Abstract
SARS-CoV-2, a single stranded positive RNA-virus, emerged in 2019 and caused a global pandemic. Some infected patients develop severe complications, such as acute respiratory distress syndrome (ARDS) and pulmonary fibrosis. The mechanisms leading to lung fibrosis in the context of COVID-19 are still unclear. However, there are correlations between this condition and certain inflammatory mediators that are elevated in the plasma of patients. This study addressed the question of whether SARS-CoV-2 spike protein was able to directly activate immune cells to produce inflammatory and fibrogenic cytokines, independent of viral infection. By stimulating peripheral blood mononuclear cells (PBMC) from healthy blood donors through different formulations of SARS-CoV-2 spike protein, the expression of fibrogenic and inflammatory cytokines was measured. The spike protein induced a significantly increased expression of IL-1β and IL-6 mRNA in PBMC. Both cytokines are important players in the COVID-19 cytokine storm, in ARDS and in the development of pulmonary fibrosis. Different receptors with an affinity to the spike protein may be involved. However, the exact mechanism on how the spike protein leads to a higher cytokine expression in PBMC needs further investigation.
1. Introduction
SARS-CoV-2, a single stranded positive RNA-virus, emerged in 2019. Infection is caused via the binding of the virus to the angiotensin-converting enzyme 2 (ACE2) receptors expressed on the epithelial cells in the upper respiratory tract, and in the cells of the lungs. Infected patients can develop severe complications, such as atypical pneumonia or acute respiratory distress syndrome (ARDS), which can lead to death [1,2]. A certain proportion of ARDS patients develop pulmonary fibrosis, which can create a dry cough and breathlessness, and may need pulmonary rehabilitation, oxygen therapy, and possibly bronchioalveolar lavage with exogenous pulmonary surfactant [3,4].
An important element for mortality due to SARS-CoV-2 is the dysregulated immune response [5]. The uncontrolled immune reaction is referred to as the “cytokine storm” with increased levels of proinflammatory cytokines [6]. Interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and IL-6 may be the driving forces for the uncontrolled cytokine release [7,8]. In the peripheral blood of ARDS patients, higher levels of Th17 cells and IL-17A were detected, wherein IL-17A amplifies the inflammatory immune response by inducing more proinflammatory cytokines [5]. The chemokine CXCL10 is also thought to play a key role in triggering the cytokine storm [9]. The consequence of the cytokine storm in the lungs is ARDS, a condition in which the abnormal systemic inflammatory response is sustained, with immune cells producing high levels of proinflammatory cytokines, such as IL-1β, IL-6, IL-12, IL-18, interferon α (IFN-α), IFN-γ, TNF-α, and transforming growth factor β (TGF- β), as well as various chemokines [6,7]. Similar to SARS-CoV and MERS-CoV, SARS-CoV-2 can suppress the induction of the antiviral immune response [10]. Thereby, viral proteins attenuate the production of type I interferons [11]. Intracellular aggregates of open reading frame 8 (ORF8) protein, produced by SARS-CoV-2 in lung epithelial cells, have been shown to downregulate interferon expression [12,13]. In addition, SARS-CoV-2 may influence cytokine gene expression via restructuring the chromatin architecture in infected cells [14]. However, the mechanisms of how SARS-CoV-2 controls gene expression in infected, as well as non-infected, cells are not known. Therefore, it is of interest to investigate whether the SARS-CoV-2 spike protein may directly influence immune cells.
About 20% of patients with ARDS are expected to develop lung fibrosis, a severe complication of SARS-CoV-2 [2]. This condition is considered as a sequelae of ARDS, the consequence of chronic inflammation and the upregulation of certain cytokines [15,16]. The excessive immune response to SARS-CoV-2 puts the body into a hyperinflammatory state, which leads to organ damage and to a continuous remodeling fibrotic state [17]. Fibrotic changes in the lungs have also been discussed in the post-COVID-19 syndrome [13,18].
