Mental Health Symptom Reporting to a Virtual Triage Engine Prior to and During the COVID-19 Pandemic
Abstract
1. Introduction
2. Methods
2.1. Study Objectives
2.2. Study Design
2.3. Setting and Description of Intervention/Virtual Triage Engine Utilized
2.4. Sample Selection and Inclusion/Eligibility Criteria
2.5. Data Captured and Analyses Completed
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gender Group | Age Group | Language Group | Time Interval I Weight for Each Group | Time Interval II Weight for Each Group | Time Interval III Weight for Each Group |
|---|---|---|---|---|---|
| Female | 18–39 | English | 1.0 | 1.2 | 1.3 |
| Female | 40–59 | English | 1.0 | 1.3 | 1.4 |
| Female | 60+ | English | 1.0 | 1.3 | 1.3 |
| Male | 18–39 | English | 1.0 | 0.1 | 1.2 |
| Male | 40–59 | English | 1.0 | 1.0 | 1.4 |
| Male | 60+ | English | 1.0 | 0.1 | 1.6 |
| Female | 18–39 | Other language | 1.0 | 0.8 | 0.3 |
| Female | 40–59 | Other language | 1.0 | 0.7 | 0.2 |
| Female | 60+ | Other language | 1.0 | 0.7 | 0.3 |
| Male | 18–39 | Other language | 1.0 | 0.1 | 0.4 |
| Male | 40–59 | Other language | 1.0 | 0.9 | 0.4 |
| Male | 60+ | Other language | 1.0 | 0.9 | 0.5 |
| Female | 18–39 | Spanish | 1.0 | 0.1 | 0.1 |
| Female | 40–59 | Spanish | 1.0 | 0.2 | 0.2 |
| Female | 60+ | Spanish | 1.0 | 0.1 | 0.1 |
| Male | 18–39 | Spanish | 1.0 | 0.2 | 0.2 |
| Male | 40–59 | Spanish | 1.0 | 0.2 | 0.1 |
| Male | 60+ | Spanish | 1.0 | 0.2 | 0.2 |
| Female | 18–39 | Polish | 1.0 | 1.5 | 2.3 |
| Female | 40–59 | Polish | 1.0 | 1.5 | 1.9 |
| Female | 60+ | Polish | 1.0 | 1.5 | 2.3 |
| Male | 18–39 | Polish | 1.0 | 1.5 | 2.5 |
| Male | 40–59 | Polish | 1.0 | 1.4 | 2.0 |
| Male | 60+ | Polish | 1.0 | 1.4 | 2.6 |
| Female | 18–39 | German | 1.0 | 1.0 | 0.8 |
| Female | 40–59 | German | 1.0 | 0.9 | 0.6 |
| Female | 60+ | German | 1.0 | 1.0 | 0.6 |
| Male | 18–39 | German | 1.0 | 0.1 | 0.9 |
| Male | 40–59 | German | 1.0 | 0.9 | 0.6 |
| Male | 60+ | German | 1.0 | 1.0 | 0.7 |
| Mental Health Symptom | Pre-Pandemic Interval vs. COVID-19 Pre-Vaccine Interval | Pre-Pandemic Interval vs. COVID-19 Post-Vaccine Interval | COVID-19 Pre-Vaccine Interval vs. COVID-19 Post-Vaccine Interval |
|---|---|---|---|
| Anxiety | −22.5 | 6.6 | 36.0 |
| Sleep disorder | 72.8 | 74.9 | −7.9 |
| General anxiety | 1.3 | 33.3 | 35.6 |
| Nervousness or weepiness | −10.8 | 21.3 | 37.7 |
| Agitation | −6.5 | 7.6 | 16.8 |
| Insomnia | −18.8 | −16.5 | 4.9 |
| Irritability | 5.8 | −83.5 | −102.4 |
| Gastric symptoms, stress-related | −28.5 | −58.4 | −33.8 |
| Feeling of hopelessness | −46.4 | −21.7 | 35.8 |
| Fear of dying | −16.3 | −68.9 | −64.5 |
| Suicidal intent/thoughts | 47.6 | 62.8 | 6.8 |
References
- Tsamakis, K.; Tsiptsios, D.; Ouranidis, A.; Mueller, C.; Schizas, D.; Terniotis, C.; Nikolakakis, N.; Tyros, G.; Kympouropoulos, S.; Lazaris, A.; et al. COVID-19 and its consequences on mental health (review). Exp. Ther. Med. 2021, 21, 244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, J.; Lipsitz, O.; Nasri, F.; Lui, L.M.W.; Gill, H.; Phan, L.; Chen-Li, D.; Iacobucci, M.; Ho, R.; Majeed, A.; et al. Impact of COVID-19 pandemic on mental health in the general population: A systematic review. J. Affect. Disord. 2020, 277, 55–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agrawal, S.; Dayama, S.; Galhotra, A. COVID-19 mental health challenges: A scoping review. J. Educ. Health Promot. 2022, 11, 375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindert, J.; Jakubauskiene, M.; Bilsen, J. The COVID-19 disaster and mental health—Assessing, responding and recovering. Eur. J. Public Health 2021, 31, iv31–iv35. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Pandemic Triggers 25% Increase in Prevalence of Anxiety and Depression Worldwide. Available online: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide (accessed on 2 March 2022).
