Abstract
While the impact of the pandemic has varied between and within countries, there are few published data on the relationship between social determinants of health (SDoH) and COVID-19 in Africa. This ecological cross-sectional study examines the relationship between COVID-19 risk and SDoH among 28 African countries. Included were countries with a recent demographic and health survey (years 2010 to 2018). The response variables were COVID-19 case rates and death rates (reported as of 15 August 2020); and the covariates comprised eight broad topics common to multiple SDoH frameworks aggregated to the country level: geography (urban residence), wealth index, education, employment, crowding, and access to information. A negative binomial regression was used to assess the association between aspects of SDoH and COVID-19 outcomes. Our analysis indicated that 1 in 4 (25.1%) households in study countries are without safe and clean water and a space for handwashing. The odds of COVID-19 morbidity and deaths were higher in countries with a high proportion of households without access to safe and clean water. Having a high proportional of educated women (1.003: 95% CI, 1.001–1.005) and living in a less crowded home (0.959: 95% CI, 0.920–1.000) were negatively associated with COVID-19 deaths, while being insured and owning a mobile phone predicted illness. Overall, aspects of SDoH contribute either negatively or positively to COVID-19 outcomes. Thus, addressing economic and environmental SDoH is critical for mitigating the spread of COVID-19 and re-emerging diseases on the African continent.
1. Introduction
Currently, it is estimated that 6.99 million people have died from COVID-19 worldwide, with 175,477 deaths reported in Africa and representing approximately 2.5% of global deaths [1]. These numbers place COVID-19 among the ten leading causes of death, mostly among developed countries [2]. The factors contributing to these low death rates in Africa, despite a weak healthcare infrastructure, have drawn fierce debate and raised big questions among scholars and civic leaders [2,3,4,5,6]. Several hypotheses have been provided to support the low reported death rates and case fatality rates, such as low vaccine coverage and/or underreporting [7,8,9,10,11,12,13,14,15,16].
Despite the effective intervention strategies implemented for the prevention and treatment of COVID-19, with a significant decrease in new cases globally, this reduction has varied across communities, subgroups, regions, countries, and within countries (e.g., urban versus rural) [17,18,19,20]. In Africa, complex factors, both internal and external, exacerbate disease and the healthcare burden of disease outbreaks [21,22,23,24,25]. While COVID-19 prevention via vaccines and treatment is available, it has become clear that to curtail infectious disease outbreaks, both biomedical and social issues must be addressed [26,27,28,29].
Social determinants of health include social and economic conditions that inhabitants are exposed to in their daily routines [24,26]. COVID-19 bears the direct and indirect impact of these SDoH factors, and influences its outcomes. For example, a weak healthcare system and poverty affect the dynamic of the infection and may reduce access to COVID-19 testing and personal protective equipment (PPE) [30]. Additionally, poor housing conditions including overcrowding poses serious health risks to people especially vulnerable subgroups (e.g., for people living in slums and often in close quarters) [27,31].
Consequently, low-income populations globally and minorities in the U.S. have been disproportionately affected by COVID-19, with poor outcomes [32,33,34,35]. Furthermore, although individual risk behaviors that contribute to COVID-19 have been established (e.g., close personal contact such as shaking hands) [24,36], there are few published data on how aspects of SDoH affect COVID-19 risk in the African context. Coping with disease outbreaks requires an expanded understanding of both ‘upstream’ causes such as social determinants [37,38] and ‘downstream’ causes such as responses—vaccines [39,40]. Thus, for those working in disease prevention, a deeper understanding of whether and how SDoH affect infectious diseases such as COVID-19 is increasingly important for designing targeted prevention interventions and for preparing for current and future disease outbreaks [41,42].
Given that improving the response to future pandemics requires an improved understanding of early disease dynamics and the role played by local, national, and international agencies [40], we conducted an ecological study to examine the associations between social determinants of health (SDoH) across 28 African countries and COVID-19 outcomes. The goals of the study are to characterize the potential relationships between SDoH and COVID-19 measures at the country level at a time when governments were scrambling to curtail the crisis.
2. Methods
2.1. Design and Setting
We conducted an ecological cross-sectional study comprising 28 African countries (Figure 1)—countries with a recent standard demographic and health survey completed conducted within the past ten years [43]. To be included, countries also had to have COVID-19 data publicly available. Initiated in 1984, the DHS was used because it captures comparable population-based data on indicators of household-level living conditions, as well as the broader socioeconomic, demographic, and environmental conditions in low- and middle-income countries (LMICs) [44] allowing a comprehensive assessment of a countries health situation, including comparison between and within countries (urban, rural). Moreover, the DHS questionnaires are consistent and this enhances the comparability of indicators across populations and time. The DHS survey participants are selected from clusters and households within a fully covered geographic sampling frame using multistage design, providing opportunities for examining both ecological and individual-level factors that relate to the distribution of health outcomes [45]. We excluded countries if they did not have a survey conducted in 2010 or later or if they did not include likely significant measures that contributed to differential COVID-19 outcomes as part of the information collected.
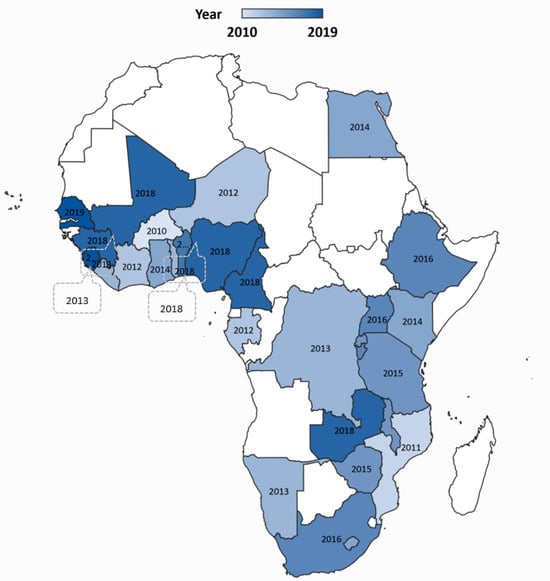
Figure 1.
Location of the 28 study countries and the Demographic and Health Survey year conducted, 2010–2018.