The exact mechanisms leading to fibrosis due to SARS-CoV-2 are still unclear, but proinflammatory cytokines, systemically or locally produced (by, e.g., type II pneumocytes) [4], are thought to play an important role, since some of them correlate with fibrotic changes [19]. Particularly high levels of IL-6, IL-10, TGF- β, TNF-α, and CXCL10 are suspected to induce lung injury, whereas elevated IFN-γ or IGF-1 may be protective [15]. In patients with fibrotic areas on CT scans, certain inflammatory mediators were upregulated in plasma, such as IL-1α, TGF-β, and CXCL10 [19]. Another important aspect is the downregulation of ACE2 receptors, through the binding of the virus, with the consequence that less angiotensin II (ANG II) can be degraded [17]. ANG II plays a role in inflammation and in the pathogenesis of fibrosis [20]. It leads to the activation of profibrotic cytokines such as IL-6, TNF-α, and TGF-β. Additionally, it can directly regulate the collagen 1 gene [2].
Generally, pulmonary fibrosis results from a dysregulation in the wound healing process and thus can form scars due to an excess of extracellular matrix deposition. TGF-β is a key player in the development of fibrosis; it induces epithelial–mesenchymal transition (EMT) and promotes the proliferation and activation of myofibroblasts, which produce extracellular matrix (ECM). Increased TNF-α and IL-1β expression is also associated with lung injury and is suspected to support fibrosis progression. Furthermore, IL-1β can upregulate TGF-β [21].
Organ fibrosis is an important public health issue, which is responsible for 45% of the overall deaths in the general population [22]. While fibrosis is associated with almost every type of organ damage, the lack of information on the basic mechanisms underlying the fibrosis process poses a major challenge in the development of targeted therapy against fibrotic diseases [23]. There is currently no treatment available that effectively stops progression or even reverses SARS-CoV-2 pulmonary fibrosis [24]. This condition is irreversible and has a poor prognosis; the only existing cure is lung transplantation [21].
The aim of this in vitro human study was to identify the expression of potentially fibrogenic cytokines by human peripheral blood mononuclear cells (PBMCs) in response to the SARS-CoV-2 spike protein. In addition, the expression of cytokines that may be protective against fibrosis was also investigated. For that purpose, PBMCs were stimulated with various formulations of spike protein. For that purpose, recombinant SARS-CoV-2 spike proteins containing virus-like particles (VLPs) were used, as well as recombinant spike trimer. Recombinant SARS-CoV-2 nucleoprotein, as well as VLPs representing dengue virus were used as negative control conditions. The expressions of the cytokines of interest were measured by using quantitative reverse transcription PCR (RT-qPCR) and ELISA.
2. Materials and Methods
2.1. Blood Samples and Reagents
Buffy coats provided by the Swiss Red Cross Blood Bank from 12 healthy donors were used. A power calculation was performed to decide on the sample size required for statistical purpose for the gene expression part using RT-qPCR. The blood samples were anonymous and therefore in accordance with the Swiss ethics legislation; no approval was required from the Ethics Committee. The following reagents were used: phytohemagglutinin (PHA: Lectin from Phaseolus vulgaris, N.61764, Sigma-Aldrich, St. Louis, MO, USA); spike peptides mixture representing all potential antigenic peptides of S1 protein (PepTivator Sars-CoV-2 Prot-S, Miltenyi Biotech, Bergisch Gladbach, Germany); recombinant SARS-CoV-2 spike trimer (generated in HEK cells, Miltenyi Biotech, Bergisch Gladbach, Germany); recombinant SARS-CoV-2 nucleoprotein (generated in HEK cells, Miltenyi Biotech, Bergisch Gladbach, Germany); SARS-CoV-2 virus-like particles (VLP: generated in HEK cells, Abnova, Tapei City, Taiwan); Dengue Virus Serotype 2 virus-like particles (VLP: generated in HEK cells, Abnova, Tapei City, Taiwan).
2.2. Blood Cell Separation and Culture of PBMC
Peripheral blood mononuclear cells (PBMC) were separated via Ficoll density gradient centrifugation. For this process, blood cells from buffy coats were placed on the gradient medium Ficoll-Paque Plus (density 1.077, CYTIVA, Uppsala, Sweden), which was used for the enrichment of monocytes and lymphocytes. After centrifugation, the resulting cell pellet was resuspended in culture medium (RPMI 1640, PAN-Biotech, Aidenbach, Germany) supplemented with antibiotic/antimycotic solution (Cat NO AAS-B, CAPRICORN SCIENTIFIC, Ebsdorfergrund, Germany). A 5% human serum (Swiss Red Cross Blood Bank) was added for the proliferation assays, whereas otherwise no serum was added to any other experimental conditions. The cells were cultured under standard conditions, i.e., 37 °C, 5% CO2, atmospheric O2, and humidified air.