- Dragioti, E.; Li, H.; Tsitsas, G.; Lee, K.H.; Choi, J.; Kim, J.; Choi, Y.J.; Tsamakis, K.; Estradé, A.; Agorastos, A.; et al. A large-scale meta-analytic atlas of mental health problems prevalence during the COVID-19 early pandemic. J. Med. Virol. 2022, 94, 1935–1949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.X.; Miller, S.O.; Xu, W.; Yin, A.; Chen, B.Z.; Delios, A.; Dong, R.K.; Chen, R.Z.; McIntyre, R.S.; Wan, X.; et al. Meta-analytic evidence of depression and anxiety in Eastern Europe during the COVID-19 pandemic. Eur. J. Psychotraumatol. 2022, 13, 2000132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.X.; Batra, K.; Xu, W.; Liu, T.; Dong, R.K.; Yin, A.; Delios, A.Y.; Chen, B.Z.; Chen, R.Z.; Miller, S.; et al. Mental disorder symptoms during the COVID-19 pandemic in Latin America—A systematic review and meta-analysis. Epidemiol. Psychiatr. Sci. 2022, 31, e23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, Y.; Xu, E.; Al-Aly, Z. Risks of mental health outcomes in people with COVID-19: Cohort study. BMJ 2022, 76, e068993. [Google Scholar] [CrossRef]
- Janitra, F.E.; Jen, H.J.; Chu, H.; Chen, R.; Pien, L.C.; Liu, D.; Lai, Y.J.; Banda, K.J.; Lee, T.Y.; Lin, H.C.; et al. Global prevalence of low resilience among the general population and health professionals during the COVID-19 pandemic: A meta-analysis. J. Affect. Disord. 2023, 332, 29–46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawohl, W.; Nordt, C. COVID-19, unemployment, and suicide. Lancet Psychiatry 2020, 7, 389–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, P.J.; Pusica, Y.; Sohaei, D.; Prassas, I.; Diamandis, E.P. An overview of mental health during the COVID-19 pandemic. Diagnosis 2021, 8, 403–412. [Google Scholar] [CrossRef]
- World Health Organization. Older People and COVID-19. Available online: https://www.who.int/teams/social-determinants-of-health/demographic-change-and-healthy-ageing/covid-19 (accessed on 22 November 2020).