2.2. Country-Level COVID-19 Data Source
We obtained reported COVID-19 hospitalizations and death data as of 15 August 2020 for 28 African countries from the Africa Centers for Disease Control and Prevention (CDC) Dashboard (accessible at: https://africacdc.org/covid-19/, accessed on 16 August 2023). The Africa CDC Dashboard compiles COVID-19 testing data from official regional collaborating centers and member country reports. Therefore, these data may not be indicative of the actual tests as of the date of the dashboard update. To determine the country-level case and death rates, we calculated the number of COVID-19 deaths and/or cases per country in each period and divided it by the population exposed to risk of infection and death in that period multiplied by 10,000.
2.3. Country-Level Social Determinants of Health Data Source, Measures
To determine the association between SDoH and COVID-19 outcomes, we abstracted from the most recent DHS in each country (n = 28 countries) 9 variables commonly reported as shaping health outcomes. The measures comprised nine broad topics common to multiple SDoH frameworks, which were aggregated to the country level [24]. Because there are gender differences in educational attainment and because COVID-19 has affected girls and women differently compared to boys and men, a few independent indicators focus on women. Measures of interest represented geography (urban residence), wealth (Human Development Index or HDI rather than “GDP per capita”. HDI was used as it has been reported to be better indicator in measuring the progress of nations, particularly LMICs. Other indicators included a wealth index—poor or average living) [46,47], education (women with no education, primary, secondary, or higher, percentage, %), sanitation (women living in households with safe and clean water access, having a place to wash hands, with soap or detergent present, %), employment (currently working, %), insurance coverage (%), crowding (average number of household members based on more than three people per sleeping room), distance to safe clean water (average time to get to drinking water), access to information (women owning a mobile phone (%)), and women listening to the radio at least once a week (%). These SDOH measures were selected based on the literature and conceptual frameworks [26,48,49]. Studies have linked some of these measures to COVID-19 screening and testing outcomes [44,50].
The DHS dataset was used because it represents the primary source of data in many low–middle income countries on the demographic, socioeconomic, and environmental conditions and trends in a country [51], and datasets are available to the public upon request to the DHS Program [52]. The DHS Program uses a two-stage cluster sampling design to capture representativeness of the data at the national and sub-national level. The variables collected via the DHS are often the same across countries and period, enabling comparison over space and time.
2.4. Data Processing
Country-level measures: To abstract the SDoH measures of interest from the DHS, we first put together a full continent database, drawn from the DHS program [52]. We included only countries with the most recent DHS survey (conducted in the past 10 years) that had COVID-19 outcomes. We then converted the DHS household survey data to country level by using data processing and aggregation methods documented in detail elsewhere by LivWell; in Ref. [53]. Briefly, the household-level data were first aggregated to the sub-national level; with a corresponding R function used to transform the raw data into the final measures at the country level. The data were then validated using data obtained from other sources (e.g., the DHS complier, Livwell data repository, the subnational human development database) [53,54,55]. Sampling weights were used in calculations as provided by the DHS Program [51], and to account for sampling weight, the weight in the pooled data was divided by the number of surveys available for the respective countries, as used in previous studies [44].
This study uses publicly available secondary data; as such, ethical approvals are not necessary.
2.5. Statistical Analysis
We used ArcGIS Software version 10.5 [56] for generating maps. Negative binomial regression was used to assess the association between COVID-19 risks and SDoH measures. Negative binomial regression was chosen over Poisson regression because the COVID-19 death and case rates (dependent variable) were over-dispersed. The approach was adopted only after performing the likelihood ratio test for over-dispersion for both negative binomial regression and Poisson regression. The denominator included all surveyed households in the DHS study countries with measures aggregated to the country-level (n = 28 countries). Predictors were added sequentially to the model, in which the fixed effects can be interpreted as conditional on countries, with all random effects fixed at zero (e.g., unit-specific models). The model goodness-of-fit and the assessment of outliers were evaluated by the Akaike information criterion (AIC), the Bayesian information criterion (BIC), and McFadden’s pseudo-R-squared statistic. All the analyses were conducted using IBM SPSS statistics version 26.0 [57].
3. Results
3.1. Characteristics of Study Countries
Table 1 presents the characteristics of the DHS SDOH measures, count, and percentages for the study countries. The study sample (n = 416,459 surveyed households in 28 study countries). Of the 63.9% surveyed countries, inhabitants live in rural areas and 36.1% are urban dwellers. One in four (25.1%) respondents are without a designated place for household members to wash their hands, and a staggering 41% of residents reported not having soap or detergent for handwashing. The share of countries reporting households with more than three people per sleeping room is 21.4%, while approximately 36.0% of countries report that inhabitants do not have any education, and 42.6% of the surveyed countries have inhabitants living in poverty.

Table 1.
Summary Statistics of Social Determinants of Health Typologies for 28 Study Countries with recent Demographic and Health Surveys, 2010–2018 (n = 486,173 surveyed households).
We observed country differences in the rates of COVID-19 deaths and cases. Overall deaths from COVID-19 across the study African countries ranged from 0–1.99 per 10,000 population during the study period (Figure 2). As of 15 August 2020, South Africa had the highest COVID-19 case and death rates in Africa and still has the highest COVID-19 related cases and deaths in Africa. For example, as of 23 December 2022, there were 4,046,603 confirmed cases of COVID-19 with 102,550 deaths reported to WHO.
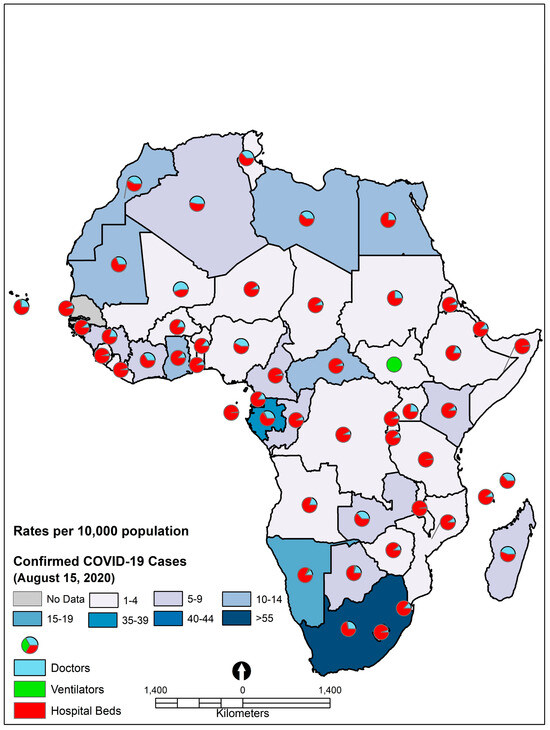
Figure 2.