2.3. Proliferation Assays
The proliferation assays were used to determine the immune status of the donors. For that purpose, PBMC were stimulated with recombinant SARS-CoV-2 S1 antigen in different formulations, or nuclear virus protein, to assess whether the blood donor had been vaccinated, infected, or none of these. No stimulation was used as the negative control condition. Stimulation with phytoheamagglutinin (PHA) was used as a positive control. This assay was performed with BrdU Labeling and the Detection Kit III (Roche, Basel, Switzerland). The T cell proliferation was measured using the reader Multiskan SkyHigh (ThermoFisher SCIENTIFIC, Waltham, MA, USA). The cells were cultured in 96-well plates and were stimulated under the following conditions (2 × 105 cells per well): negative control: PBMC without stimulation; positive control: PHA [1 ng/μL]; test for previous infection or vaccination: recombinant SARS-CoV-2 spike peptide [150 pmol/μL]; test for previous infection or vaccination: recombinant SARS-CoV-2 spike trimer [1 ng/μL]; test for infection: recombinant SARS-CoV-2 nucleoprotein [1 ng/μL]; test for previous infection or vaccination: VLP of SARS-CoV-2 [1 ng/μL]; negative control for VLP formulation: VLP of dengue [1 ng/μL], as the probability of blood donors in the Swiss population having been in contact with dengue virus is extremely low. By observing T cell proliferation under the different conditions, the following immune statuses could be deduced: 1. uninfected/not vaccinated, 2. infected, 3. vaccinated.
2.4. Measuring Cytokine Gene Expression at mRNA and Protein Level
After PBMC separation by Ficoll, the cells were stimulated under the same conditions used for the proliferation assays, except for the spike peptides. After incubation for 24 h (107 cells in 5 mL RPMI 1640 per condition, in 25 cm2 culture flasks), the cells were harvested into 15 mL tubes and centrifuged. The pellets and supernatants were frozen separately for further processing. For the standard RNA extraction, TRI Reagent® (TR 118) (Molecular Research Center, Cincinnati, OH, USA) and chloroform (Honeywell, Charlotte, NC, USA) were added to the frozen pellets, and the cells were lysed and the total RNA was obtained, according to the standard protocol. The GOScriptTM Transcription mix by Promega (Madison, WI, USA), which includes a mix of random primers, was used to transcribe the extracted RNA into cDNA. cDNAs were processed for quantitative RT-PCR using specific primer pairs for detection of the expression of the inflammatory mediators of interest: IFN-γ, TGF-β, TNF-α, IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12p40, IL-13, IL-17A, IL-18, IL-22, IL-23, and CXCL10 (Microsynth, Balgach, Switzerland). The sequence for the forward and reverse primers for each cytokine is listed in Table 1. To perform the qPCR, the KAPA SYBR® FAST qPCR Master Mix (2×) Kit (Kapa Biosystems, Potters Bar, UK) was used and was run on the C1000 touch thermal cycler CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). The following thermocycle protocol was used: enzyme activation for 2 min at 95 °C, denaturation for 15 s at 95 °C, annealing for 30 s at 60 °C, elongation 40 s at 72 °C. After 40 cycles, a melting curve was generated as a quality control. The amplification curve gave the threshold value , which served as basis for the analysis.

Table 1.
List of RT-qPCR forward (F) and reverse (R) primers.
For further analysis and as a basis for statistical tests, the relative quantification value RQ was used. The housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and actin were chosen (Microsynth, Balgach, Switzerland). As the difference in mRNA expression was minimal between these two genes, the mean value of the RQ was used. The calculation was performed using Microsoft® Excel, by applying the following formula:
∆Ct = Ctgene − Ct reference gene
∆∆Ct = ∆Ct sample − ∆Ctctrl
RQ = 2−∆∆Ct
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
Various cytokines that were expected to be secreted into the culture supernatants were measured. The direct sandwich-ELISA principle was used to determine the concentration of the cytokines of interest in the collected samples (frozen supernatants). Different kits from the following manufacturers were used according to the recommended instructions: ELISA MAX™ Deluxe set (BioLegend®, San Diego, CA, USA) for CXCL10, uncoated ELISA (Invitrogen from ThermoFisher SCIENTIFIC, Waltham, MA, USA) for IFN-γ, IL-1β, IL-2, IL-6, IL-10, TGF-β, and TNF-α, ELISA Kit (MyBioSource, San Diego, CA, USA) for Human IL-12, IL-17A, IL-18, and IL-23. Unfortunately, the secretion of IL-13, IL-18, and IL22 could not be measured due to insufficient funding.
