5.0 (3.3) | 0.182 | |
| Length of ICU Stay (Days) | 3.0 (-) | 5.50 (3.5) | 0.333 | |
| Laboratory Parameters | ||||
| Hemoglobin (Hb) (g/dl) | 13.40 (2.18) | 14.53 (6.83) | 0.207 | |
| White Blood Cells (WBCs) (109/L) | 7.91 (4.10) | 6.15 (3.05) | <0.001 | |
| Platelets (109/L) | 226.23 (77.03) | 218.29 (84.19) | 0.506 | |
| Erythrocyte Sedimentation Rate (ESR) (mmh/h) | 76.19 (31.68) | 68.91 (29.36) | 0.217 | |
| D-Dimer (mg/L) | 3.18 (7.53) | 3.18 (30.45) | 0.999 | |
| Lactic Acid Dehydrogenase (LDH) (U/L) | 346.30 (203.06) | 291.10 (112.51) | 0.007 | |
| Aspartate Aminotransferase (AST) (U/L) | 50.49 (36.16) | 40.91 (21.55) | 0.010 | |
| Creatinine (µmol/L) | 114.28 (72.36) | 96.45 (60.27) | 0.052 | |
| Urea (mmol/L) | 8.85 (5.46) | 6.93 (4.88) | 0.009 | |
| C-Reactive Protein (CRP) (mg/l) | 88.50 (60.19) | 67.77 (53.66) | 0.011 | |
| Alkaline Phosphatase (ALP) (U/L) | 86.93 (48.87) | 66.13 (29.15) | <0.001 | |
| Gamma-Glutamyl Transferase (GGT) (U/L) | 72.58 (102.13) | 45.85 (40.64) | 0.002 | |
| Total Bilirubin (µmol/L) | 13.44 (7.43) | 14.73 (20.10) | 0.628 | |
| FiO2 | 73.80 (20.50) | 83.35 (17.06) | 0.035 | |
| Vital Parameters | ||||
| Temperature, “°C” | 37.29 (0.78) | 39.04 (23.06) | 0.620 | |
| Heart Rate | 98.49 (17.22) | 96.04 (17.38) | 0.400 | |
| Respiratory Rate | 20.39 (1.64) | 20.53 (1.42) | 0.572 | |
| Mean Arterial Pressure (MAP) | 85.67 (10.36) | 88.90 (11.14) | 0.346 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, S.; Alqahtani, M.; Amer, K.; Ur Rahman, F.; AlMasoudi, R.; Al-Otaibi, S.; Alahmary, B.; Asiri, O.; Alshamrani, A.; Alshehri, R.; et al. Favipiravir for COVID-19 Pneumonia: Effectiveness, Safety, and Clinical Outcomes: A Retrospective Single-Center Experience. COVID 2024, 4, 1971-1984. https://doi.org/10.3390/covid4120139
Alqahtani S, Alqahtani M, Amer K, Ur Rahman F, AlMasoudi R, Al-Otaibi S, Alahmary B, Asiri O, Alshamrani A, Alshehri R, et al. Favipiravir for COVID-19 Pneumonia: Effectiveness, Safety, and Clinical Outcomes: A Retrospective Single-Center Experience. COVID. 2024; 4(12):1971-1984. https://doi.org/10.3390/covid4120139
Chicago/Turabian StyleAlqahtani, Saad, Mushary Alqahtani, Khaled Amer, Fasih Ur Rahman, Razan AlMasoudi, Sahar Al-Otaibi, Batool Alahmary, Osama Asiri, Abdulaziz Alshamrani, Razan Alshehri, and et al. 2024. "Favipiravir for COVID-19 Pneumonia: Effectiveness, Safety, and Clinical Outcomes: A Retrospective Single-Center Experience" COVID 4, no. 12: 1971-1984. https://doi.org/10.3390/covid4120139
APA StyleAlqahtani, S., Alqahtani, M., Amer, K., Ur Rahman, F., AlMasoudi, R., Al-Otaibi, S., Alahmary, B., Asiri, O., Alshamrani, A., Alshehri, R., Asiri, F., Alqahtani, M., Alshahrani, A., & Elsharif, Y. (2024). Favipiravir for COVID-19 Pneumonia: Effectiveness, Safety, and Clinical Outcomes: A Retrospective Single-Center Experience. COVID, 4(12), 1971-1984. https://doi.org/10.3390/covid4120139