Confirmed COVID-19 case rates in 54 African countries, 2020.
3.2. Multivariate Analysis of Correlates for COVID-19 Case and Death Rates in the 28 Study Countries
The results of a binomial negative regression model on COVID-19 case rates across the 28 African study countries are shown in Table 2. Not having access to quality water or safe and clean water (1.153; 95% CI, 1.036–1.284), health insurance (1.262: 95% CI, 1.124–1.417), women not owning a mobile phone was positively associated with COVID-19 case rates.

Table 2.
Negative binomial regression of 28 African countries social determinants of health factors on COVID-19 case rates.
Regarding COVID-19 death rates, we also observed that the odds of COVID-19 death rates were higher in countries with a high proportion of households without access to quality water (1.004: 95% CI, 1.002–1.006) and uneducated women (1.003: 95% CI, 1.001–1.005 (Table 3). Having household crowding especially having fewer than three people sharing a room was negatively associated with COVID-19 death rates.

Table 3.
Negative binomial regression of 28 African countries and social determinants of health factors on COVID-19 death rates.
4. Discussion
This study augments the existing literature on the effects of the pandemic in Africa by examining the effects of country-level (n = 28 countries) SDoH measures on COVID-19 outcomes (cases and death rates) during a time when countries were scrambling to curtail the crisis. Consistent with the literature, this study found a link between country level SDoH measures and COVID-19 outcomes [23,25,58,59,60,61]. Overall, death rates and case rates varied by country, with South Africa exhibiting the highest COVID-19 case and death rates across sub-Saharan Africa. This finding supports evidence from previous observations in South Africa [62,63,64,65,66]. Notably, the low COVID-19 rates reported in other African countries may be somewhat limited by data gaps inherent in most health information systems in Africa’s ministries of health [67]. The need for better data during pandemics extends to a need for better demographic surveillance systems for all vulnerable populations [61].
In our study, household overcrowding was negatively associated with both cases and death rates. The findings are consistent with those of previous studies that found household crowding or when the number of persons surpasses the number of rooms as a risk factor for COVID-19 [68,69,70,71,72,73,74] and those that linked crowding to infectious disease transmission [75,76]. A study conducted in Kenya by researchers from the University of Pécs on urban slums and COVID-19 provides recommendations for what non-governmental and government actors can do to prepare for future interventions on infectious disease outbreaks, including better coordination of efforts and expertise [77]. This finding is also consistent with those who noted a need for an integrated policy approach as a solution to Kenya’s slum residents’ risk to COVID-19 [73], and that any pandemic responses “that do not recognize these realities will further jeopardize the survival of large segments of the urban population globally [73]”.
Not surprising, the percentage of women with an education was a significant explanatory variable for the variation in COVID-19 across the 28 African countries. However, a closer examination reveals the presence of several preventative strategies that, when seen in their totality, have influenced significantly COVID-19 outcomes across the African continent [78]. Efforts and reasons as to why African exhibited low COVID-29 deaths and cases are detailed elsewhere [77,79,80,81,82]. This result also suggests the importance of educating women in developing countries, as this can improve health outcomes. In the context of Africa, women’s educational level has been reported as shaping healthcare seeking behaviors, and among the most effective intervention strategies, those to do with women’s education and health literacy have been the most effective at changing health behaviors and improving outcomes [44,83,84,85,86]. Moreover, a recent review noted that COVID-19 disproportionately affected girls and women differently compared to boys and men [87], and women are more vulnerable to COVID-19-related economic impacts because of existing gender inequalities [24,44,88].
We also observed that being uninsured was associated with COVID-19 risks, a finding supported by studies conducted in Germany [89], U.S. [90], and Korea [91]. The finding alludes to the relationship between socioeconomic status and COVID-19 risk. This finding is not surprising considering that those low-income people are more likely to be unemployed, have unstable work conditions and incomes, or to be employed in jobs that do not provide insurance, exacerbating their risks [92].
Women not owning a mobile phone considerably influenced the odds of COVID-19 case rates. The use of mobile phones has gained popularity as a low cost method of addressing health system needs in Sub-Saharan Africa [93], and a lack of mobile phone ownership is noted as a facilitator or hindrance to the implementation of health interventions [94,95]. For example, a study conducted in Malawi of mobile/cellular phone ownership and health behaviors in postpartum mothers noted low depressive symptoms among women who owned mobile phones. Moise et al., ref. [96]’s scoping review (n = 22 studies) of digital-technology-enabled health interventions implemented during the pandemic to improve maternal and birth outcomes noted high use of mobile phones for service delivery and case management [95]. Elsewhere, access to mobile devices has been linked to health literacy [97], empowerment [67], improved self-efficacy and communication between healthcare workers, clients, and better adherence outcomes [98,99,100]. Thus, the results of this study suggest that access to a mobile phone is a social determinant of health and can influence women’s health outcomes.
These findings suggest key implications for addressing future infectious disease outbreaks in Sub-Saharan Africa. For example, another finding is that access to high quality water is positively linked to COVID-19 cases. However, although access to water, sanitation, and hygiene (WASH) is vital to “protect human health during infectious disease outbreaks”, while “hand hygiene is a critical factor of the wider WASH framework and is highly recommended by WHO as a significant control measure to control infectious disease transmission [101,102,103,104]”, its effect on reducing the incidence of COVID-19 has not been widely investigated. A recent mini systematic review of thirteen studies noted not finding any studies on COVID-19 health outcomes and WASH [105]. Further studies, which take these variables into account, will need to be undertaken. One such study identified vital knowledge gaps and priorities for the research that can be used for current and future pandemics [106].
The most unanticipated finding of this study was the apparent negative relationship between the Human Development Index (HDI) and COVID-19 outcomes. This outcome is contrary to that in recent studies that noted an association between the HDI and COVID-19 incidence [107,108,109,110] but collaborates Roghani and Panahi’s findings that noted GDP and not HDI as a significator measure for COVID-19 outcomes [111]. Notably, although we found a negative association between COVID-19 outcomes and HDI, higher country HDI has been linked to enhanced vaccine distribution and health infrastructure [112]. Given that economic measures are an aspect of SDoH concepts, they play a significant role in contributing to a country’s capacity for implementing effective pandemic interventions, such as vaccine distribution and health infrastructure. Future studies on the current topic are, therefore, recommended.