- Khademi, F.; Moayedi, S.; Golitaleb, M.; Karbalaie, N. The COVID-19 pandemic and death anxiety in the elderly. Int. J. Ment. Health Nurs. 2020, 30, 346–349. [Google Scholar] [CrossRef]
- Péterfi, A.; Mészáros, Á.; Szarvas, Z.; Pénzes, M.; Fekete MFehér, Á.; Lehoczki, A.; Csípő, T.; Fazekas-Pongo, V. Comorbidities and increased mortality of COVID-19 among the elderly: A systematic review. Physiol. Int. 2022, 109, 163–176. [Google Scholar] [CrossRef]
- Cocuzzo, B.; Wrench, A.; O’Malley, C. Effects of COVID-19 on older adults: Physical, mental, emotional, social, and financial problems seen and unseen. Cureus 2022, 14, e29493. [Google Scholar] [CrossRef]
- Harris, E. Most COVID-19 deaths worldwide were among older people. JAMA 2023, 329, 704. [Google Scholar] [CrossRef]
- Gerlach, L.B.; Solway, E.; Maust, D.T.; Kirch, M.; Kullgren, J.T.; Singer, D.C.; Malani, P.N. The COVID-19 pandemic and mental health symptoms among US adults. J. Gen. Intern. Med. 2021, 36, 3285–3288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaudhuri, K.; Howley, P. The impact of COVID-19 vaccination for mental well-being. Eur. Econ. Rev. 2022, 150, 104293. [Google Scholar] [CrossRef]
- Bilge, Y.; Keles, E.; Baydili, K.N. The impact of COVID-19 vaccination on mental health. J. Loss Trauma 2022, 27, 285–288. [Google Scholar] [CrossRef]
- Chen, S.; Aruldass, A.R.; Cardinal, R.N. Mental health outcomes after SARS-CoV-2 vaccination in the United States: A national cross-sectional study. J. Affect. Disord. 2022, 298, 396–399. [Google Scholar] [CrossRef]
- Gellert, G.A.; Garber, L.; Kabat-Karabon, A.; Kuszczyński, K.; Price, T.; Trybucka, K.; Nichols, M.W.; Pike, J.M.; Powers, M.J.; Markiewicz, N.; et al. Using AI-based virtual triage to improve acuity-level alignment of patient care seeking in an ambulatory care setting. Int. J. Healthc. 2024, 10, 41. [Google Scholar] [CrossRef]
- Gellert, G.A.; Almeida Carvalho, D.; Price, T.; Kabat-Karabon, A.; Galvão, P.; Gellert, G.L.; Orzechowski, P.M. Impact of integrated virtual and live nurse triage on patient care seeking and health care delivery effectiveness and efficiency. Telemed. Rep. 2024, 5, 330–338. [Google Scholar] [CrossRef]