For each condition (negative control, PHA, VLP Dengue, VLP SARS, SARS Nucleoprotein, Spike Trimer), measurements were conducted in duplicate. The optical density of the colorimetric reaction was measured by the reader Multiskan SkyHigh (ThermoFisher SCIENTIFIC, Waltham, MA, USA) at a wavelength of 450 nm and 570 nm, where for the analysis the optical density of the wavelength 570 was subtracted from that of the wavelength 450. The obtained standard curve was used to convert the optical density into cytokine concentrations.
2.6. Statistical Analysis
Microsoft Excel was used for data collection and descriptive statistics. For the proliferation assay, t-tests, using Microsoft Excel, were performed for the comparison of the different activation conditions with the negative control. The statistical analysis of the RT-qPCR results was performed using the software jamovi. For each cytokine, a one-way repeated measures ANOVA was performed. The Tukey post hoc test was used to compare the different conditions with each other. Descriptive statistics were used to interpret the ELISA results. Because of the limited data volume and heterogeneity of the ELISA data, no further statistical testing was performed.
3. Results
3.1. Proliferation Assay
The unknown immune status towards SARS-CoV-2 was assessed through T cell proliferation assays. PBMC were accordingly cultured in the presence (PHA for maximum positive stimulation, various formulations of SARS-CoV-2 or dengue virus antigens) or absence (negative control) of stimulation. The stimulatory conditions were statistically tested using a t-test against the negative control. The various formulations of spike protein gave us information about the vaccination status, as the currently available SARS-CoV-2 vaccines are based on the spike protein [25]. The results are summarized in Table 2. Blood donors 1, 3, 6, and 7 had significantly increased T cell proliferation under PHA stimulation, but not under the various SARS-CoV-2 antigen stimulation. Therefore, it can be assumed that these blood donors had never been in contact with the SARS-CoV-2 antigens, i.e., neither infected nor vaccinated. Blood donor 11 and 12 showed a specific T lymphocyte proliferation through the SARS-CoV-2 spike peptide, but no proliferation when stimulated with SARS-CoV-2 nucleoprotein as an antigen. These two individuals were probably vaccinated but never infected. The nucleoprotein was used to identify blood donors that had possibly been infected. In blood donor 2, 4 and 8, it triggered higher T cell proliferation; therefore, it can be assumed that these individuals were already in contact with the virus before. For individuals 5 (reaction against spike peptide did not show statistical significance), 9, and 10 (no statistical significance for any of the conditions), the assay was inconclusive for technical reasons. In summary, four blood donors had never been in contact with the virus and were not vaccinated, two were vaccinated, and three had been infected. In three blood donors, it was not possible to draw clear conclusions about their immune status.

Table 2.
A significant increase in T cell proliferation was observed for the 12 blood donors and the corresponding stimulatory condition (t-test: p < 0.05). VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 spike peptide (SSP), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
3.2. RT-qPCR
In the following RT-qPCR results section, cytokines are classified according to their role in fibrosis into either profibrotic or antifibrotic. This classification is based on the review article of Sziksz et al. [26], where individual cytokines were discussed in terms of their function in organ fibrosis.
However, functional classifications of cytokines, such as the classification into proinflammatory and anti-inflammatory, are also considered as problematic since TNF-α can act in both directions depending on the context [27]. Depending on the model, the inflammatory mediators can be classified differently regarding their fibrogenic potential. This is exemplified by the chemokine CXCL10, which has an antifibrotic effect in the bleomycin-induced lung fibrosis model in mice [28], but a profibrotic effect in human lung transplants [29].