5. Limitations
The current study adds to our knowledge regarding the influence of social determinants of health on the health outcomes in Africa. However, this study has limitations. First, a limitation of this study is the issue of omitted variable bias. For example, of all potential risk and protective factors (e.g., use of PPE) that can moderate an individual’s exposure to the COVID-19, we only include select SDoH measures. Further, the DHS data on healthcare services are limited to evaluating availability and utilization, and no indicators on quality of care are collected. Further research should be undertaken to assess the quality of care beyond the use of cross-sectional surveys. In addition, the available DHS vary by country, capturing only a brief snapshot of SDOH overtime and shedding little light on how SDOH change over time. This limitation is largely unavoidable due to a dearth of longitudinal data on COVID-19 and protective factors in Africa. A further study with more focus on protective factors, such as the use of PPE, that can be generated via focus groups and surveys is, therefore, suggested.
6. Conclusions
Although many countries have managed to decrease the effect of the pandemic, variation in COVID-19 outcomes between countries is largely correlated with aspects of social determinant of health measures. The main predictors of case rates were access to quality water, not having health insurance, and ownership of mobile phones by women, while predicators of COVID-19 mortality rates include ownership of mobile phones by women, access to quality water, and the percentage of educated women in a county. These findings have implications for addressing healthcare systems in Africa and underscore the need to include and integrate capacity, economic, and environmental determinants in healthcare reform. This is critical for planning, preparedness, and response to the next major re/emerging disease outbreak. Additionally, ministries of health should recognize the importance of effectively allocating resources to areas with overcrowded housing, poor WASH, and education of girls, as this may help during future disease outbreaks. Our findings emphasize an imperative for further studies to explore the association between cell phone ownership and health outcomes, as well as potential risk and protective factors.
Author Contributions
Conceptualization, I.K.M. and L.R.O.-W.; methodology, I.K.M.; software, I.K.M.; validation, L.R.O.-W.; formal analysis, B.A.M. and L.R.O.-W.; data curation, L.R.O.-W.; writing—original draft preparation, I.K.M.; writing—review and editing, H.H.; visualization, K.O.; supervision, I.K.M.; project administration, I.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study used publicly available secondary data sources.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- COVID WHO. Dashboard. World Health Organzation (WHO). Available online: https://data.who.int/dashboard/covid19 (accessed on 1 August 2022).
- Okonji, E.F.; Okonji, O.C.; Mukumbang, F.C.; Van Wyk, B. Understanding varying COVID-19 mortality rates reported in Africa compared to Europe, Americas and Asia. Trop. Med. Int. Health 2021, 26, 716–719. [Google Scholar] [CrossRef]
- Maeda, J.M.; Nkengasong, J.N. The puzzle of the COVID-19 pandemic in Africa. Science 2021, 371, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Mwananyanda, L.; Gill, C.J.; MacLeod, W.; Kwenda, G.; Pieciak, R.; Mupila, Z.; Lapidot, R.; Mupeta, F.; Forman, L.; Ziko, L. COVID-19 deaths in Africa: Prospective systematic postmortem surveillance study. BMJ 2021, 372, n334. [Google Scholar] [CrossRef]
- Moise, I.K.; Verity, J.F.; Kangmennaang, J. Identifying youth-friendly service practices associated with adolescents’ use of reproductive healthcare services in post-conflict Burundi: A cross-sectional study. Int. J. Health Geogr. 2017, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Moise, I.K. Causes of Morbidity and Mortality among Neonates and Children in Post-Conflict Burundi: A Cross-Sectional Retrospective Study. Children 2018, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Marbot, O. Coronavirus: Unpacking the Theories behind Africa’s Low Infection Rate. Africa Report. 5 May 2020. Available online: https://www.theafricareport.com/27470/coronavirus-unpacking-the-theories-behind-africas-low-infection-rate/ (accessed on 15 July 2022).
- Harding, A. Coronavirus in South Africa: Scientists explore surprise theory for low death rate. BBC News, 3 September 2020. [Google Scholar]
- Winning, A. Puzzled scientists seek reasons behind Africa’s low fatality rates from pandemic. Reuters, 29 September 2020. [Google Scholar]
- Makoni, M. COVID-19 vaccine trials in Africa. Lancet Respir. Med. 2020, 8, e79–e80. [Google Scholar] [CrossRef]
- Cabore, J.W.; Karamagi, H.C.; Kipruto, H.K.; Mungatu, J.K.; Asamani, J.A.; Droti, B.; Titi-Ofei, R.; Seydi, A.B.W.; Kidane, S.N.; Balde, T.; et al. COVID-19 in the 47 countries of the WHO African region: A modelling analysis of past trends and future patterns. Lancet Glob. Health 2022, 10, e1099–e1114. [Google Scholar] [CrossRef]
- Bradshaw, D.; Dorrington, R.; Moultrie, T.; Groenewald, P.; Moultrie, H. Underestimated COVID-19 mortality in WHO African region. Lancet Glob. Health 2022, 10, e1559. [Google Scholar] [CrossRef]
- Soko, N.D.; Dlamini, S.; Ntsekhe, M.; Dandara, C. The COVID-19 Pandemic and Explaining Outcomes in Africa: Could Genomic Variation Add to the Debate? OMICS J. Integr. Biol. 2022, 26, 594–607. [Google Scholar] [CrossRef]