- Gellert, G.A.; Kabat-Karabon, A.; Gellert, G.L.; Rasławska-Socha, J.; Gorski, S.; Price, T.; Kuszczyński, K.; Palczewski, M.; Jaszczak, J.; Orzechowski, P.M. The potential of virtual triage AI to improve early detection, care acuity alignment and emergent care referral of life-threatening conditions. Front. Public Health 2024, 12, 1362246. [Google Scholar] [CrossRef]
- Gellert, G.A.; Palczewski, M.; Marecka, M.; Paczkowska, K.; Suwińska, A.; Price, T.; Jaszczak, J.; Gellert, G.L.; Orzechowski, P.M.; Gorski, S. AI-based virtual triage detection of inappropriate care seeking intent among patients experiencing potentially life-threatening cardiac symptoms. Medinformatics 2024, 1, 176–183. [Google Scholar] [CrossRef]
- Lohr, S.L. Sampling: Design and Analysis, 2nd ed.; Brooks/Cole: Boston, MA, USA, 2010. [Google Scholar]
- Gellert, G.A.; Orzechowski, P.M.; Price, T.; Jaszczak, J.; Marcjasz, N.; Mlodawska, A.; Kwiecien, A.K.; Kurkiewicz, P. A multinational survey of patient utilization of and value conveyed through virtual symptom triage and healthcare referral. Front. Public Health 2023, 10, 1047291. [Google Scholar] [CrossRef]
- Du, M.; Liu, M.; Wang, Y.; Qin, C.; Liu, J. Global burden of sleep disturbances among older adults and the disparities by geographical regions and pandemic periods. SSM Popul. Health 2023, 25, 101588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.S.; Hoffman, Y.S.G.; Palgi, Y.; Shrira, A. COVID-19 related loneliness and sleep problems in older adults: Worries and resilience as potential moderators. Personal. Individ. Differ. 2021, 168, 110371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- American Psychological Association. Stress in America 2019; Stress in America: Stress and Current Events; American Psychological Association: Washington, DC, USA, 2019. [Google Scholar]
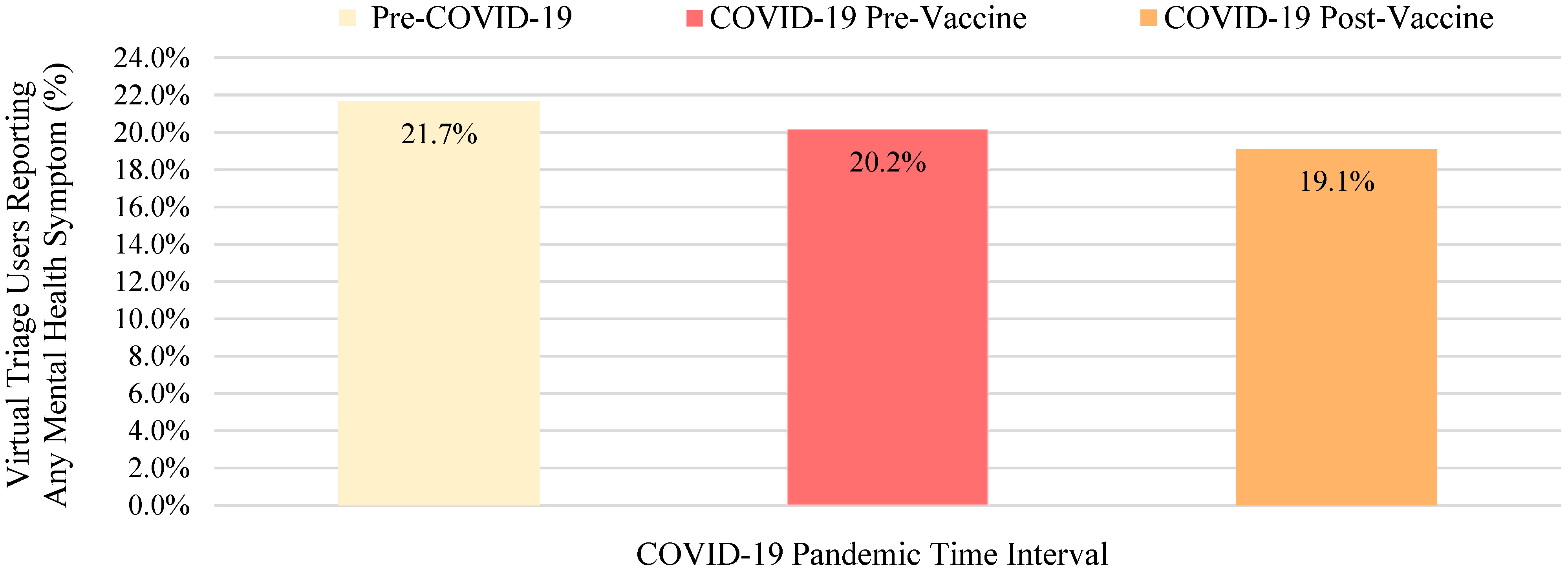
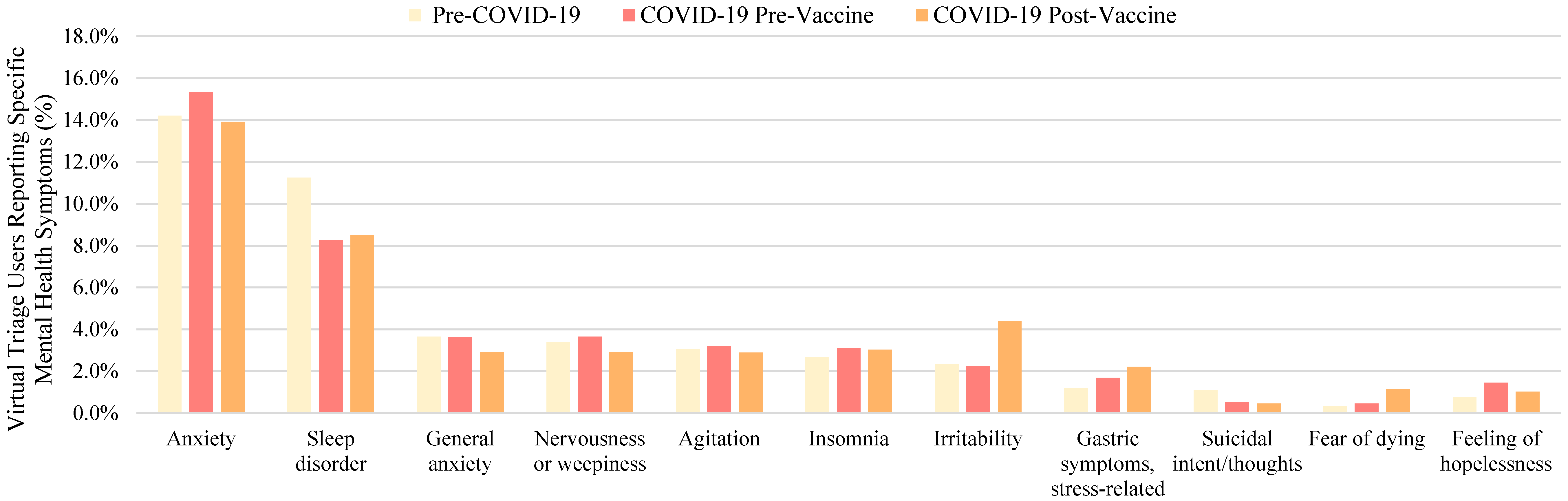
| Mental Health Symptom | Pre-Pandemic Interval (Percent Reported in Encounters During Time Interval I) | COVID-19 Pre-Vaccine Interval (Percent Reported in Encounters During Time Interval II) | COVID-19 Post-Vaccine Interval (Percent Reported in Encounters During Time Interval III) | Total Encounters in Which Symptom Was Reported (Percent Reported in All Encounters Across All Time Intervals) |