3.2.1. RT-qPCR: Profibrotic Cytokines
In the following paragraphs, the mRNA expressions of the profibrotic cytokines IL-1β, IL-6, IL-13, IL-17, TNF-α, and the key cytokine TGF-β are presented. The results with the mean values are summarized in Table 3.

Table 3.
Gene expression of profibrotic cytokines measured with RT-qPCR: mean values of 12 blood donors are listed; * p < 0.05; negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
IL-1β plays a key role in the induction of the innate and adaptive immune responses [27] and activates Th17 differentiation [10]. Together with IL-6 and TNF-α, IL-1β is one of the most important cytokines for the cytokine release syndrome in the advanced stages of SARS-CoV-2 [8]. In our study, the F-statistic was significant (p < 0.001), which indicates differences in IL-1β mRNA expression between the individual conditions. Compared to the negative control, IL-1β mRNA expression was significantly increased under the conditions with VLP SARS (p < 0.001) and PHA (p < 0.001). No significant difference could be shown when testing VLP SARS against VLP dengue (p = 0.21), which is the negative control for VLPs, although IL-1β mRNA expression with VLP dengue stimulation was upregulated but did not reach a significant level (p = 0.066). In addition, no significant results were found for stimulation with the spike trimer and the nucleoprotein (Figure 1).
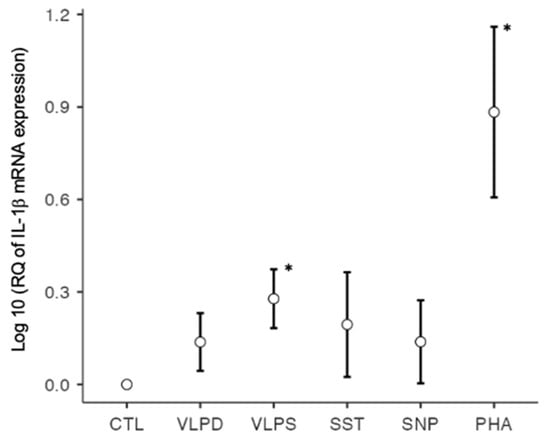
Figure 1.
IL-1β mRNA expression on a logarithmic scale, * p < 0.05; six conditions: negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
IL-6 is produced by various cells, including T cells, and leads to the proliferation of B cells and to Th17 differentiation [10]. Its production is stimulated by IL-1β and TNF-α. Due to its pleiotropic effects, it plays a key role in the development of the COVID-19 cytokine storm [6]. Studies have shown that high levels of circulating IL-6 are associated with the severity of SARS-CoV-2 infection. Furthermore, it has been reported that IL-6 is involved in fibrotic lung injury; therefore, this cytokine may be an important target for therapy [2,30]. For IL-6 mRNA, the ANOVA’s F-statistic indicated a significant difference between the different experimental conditions (p < 0.001). Significantly increased IL-6 mRNA expression was observed under the conditions with the stimulation through the VLP SARS (p = 0.003) and PHA (p < 0.001). Same as for IL-1β, there was no significant difference in IL-6 mRNA expression between the two VLPs (p = 0.336), although there was a non-significant increase in the VLP dengue condition (p = 0.055). Under the spike trimer and nucleoprotein stimulation, no significant increase in IL-6 mRNA expression was observed (Figure 2).
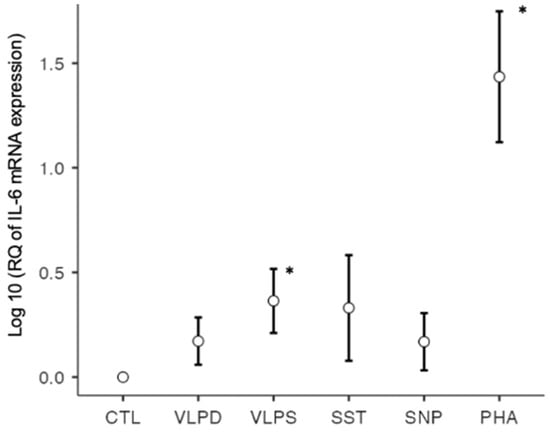
Figure 2.
IL-6 mRNA expression on a logarithmic scale, * p < 0.05; six conditions: negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
Interleukin 13 is a cytokine of the Th2 response and has, similar to IL-4, strong profibrotic properties [26]. Except for PHA, the positive control, there were no other significant RT-qPCR results.