- Rice, B.L.; Annapragada, A.; Baker, R.E.; Bruijning, M.; Dotse-Gborgbortsi, W.; Mensah, K.; Miller, I.F.; Motaze, N.V.; Raherinandrasana, A.; Rajeev, M.; et al. Variation in SARS-CoV-2 outbreaks across sub-Saharan Africa. Nat. Med. 2021, 27, 447–453. [Google Scholar] [CrossRef]
- Singini, G.C.; Manda, S.O.M. Inter-Country COVID-19 Contagiousness Variation in Eight African Countries. Front. Public Health 2022, 10, 796501. [Google Scholar] [CrossRef] [PubMed]
- Dalal, J.; Triulzi, I.; James, A.; Nguimbis, B.; Dri, G.G.; Venkatasubramanian, A.; Noubi Tchoupopnou Royd, L.; Botero Mesa, S.; Somerville, C.; Turchetti, G.; et al. COVID-19 mortality in women and men in sub-Saharan Africa: A cross-sectional study. BMJ Glob. Health 2021, 6, e007225. [Google Scholar] [CrossRef] [PubMed]
- Messner, W.; Payson, S.E. Variation in COVID-19 outbreaks at the US state and county levels. Public Health 2020, 187, 15–18. [Google Scholar] [CrossRef]
- Samadizadeh, S.; Masoudi, M.; Rastegar, M.; Salimi, V.; Shahbaz, M.B.; Tahamtan, A. COVID-19: Why does disease severity vary among individuals? Respir. Med. 2021, 180, 106356. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; St. Pierre, J.M.; Pickering, T.A.; Demirjian, N.L.; Fields, B.K.K.; Desai, B.; Gholamrezanezhad, A. Coronavirus disease 2019 (COVID-19): A modeling study of factors driving variation in case fatality rate by country. Int. J. Environ. Res. Public Health 2020, 17, 8189. [Google Scholar] [CrossRef]
- Onovo, A.A.; Kalaiwo, A.; Obanubi, C.; Odezugo, G.; Estill, J.; Keiser, O. Estimates of the COVID-19 Infection Fatality Rate for 48 African Countries: A Model-Based Analysis. BioMed 2021, 1, 63–79. [Google Scholar] [CrossRef]
- Zheng, Z.; Peng, F.; Xu, B.; Zhao, J.; Liu, H.; Peng, J.; Li, Q.; Jiang, C.; Zhou, Y.; Liu, S. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020, 81, e16–e25. [Google Scholar]
- Prasannan, L.; Rochelson, B.; Shan, W.; Nicholson, K.; Solmonovich, R.; Kulkarni, A.; Lewis, D.; Greenberg, M.; Nimaroff, M.; Blitz, M.J. Social determinants of health and coronavirus disease 2019 in pregnancy. Am. J. Obstet. Gynecol. MFM 2021, 3, 100349. [Google Scholar] [CrossRef]
- Abrams, E.M.; Szefler, S.J. COVID-19 and the impact of social determinants of health. Lancet Respir. Med. 2020, 8, 659–661. [Google Scholar] [CrossRef]
- Moise, I. Variation in Risk of COVID-19 Infection and Predictors of Social Determinants of Health in Miami–Dade County, Florida. Prev. Chronic Dis. 2020, 17, E124. [Google Scholar] [CrossRef]
- Ngepah, N. What lessons can Africa learn from the social determinants of COVID-19 spread, to better prepare for the current and future pandemics in the continent? Afr. Dev. Rev. 2021, 33, S45–S59. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.; Melo, A.; Moise, I.K.; Saavedra, J.; Szapocznik, J. The Association Between the Social Determinants of Health and HIV Control in Miami-Dade County ZIP Codes, 2017. J. Racial Ethn. Health Disparities 2021, 8, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Singu, S.; Acharya, A.; Challagundla, K.; Byrareddy, S.N. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front. Public Health 2020, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Mwisongo, A.; Nabyonga-Orem, J. Global health initiatives in Africa—Governance, priorities, harmonisation and alignment. BMC Health Serv. Res. 2016, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- El-Sadr, W.M.; Justman, J. Africa in the Path of COVID-19. N. Engl. J. Med. 2020, 383, e11. [Google Scholar] [CrossRef] [PubMed]
- Ihekweazu, C.; Agogo, E. Africa’s response to COVID-19. BMC Med. 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Kamis, C.; Stolte, A.; West, J.S.; Fishman, S.H.; Brown, T.; Brown, T.; Farmer, H.R. Overcrowding and COVID-19 mortality across U.S. counties: Are disparities growing over time? SSM-Popul. Health 2021, 15, 100845. [Google Scholar] [CrossRef]
- Louis-Jean, J.; Cenat, K.; Njoku, C.V.; Angelo, J.; Sanon, D. Coronavirus (COVID-19) and racial disparities: A perspective analysis. J. Racial Ethn. Health Disparities 2020, 7, 1039–1045. [Google Scholar] [CrossRef]
- Tran, B.X.; Ha, G.H.; Nguyen, L.H.; Vu, G.T.; Hoang, M.T.; Le, H.T.; Latkin, C.A.; Ho, C.S.H.; Ho, R.C.M. Studies of novel coronavirus disease 19 (COVID-19) pandemic: A global analysis of literature. Int. J. Environ. Res. Public Health 2020, 17, 4095. [Google Scholar] [CrossRef]
- Abdelhafiz, A.S.; Mohammed, Z.; Ibrahim, M.E.; Ziady, H.H.; Alorabi, M.; Ayyad, M.; Sultan, E.A. Knowledge, perceptions, and attitude of Egyptians towards the novel coronavirus disease (COVID-19). J. Community Health 2020, 45, 881–890. [Google Scholar] [CrossRef]
- Koma, W.; Artiga, S.; Claxton, G.J.; Rae, M.; Neuman, T.; Michaud, J.; Kates, J. Low-income and communities of color at higher risk of serious illness if infected with coronavirus. KFF, 7 May 2020. [Google Scholar]
- Kavanagh, M.M.; Erondu, N.A.; Tomori, O.; Dzau, V.J.; Okiro, E.A.; Maleche, A.; Aniebo, I.C.; Rugege, U.; Holmes, C.B.; Gostin, L.O. Access to lifesaving medical resources for African countries: COVID-19 testing and response, ethics, and politics. Lancet 2020, 395, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.; Chaves, L.F.; Bergmann, L.R.; Ayres, C.; Hogerwerf, L.; Kock, R.; Wallace, R.G. Clear-Cutting Disease Control: Capital-Led Deforestation, Public Health Austerity, and Vector-Borne Infection; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Redding, D.W.; Atkinson, P.M.; Cunningham, A.A.; Lo Iacono, G.; Moses, L.M.; Wood, J.L.N.; Jones, K.E. Impacts of environmental and socio-economic factors on emergence and epidemic potential of Ebola in Africa. Nat. Commun. 2019, 10, 4531. [Google Scholar] [CrossRef] [PubMed]