|---|---|---|---|---|
| Anxiety 1 | 122,760 (14.2%) | 187,900 (15.3%) | 314,176 (13.9%) | 624,836 (14.4%) |
| Sleep disorder 2 | 97,225 (11.3%) | 101,169 (8.3%) | 191,968 (8.5%) | 390,362 (9.0%) |
| Irritability | 20,351 (2.4%) | 27,363 (2.2%) | 98,876 (4.4%) | 146,590 (3.4%) |
| General anxiety 3 | 31,547 (3.7%) | 44,325 (3.6%) | 65,872 (2.9%) | 141,744 (3.3%) |
| Nervousness or weepiness | 29,112 (3.4%) | 44,719 (3.6%) | 65,647 (2.9%) | 139,477 (3.2%) |
| Agitation | 26,343 (3.0%) | 39,298 (3.2%) | 65,152 (2.9%) | 130,793 (3.0%) |
| Insomnia | 23,082 (2.7%) | 38,201 (3.1%) | 68,239 (3.0%) | 129,523 (3.0%) |
| Gastric symptoms, stress-related | 10,359 (1.2%) | 20,624 (1.7%) | 50,052 (2.2%) | 81,035 (1.9%) |
| Feeling of hopelessness | 6485 (0.8%) | 17,743 (1.4%) | 22,935 (1.0%) | 47,163 (1.1%) |
| Fear of dying 1 | 2691 (0.3%) | 5583 (0.5%) | 25,700 (1.1%) | 33,975 (0.8%) |
| Suicidal intent/thoughts | 9406 (1.1%) | 6269 (0.5%) | 10,357 (0.5%) | 26,032 (0.6%) |
| Total Encounters reporting 1+ mental health symptom | 187,365 (21.7%) | 247,160 (20.2%) | 431,693 (19.1%) | 866,218 (100.0%) |
| Mental Health Symptom | Pre-Pandemic Interval | COVID-19 Pre-Vaccine Interval | COVID-19 Post-Vaccine Interval | All Encounters and Intervals |
|---|---|---|---|---|
| Anxiety 1 | 65.5% | 76.0% | 72.8% | 72.1% |
| Sleep disorder 2 | 51.9% | 40.9% | 44.5% | 45.1% |
| General anxiety 3 | 16.8% | 17.9% | 15.3% | 16.4% |
| Nervousness or weepiness | 15.5% | 18.1% | 15.2% | 16.1% |
| Agitation | 14.1% | 15.9% | 15.1% | 15.1% |
| Insomnia | 12.3% | 15.5% | 15.8% | 15.0% |
| Irritability | 10.9% | 11.1% | 22.9% | 16.9% |
| Gastric symptoms, stress-related | 5.5% | 8.3% | 11.6% | 9.4% |
| Feeling of hopelessness | 3.5% | 7.2% | 5.3% | 5.4% |
| Fear of dying 1 | 1.4% | 2.3% | 6.0% | 3.9% |
| Suicidal intent/thoughts | 5.0% | 2.5% | 2.4% | 3.0% |
| Gender of Patient-User | Pre-COVID-19 Encounters | COVID-19 Pre-Vaccine Encounters | COVID-19 Post-Vaccine Encounters | Total Encounters |
|---|---|---|---|---|
| Gender | ||||
| Female | 603,635 (13.9%) | 856,072 (19.7%) | 1,577,157 (36.3%) | 3,036,864 (69.9%) |
| Male | 260,412 (6.0%) | 369,315 (8.5%) | 680,396 (15.7%) | 1,310,123 (30.1%) |
| Gender Totals 1 | 864,047 (19.9%) | 1,225,387 (28.2%) | 2,257,553 (51.9%) | 4,346,987 (100.0%) |