IL-17 plays an important role in the defense against extracellular bacteria and fungi. It is produced by Th17 cells and γδ T cells, often together with IL-22 [27]. In severe SARS-CoV-2 infection, IL-17 is elevated and induces other proinflammatory cytokines such as IL-1, IL-6, and TNF-α, which can lead to tissue damage [5]. IL-17 causes lung remodeling in different pathologies, such as in asthma or COPD, and is suspected to cause pulmonary fibrosis [31]. For example, less bleomycin-induced pulmonary fibrosis was found in IL-17-deficient mice compared to wild types [23]. In our RT-qPCR results, there was a significant F-value for IL-17 (p = 0.010). The cytokine mRNA expression was only significantly upregulated for the VLP dengue stimulation (p = 0.036) (Figure 3). An almost significant p-value was observed for the increased mRNA expression by the nucleoprotein stimulation (p = 0.072). However, the positive control PHA (p = 0.488) and the remaining conditions were not significant.
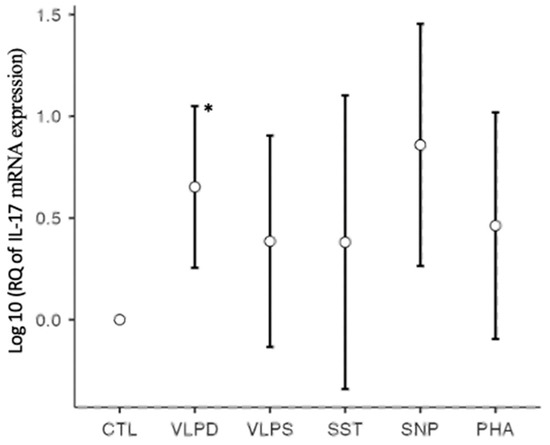
Figure 3.
IL-17 mRNA expression on a logarithmic scale, * p < 0.05; six conditions: negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
TGF-β is produced by various cells, including T lymphocytes. It induces Th17 cells and leads to higher collagen synthesis in fibroblasts [10]. TGF-β is considered to be one of the main stimuli for fibrosis. In SARS-CoV-2, TGF-β is also thought to be upregulated by angiotensin II, which is increased because of the virus-induced reduction in ACE2 receptors [2]. In our results, no significant change was detected for TGF-β mRNA expression by RT-qPCR, except a reduction in the TGF-β mRNA expression under the PHA condition compared to the negative control (p < 0.001).
TNF-α is produced by a variety of different cells. Like IL-6 and IL-1, TNF-α contributes to the COVID-19 cytokine storm [5]. Together with IL-6, it is one of the major cytokines inducing post-ARDS pulmonary fibrosis [16]. In our study, except for the positive control, there were no significant values for TNF-α mRNA expression, independent of the stimulation condition.
3.2.2. RT-qPCR: Antifibrotic Cytokines
The mRNA expression of the antifibrotic cytokines IL10, IL-12, IL-22, and IFN-γ is presented in the following section. IFN-γ has a dual role, as it also represents an important antiviral cytokine [15]. The results are summarized in Table 4.

Table 4.
Gene expression of antifibrotic cytokines measured with RT-qPCR: mean values of 12 blood donors are listed; * p < 0.05; negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
IFN-γ is exclusively produced by CD4+ Th1 cells, by CD8+ cytotoxic T cells, and by NK cells [10]. It has both antiviral and antifibrotic properties [15]. However, it also has a role in the COVID-19 cytokine storm, where persistently high levels of this cytokine can lead to tissue damage [5,15]. In our study, no significant results, except for the positive control, could be observed.
IL-12 is not produced by T cells, but by macrophages and dendritic cells. However, it plays an important role in the Th1 differentiation and in the induction of IFN-γ synthesis [10]. It has antifibrotic properties [26], but is discussed in the context of SARS-CoV-2 due to its role in the cytokine storm [5]. The RT-qPCR did not reveal significant differences in IL-12 mRNA expression.
IL-10 is mainly produced by regulatory T cells (Treg) and macrophages. It has immunosuppressive functions and is also considered to have repressive effects on collagen synthesis. IL-10 has inhibitory effects on antigen-presenting cells and on the expression of major histocompatibility complex II (MHC II) [27]. In our experiment, no significant changes in IL-10 mRNA expression were observed.