- Waitzkin, H. Confronting the Upstream Causes of COVID-19 and Other Epidemics to Follow. Int. J. Health Serv. 2021, 51, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Berman, P.; Cameron, M.A.; Gaurav, S.; Gotsadze, G.; Hasan, M.Z.; Jenei, K.; Keidar, S.; Kornreich, Y.; Lovato, C.; Patrick, D.M. Improving the response to future pandemics requires an improved understanding of the role played by institutions, politics, organization, and governance. PLOS Glob. Public Health 2023, 3, e0001501. [Google Scholar] [CrossRef]
- Nuwagira, E.; Muzoora, C. Is Sub-Saharan Africa prepared for COVID-19? Trop. Med. Health 2020, 48, 18. [Google Scholar] [CrossRef]
- Braveman, P.; Gottlieb, L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014, 129 (Suppl. S2), 19–31. [Google Scholar] [CrossRef]
- MEASURE DHS. MEASURE DHS: Demographic and Health Surveys; DHS Survey Types; MEASURE DHS, ICF International: Calverton, MD, USA, 2013. [Google Scholar]
- Moise, I.K.; Kangmennaang, J.; Halwiindi, H.; Grigsby-Toussaint, D.S.; Fuller, D.O. Increase in Obesity Among Women of Reproductive Age in Zambia, 2002–2014. J. Womens Health 2019, 28, 1679–1687. [Google Scholar] [CrossRef]
- Ozodiegwu, I.D.; Ambrose, M.; Battle, K.E.; Bever, C.; Diallo, O.; Galatas, B.; Runge, M.; Gerardin, J. Beyond national indicators: Adapting the Demographic and Health Surveys’ sampling strategies and questions to better inform subnational malaria intervention policy. Malar. J. 2021, 20, 122. [Google Scholar] [CrossRef]
- Islam, S. The human development index and per capita GDP. Appl. Econ. Lett. 1995, 2, 166–167. [Google Scholar] [CrossRef]
- Ghosh, J.; Moise, I.K.; Kalipeni, E. The role of regional integration in foreign direct investment in Southern Africa. In Advances in Geoeconomics; Routledge: London, UK, 2017; pp. 151–159. [Google Scholar]
- Moise, I.K. Geographic gender differences in traumatic unintentional injury hospitalization and youth drinking. Drug Alcohol. Depend. 2019, 205, 107701. [Google Scholar] [CrossRef]
- Kolak, M.; Bhatt, J.; Park, Y.H.; Padrón, N.A.; Molefe, A. Quantification of Neighborhood-Level Social Determinants of Health in the Continental United States. JAMA Netw. Open 2020, 3, e1919928. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Wilson, M. COVID-19: A potential public health problem for homeless populations. Lancet Public Health 2020, 5, e186–e187. [Google Scholar] [CrossRef] [PubMed]
- DHS Program. Description of the Demographic and Health Surveys Individual Recode Data File; MEASURE DHS, ICF International: Calverton, MD, USA, 2008. [Google Scholar]
- The DHS Program. Demographic and Health Surveys. 2017. Available online: https://dhsprogram.com/ (accessed on 19 August 2019).
- Belmin, C.; Hoffmann, R.; Elkasabi, M.; Pichler, P.-P. LivWell: A sub-national Dataset on the Living Conditions of Women and their Well-being for 52 Countries. Sci. Data 2022, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.; Permanyer, I. The Subnational Human Development Database. Sci. Data 2019, 6, 190038. [Google Scholar] [CrossRef] [PubMed]
- Kummu, M.; Taka, M.; Guillaume, J.H.A. Gridded global datasets for Gross Domestic Product and Human Development Index over 1990–2015. Sci. Data 2018, 5, 180004. [Google Scholar] [CrossRef] [PubMed]
- ESRI Inc. ArcGIS Desktop: Release 10; Environmental Systems Research Institute: Redlands, CA, USA, 2010. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp.: Armonk, NY, USA, 2013. [Google Scholar]
- Rollston, R.; Galea, S. COVID-19 and the Social Determinants of Health. Am. J. Health Promot. 2020, 34, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Dukhovnov, D.; Barbieri, M. County-level socio-economic disparities in COVID-19 mortality in the USA. Int. J. Epidemiol. 2022, 51, 418–428. [Google Scholar] [CrossRef]
- Nartey, A. Afterword: Social determinants of health: Insights from COVID-19 in Ghana and sub-Saharan Africa. In Communicative Perspectives on COVID-19 in Ghana; Routledge: New York, NY, USA, 2023; pp. 234–245. [Google Scholar]
- Emina, J.; Beguy, D.; Zulu, E.M.; Ezeh, A.C.; Muindi, K.; Elung’ata, P.; Otsola, J.K.; Yé, Y. Monitoring of Health and Demographic Outcomes in Poor Urban Settlements: Evidence from the Nairobi Urban Health and Demographic Surveillance System. J. Urban. Health 2011, 88, 200–218. [Google Scholar] [CrossRef]
- Schröder, M.; Bossert, A.; Kersting, M.; Aeffner, S.; Coetzee, J.; Timme, M.; Schlüter, J. COVID-19 in South Africa: Outbreak despite interventions. Sci. Rep. 2021, 11, 4956. [Google Scholar] [CrossRef]
- Blumberg, L.H.; Jassat, W.; Mendelson, M.; Cohen, C. The COVID-19 crisis in South Africa: Protecting the vulnerable. S. Afr. Med. J. 2020, 110, 825–826. [Google Scholar] [CrossRef]
- Madhi, S.A.; Nel, J. Epidemiology of severe COVID-19 from South Africa. Lancet HIV 2021, 8, e524–e526. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Koegelenberg, C.F.N.; Moolla, M.S.; Louw, E.H.; Mowlana, A.; Nortjé, A.; Ahmed, R.; Brittain, N.; Lalla, U.; Allwood, B.W. High HIV prevalence in an early cohort of hospital admissions with COVID-19 in Cape Town, South Africa. SAMJ S. Afr. Med. J. 2020, 110, 982–987. [Google Scholar] [CrossRef]
- Stiegler, N.; Bouchard, J.-P. South Africa: Challenges and successes of the COVID-19 lockdown. Ann. Médico-Psychol. Rev. Psychiatr. 2020, 178, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Milan, S.; Treré, E. The Rise of the Data Poor: The COVID-19 Pandemic Seen From the Margins. Soc. Media Soc. 2020, 6, 2056305120948233. [Google Scholar] [CrossRef] [PubMed]