| Age | ||||
| 18–39 | 646,347 (14.9%) | 916,646 (21.1%) | 1,688,754 (38.8%) | 3,251,747 (74.8%) |
| 40–59 | 176,791 (4.1%) | 250,724 (5.8%) | 461,914 (10.6%) | 889,429 (20.5%) |
| 60+ | 40,909 (0.9%) | 58,017 (1.3%) | 106,886 (2.5%) | 205,812 (4.7%) |
| Age Totals 1 | 864,047 (19.9%) | 1,225,387 (28.2%) | 2,257,553 (51.9%) | 4,346,987 (100.0%) |
| Language of Patient-User | Pre-COVID-19 Encounters | Pre-Vaccine COVID-19 Pandemic Encounters | Post-Vaccine Encounters | Total Encounters |
|---|---|---|---|---|
| English | 644,006 (14.8%) | 913,326 (21.0%) | 1,682,637 (38.7%) | 3,239,969 (74.5%) |
| Polish | 103,460 (2.4%) | 146,726 (3.4%) | 270,317 (6.2%) | 520,503 (12.0%) |
| German | 52,014 (1.2%) | 73,766 (1.7%) | 135,900 (3.1%) | 261,680 (6.0%) |
| Spanish | 18,413 (0.4%) | 26,113 (0.6%) | 48,109 (1.1%) | 92,635 (2.1%) |
| Other languages | 46,154 (1.1%) | 65,455 (1.5%) | 120,590 (2.8%) | 232,199 (5.3%) |
| Total 1 | 864,047 (19.9%) | 1,225,387 (28.2%) | 2,257,553 (51.9%) | 4,346,987 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellert, G.A.; Kabat-Karabon, A.; Price, T.; Gellert, G.L.; Kuszczyński, K.; Nowak, M.; Orzechowski, P.M. Mental Health Symptom Reporting to a Virtual Triage Engine Prior to and During the COVID-19 Pandemic. COVID 2024, 4, 1908-1920. https://doi.org/10.3390/covid4120134
Gellert GA, Kabat-Karabon A, Price T, Gellert GL, Kuszczyński K, Nowak M, Orzechowski PM. Mental Health Symptom Reporting to a Virtual Triage Engine Prior to and During the COVID-19 Pandemic. COVID. 2024; 4(12):1908-1920. https://doi.org/10.3390/covid4120134
Chicago/Turabian StyleGellert, George A., Aleksandra Kabat-Karabon, Tim Price, Gabriel L. Gellert, Kacper Kuszczyński, Mateusz Nowak, and Piotr M. Orzechowski. 2024. "Mental Health Symptom Reporting to a Virtual Triage Engine Prior to and During the COVID-19 Pandemic" COVID 4, no. 12: 1908-1920. https://doi.org/10.3390/covid4120134
APA StyleGellert, G. A., Kabat-Karabon, A., Price, T., Gellert, G. L., Kuszczyński, K., Nowak, M., & Orzechowski, P. M. (2024). Mental Health Symptom Reporting to a Virtual Triage Engine Prior to and During the COVID-19 Pandemic. COVID, 4(12), 1908-1920. https://doi.org/10.3390/covid4120134