IL-22 is produced by Th17 cells [10]. The F-test detected significant differences. However, at the level of the different conditions, only the VLP dengue condition led to an almost significant increase in cytokine expression (p = 0.06).
3.2.3. RT-qPCR: Antiviral Cytokines
Same as for the cytokines IL-12 and IFN-γ discussed in the previous paragraph, IL-2 and IL-18 are classified as antiviral cytokines [32,33]. The results are summarized in Table 5.

Table 5.
Gene expression of antiviral cytokines measured with RT-qPCR: mean values of 12 blood donors are listed; * p < 0.05; negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
IL-2 is produced only by T cells and stimulates the proliferation and differentiation of effector T cells [10]. Except for the positive control, IL-2 mRNA expression was not significantly increased for any of the experimental conditions.
IL-18 is produced by various cells, but not by T cells. Nevertheless, it plays an important role in the induction of the Th1 immune response by stimulating T cells to produce IFN-γ [10]. No significant difference in IL-18 mRNA expression was found between the different conditions by performing RT-qPCR, except for the PHA, where it was significantly decreased.
3.2.4. RT-qPCR: Unclassified Cytokines/Chemokines
In the following section, the RT-qPCR results of the chemokine CXCL10 and the cytokine IL-23 are shown. IL-23 could not be classified based on the available literature. The results are summarized in Table 6.

Table 6.
Gene expression of unclassified cytokines measured with RT-qPCR: mean values of 12 blood donors are listed; * p < 0.05; negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
The chemokine CXCL10 is secreted by many cells of the inflamed tissue and recruits Th1 cells which secrete IFN-γ. CXCL10 has pro- and antifibrotic effects [34]. In the context of SARS-CoV-2, the chemokine is considered a key chemokine for the COVID-19 cytokine storm [9] and its secretion is stimulated by IL-6 [35]. In our study there was a difference between the different conditions (F-statistic, p < 0.001). A significant upregulation of CXCL10 mRNA was observed by the nucleoprotein (p = 0.034), in addition to the positive control with PHA (p < 0.001). The mRNA levels were elevated, but not significant, upon stimulation with VLP SARS (p = 0.112) and spike trimer (p = 0.062) (Figure 4).
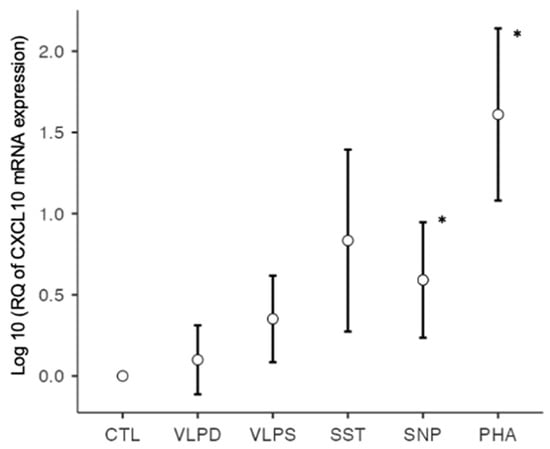
Figure 4.
CXCL10 mRNA expression on a logarithmic scale, * p < 0.05; six conditions: negative control (CTL), VLP dengue (VLPD), VLP SARS-CoV-2 (VLPS), SARS-CoV-2 spike trimer (SST), SARS-CoV-2 nucleoprotein (SNP), positive control (PHA).
IL-23 is not produced by T cells, but by macrophages and dendritic cells, and it plays a role in Th17 differentiation [10]. No significant RT-qPCR results could be observed for IL-23.
3.3. ELISA
ELISA was performed for the same cytokines as for the RT-qPCR, except for IL-13, IL-18, and IL-22 (Table 7). Samples 1–4 and 8–12 were included. Protein concentrations were measured after 24 h of culture, at the same time point as for the determination of mRNA expression in RT-qPCR.

Table 7.
Mean cytokine concentration values under the different conditions in [pg/mL]; each sample was measured in duplicate; ND indicates not done.