- Rader, B.; Scarpino, S.V.; Nande, A.; Hill, A.L.; Adlam, B.; Reiner, R.C.; Pigott, D.M.; Gutierrez, B.; Zarebski, A.E.; Shrestha, M. Crowding and the shape of COVID-19 epidemics. Nat. Med. 2020, 26, 1829–1834. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeb, A.; Alzahrani, E.; Iqbal, S. Crowding effects on the dynamics of COVID-19 mathematical model. Adv. Differ. Equ. 2020, 2020, 675. [Google Scholar] [CrossRef] [PubMed]
- Rader, B.; Nande, A.; Adlam, B.; Hill, A.L.; Reiner, R.C.; Pigott, D.M.; Gutierrez, B. COVID-19 data working group; Brownstein, J.S.; Castro, M.C.; et al. Crowding and the epidemic intensity of COVID-19 transmission. medRxiv 2020. [Google Scholar] [CrossRef]
- Brown, K.A.; Jones, A.; Daneman, N.; Chan, A.K.; Schwartz, K.L.; Garber, G.E.; Costa, A.P.; Stall, N.M. Association between nursing home crowding and COVID-19 infection and mortality in Ontario, Canada. JAMA Intern. Med. 2021, 181, 229–236. [Google Scholar] [CrossRef]
- Ríos, V.; Denova-Gutiérrez, E.; Barquera, S. Association between living in municipalities with high crowding conditions and poverty and mortality from COVID-19 in Mexico. PLoS ONE 2022, 17, e0264137. [Google Scholar] [CrossRef]
- Solymári, D.; Kairu, E.; Czirják, R.; Tarrósy, I. The impact of COVID-19 on the livelihoods of Kenyan slum dwellers and the need for an integrated policy approach. PLoS ONE 2022, 17, e0271196. [Google Scholar] [CrossRef]
- Corburn, J.; Vlahov, D.; Mberu, B.; Riley, L.; Caiaffa, W.T.; Rashid, S.F.; Ko, A.; Patel, S.; Jukur, S.; Martínez-Herrera, E.; et al. Slum Health: Arresting COVID-19 and Improving Well-Being in Urban Informal Settlements. J. Urban Health 2020, 97, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Neiderud, C.-J. How urbanization affects the epidemiology of emerging infectious diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef]
- Varshney, K.; Glodjo, T.; Adalbert, J. Overcrowded housing increases risk for COVID-19 mortality: An ecological study. BMC Res. Notes 2022, 15, 126. [Google Scholar] [CrossRef]
- Solymári, D.; Kairu, E.; Czirják, R.; Tarrósy, I. Caring Interventions for the Most Vulnerable Populations in Economically Disadvantaged Areas during the Coronavirus (COVID-19) Pandemic by Non-Governmental Organizations in Kenya. Soc. Sci. 2023, 12, 284. [Google Scholar] [CrossRef]
- Anjorin, A.A. The coronavirus disease 2019 (COVID-19) pandemic: A review and an update on cases in Africa. Asian Pac. J. Trop. Med. 2020, 13, 199–203. [Google Scholar] [CrossRef]
- Nguimkeu, P.; Tadadjeu, S. Why is the number of COVID-19 cases lower than expected in Sub-Saharan Africa? A cross-sectional analysis of the role of demographic and geographic factors. World Dev. 2021, 138, 105251. [Google Scholar] [CrossRef] [PubMed]
- Bamgboye, E.L.; Omiye, J.A.; Afolaranmi, O.J.; Davids, M.R.; Tannor, E.K.; Wadee, S.; Niang, A.; Were, A.; Naicker, S. COVID-19 Pandemic: Is Africa Different? J. Natl. Med. Assoc. 2021, 113, 324–335. [Google Scholar] [CrossRef]
- Bouba, Y.; Tsinda, E.K.; Fonkou, M.D.M.; Mmbando, G.S.; Bragazzi, N.L.; Kong, J.D. The Determinants of the Low COVID-19 Transmission and Mortality Rates in Africa: A Cross-Country Analysis. Front. Public Health 2021, 9, 751197. [Google Scholar] [CrossRef]
- Tsinda, E.K.; Mmbando, G.S. Recent updates on the possible reasons for the low incidence and morbidity of COVID-19 cases in Africa. Bull. Natl. Res. Cent. 2021, 45, 133. [Google Scholar] [CrossRef]
- Moise, I.K.; Kalipeni, E.; Jusrut, P.; Iwelunmor, J.I. Assessing the reduction in infant mortality rates in Malawi over the 1990–2010 decades. Glob. Public Health 2017, 12, 757–779. [Google Scholar] [CrossRef]
- Bicego, G.T.; Boerma, J.T. Maternal education and child survival: A comparative study of survey data from 17 countries. Soc. Sci. Med. 1993, 36, 1207–1227. [Google Scholar] [CrossRef] [PubMed]
- Dallolio, L.; Di Gregori, V.; Lenzi, J.; Franchino, G.; Calugi, S.; Domenighetti, G.; Fantini, M.P. Socio-economic factors associated with infant mortality in Italy: An ecological study. Int. J. Equity Health 2012, 11, 45. [Google Scholar] [CrossRef]
- Kalipeni, E. Determinants of infant mortality in Malawi: A spatial perspective. Soc. Sci. Med. 1993, 37, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.; Madhavan, S.; Mokashi, M.; Amanuel, H.; Johnson, N.R.; Pace, L.E.; Bartz, D. Health risks and outcomes that disproportionately affect women during the COVID-19 pandemic: A review. Soc. Sci. Med. 2020, 266, 113364. [Google Scholar] [CrossRef] [PubMed]
- Flor, L.S.; Friedman, J.; Spencer, C.N.; Cagney, J.; Arrieta, A.; Herbert, M.E.; Stein, C.; Mullany, E.C.; Hon, J.; Patwardhan, V.; et al. Quantifying the effects of the COVID-19 pandemic on gender equality on health, social, and economic indicators: A comprehensive review of data from March, 2020, to September, 2021. Lancet 2022, 399, 2381–2397. [Google Scholar] [CrossRef]
- Wahrendorf, M.; Rupprecht, C.J.; Dortmann, O.; Scheider, M.; Dragano, N. Erhöhtes Risiko eines COVID-19-bedingten Krankenhausaufenthaltes für Arbeitslose: Eine Analyse von Krankenkassendaten von 1,28 Mio. Versicherten in Deutschland. Bundesgesundheitsblatt-Gesundheitsforsch.-Gesundheitsschutz 2021, 64, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, A.W.; Hawks, L.; Bor, D.H.; Woolhandler, S.; Himmelstein, D.U.; McCormick, D. 18.2 Million Individuals at Increased Risk of Severe COVID-19 Illness Are Un- or Underinsured. J. Gen. Intern. Med. 2020, 35, 2487–2489. [Google Scholar] [CrossRef]
- Oh, T.K.; Choi, J.-W.; Song, I.-A. Socioeconomic disparity and the risk of contracting COVID-19 in South Korea: An NHIS-COVID-19 database cohort study. BMC Public Health 2021, 21, 144. [Google Scholar] [CrossRef]
- Patel, J.A.; Nielsen, F.B.H.; Badiani, A.A.; Assi, S.; Unadkat, V.A.; Patel, B.; Ravindrane, R.; Wardle, H. Poverty, inequality and COVID-19: The forgotten vulnerable. Public Health 2020, 183, 110–111. [Google Scholar] [CrossRef]
- Déglise, C.; Suggs, L.S.; Odermatt, P. SMS for disease control in developing countries: A systematic review of mobile health applications. J. Telemed. Telecare 2012, 18, 273–281. [Google Scholar] [CrossRef]
- Okano, J.T.; Ponce, J.; Krönke, M.; Blower, S. Lack of ownership of mobile phones could hinder the rollout of mHealth interventions in Africa. eLife 2022, 11, e79615. [Google Scholar] [CrossRef] [PubMed]
- Moise, I.K.; Ivanova, N.; Wilson, C.; Wilson, S.; Halwindi, H.; Spika, V.M. Lessons from digital technology-enabled health interventions implemented during the coronavirus pandemic to improve maternal and birth outcomes: A global scoping review. BMC Pregnancy Childbirth 2023, 23, 195. [Google Scholar] [CrossRef] [PubMed]
- Anto-Ocrah, M.; Latulipe, R.J.; Mark, T.E.; Adler, D.; Zaihra, T.; Lanning, J.W. Exploring association of mobile phone access with positive health outcomes and behaviors amongst post-partum mothers in rural Malawi. BMC Pregnancy Childbirth 2022, 22, 485. [Google Scholar] [CrossRef] [PubMed]
- Moon, Z.; Zuchowski, M.; Moss-Morris, R.; Hunter, M.S.; Norton, S.; Hughes, L.D. Disparities in access to mobile devices and e-health literacy among breast cancer survivors. Support. Care Cancer 2022, 30, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, K.W.; Woodard, L.M.; Woodard, T.J. Role of medication therapy management in preexposure prophylaxis therapy for HIV prevention. J. Pharm. Pract. 2015, 28, 10–12. [Google Scholar] [CrossRef]
- Friedman, R.H.; Kazis, L.E.; Jette, A.; Smith, M.B.; Stollerman, J.; Torgerson, J.; Carey, K. A telecommunications system for monitoring and counseling patients with hypertension: Impact on medication adherence and blood pressure control. Am. J. Hypertens. 1996, 9, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Pintye, J.; Rogers, Z.; Kinuthia, J.; Mugwanya, K.K.; Abuna, F.; Lagat, H.; Sila, J.; Kemunto, V.; Baeten, J.M.; John-Stewart, G.; et al. Two-Way Short Message Service (SMS) Communication May Increase Pre-Exposure Prophylaxis Continuation and Adherence Among Pregnant and Postpartum Women in Kenya. Glob. Health Sci. Pract. 2020, 8, 55–67. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Bartram, J.; Clasen, T.; Colford, J.M., Jr.; Cumming, O.; Curtis, V.; Bonjour, S.; Dangour, A.D.; De France, J.; Fewtrell, L.; et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: A retrospective analysis of data from 145 countries. Trop. Med. Int. Health 2014, 19, 894–905. [Google Scholar] [CrossRef]
- Boisson, S.; Engels, D.; Gordon, B.A.; Medlicott, K.O.; Neira, M.P.; Montresor, A.; Solomon, A.W.; Velleman, Y. Water, sanitation and hygiene for accelerating and sustaining progress on neglected tropical diseases: A new Global Strategy 2015–20. Int. Health 2016, 8 (Suppl. S1), i19–i21. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef]
- Donde, O.O.; Atoni, E.; Muia, A.W.; Yillia, P.T. COVID-19 pandemic: Water, sanitation and hygiene (WASH) as a critical control measure remains a major challenge in low-income countries. Water Res. 2021, 191, 116793. [Google Scholar] [CrossRef] [PubMed]
- Khatib, M.N.; Sinha, A.; Mishra, G.; Quazi, S.Z.; Gaidhane, S.; Saxena, D.; Gaidhane, A.M.; Bhardwaj, P.; Sawleshwarkar, S.; Zahiruddin, Q.S. WASH to control COVID-19: A rapid review. Front. Public Health 2022, 10, 976423. [Google Scholar] [CrossRef] [PubMed]
- Howard, G.; Bartram, J.; Brocklehurst, C.; Colford, J.M., Jr.; Costa, F.; Cunliffe, D.; Dreibelbis, R.; Eisenberg, J.N.S.; Evans, B.; Girones, R.; et al. COVID-19: Urgent actions, critical reflections and future relevance of ‘WaSH’: Lessons for the current and future pandemics. J. Water Health 2020, 18, 613–630. [Google Scholar] [CrossRef]
- Heo, M.H.; Kwon, Y.D.; Cheon, J.; Kim, K.B.; Noh, J.W. Association between the Human Development Index and Confirmed COVID-19 Cases by Country. Healthcare 2022, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Buheji, M.; AlDerazi, A.; Ahmed, D.; Bragazzi, N.L.; Jahrami, H.; Hamadeh, R.R.; BaHammam, A.S. The association between the initial outcomes of COVID-19 and the human development index: An ecological study. Hum. Syst. Manag. 2022, 41, 303–313. [Google Scholar] [CrossRef]
- Liu, K.; He, M.; Zhuang, Z.; He, D.; Li, H. Unexpected positive correlation between human development index and risk of infections and deaths of COVID-19 in Italy. One Health 2020, 10, 100174. [Google Scholar] [CrossRef]
- Armstrong, A.d.C.; Baggio, J.A.O.; Carmo, R.F.d.; Machado, M.F.; Santos, A.D.d.; Souza, C.D.F.d. COVID-19 in Brazil: Spatial risk, social vulnerability, human development, clinical manifestations and predictors of mortality—A retrospective study with data from 59 695 individuals. Epidemiol. Infect. 2021, 149, e100. [Google Scholar] [CrossRef]
- Ali, R.; Samin, P. The global distribution of COVID-19 vaccine: The role of macro-socioeconomics measures. medRxiv 2022. [Google Scholar] [CrossRef]
- Roghani, A. The relationship between macro-socioeconomics determinants and COVID-19 vaccine distribution. AIMS Public Health 2021, 8, 655–664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).