An elevation tendency in the cytokines IL-1β and IL-6 was observed under stimulations with both formulations of the spike protein, with the nucleoprotein, but also with the VLP dengue as well as with the positive control. This partly corresponds to the results of RT-qPCR, where IL-1β and IL-6 mRNA expression was increased under the VLP SARS condition, and was elevated under the remaining conditions on a non-significant level. The significant increase in the mRNA level of CXCL10, however, was not reflected in the protein concentration. Since the ELISA results were very heterogeneous, no statistical tests were performed, and therefore no reliable conclusion can be obtained.
4. Discussion
The aim of this in vitro human study was to identify potentially fibrogenic cytokines, including IL-1β, IL-6, IL-13, IL-17, TNF-α, and TGF-β, expressed by PBMC in response to SARS-CoV-2 spike protein. In addition, a panel of other cytokines were investigated, including potentially antifibrotic cytokines (IL10, IL-12, IL-22, and IFN-γ) and antiviral cytokines (IL-2, IL-12, IL-18, and IFN-γ). Two formulations of SARS-CoV-2 spike protein (recombinant spike trimer, VLPs containing recombinant spike protein), as well as corresponding controls (no stimulus, PHA, SARS-CoV-2 nucleoprotein, and VLPs of dengue virus) were applied as stimuli. Cytokine expression was measured by using RT-qPCR and ELISA. In the RT-qPCR, two fibrogenic cytokines emerged with statistically significant results under stimulation with the spike protein. This was the case for IL-1β and IL-6 mRNA expression under the VLP-SARS condition. Furthermore, CXCL10 mRNA expression was increased by SARS-CoV-2 nucleoprotein stimulation.
The proliferation assay allowed us to identify the possible immune status of each blood donor. It can be assumed that three out of twelve patients had been infected, two were vaccinated, and four had never been in contact with the virus. For three blood donors, we were not able to determine their immune status. To reliably evaluate the influence of the immune status on cytokine mRNA expression and cytokine synthesis, we would need to increase the sample size.
IL-1β mRNA expression was significantly increased in PBMC under the VLP SARS condition (p < 0.001) and under the positive control condition (p < 0.001). This cytokine has functions in the innate and adaptive immune responses, and has a proinflammatory effect. It can induce the production of TNF-α and IL-6 and thus amplify the immune response [36]. In the context of SARS-CoV-2 infection, together with IL-6, IL-1β induces the expression of hepatic acute phase proteins and causes fever [37]. In pulmonary fibrosis, increased IL-1β expression is often accompanied by increased TNF expression. Both cytokines can cause acute lung injury and contribute to the progression of fibrosis, among others, through the stimulation of the profibrotic cytokine TGF-β [21]. Published studies showed that IL-1β antagonists can attenuate the progression of fibrosis [21]. In the context of SARS-CoV-2, IL-1β is, together with IL-6, one of the key cytokines in the genesis of the cytokine storm [8] and is one of the cytokines most overexpressed in SARS-CoV-2 infection [38]. IL-1β plasma levels were significantly increased in intensive care unit (ICU) patients [39]. Karina et al. evaluated, in an interventional study, the effect of intravenous activated autologous platelet-rich plasma containing an IL-1 receptor antagonist. This antagonist reduced plasma IL-1β levels and lung injury in critically and severely ill patients, but not to a statistically significant level [38]. Another possibility to intervene at the level of IL-1β is by using the recombinant IL-1 receptor antagonist anakinra, which is used in rheumatoid arthritis [40]. At present, there is not enough evidence for this treatment in the context of COVID-19 and more studies are needed [41].
In our study, a similar pattern was observed for IL-6 mRNA expression as for IL-1β. There was a significant increase under the condition VLP SARS (p = 0.003), accompanied by a significant increase under PHA (p < 0.001). Under VLP dengue, the mRNA was upregulated but did not reach significance (p = 0.055).
Upregulated by IL-1β and TNF-α action, IL-6 is one of the cytokines playing a key role in the COVID-19 cytokine storm due to its pleotropic effects. The increased ANG II concentration in COVID-19 patients may also lead to an upregulation of IL-6, which is a required factor for Th17 cell proliferation [2,6]. In addition, IL-6 stimulates the secretion of various chemokines, such as CCL2 or CXCL8, which stimulate the production of vascular endothelial growth factor (VEGF). VEGF in turn leads to higher vascular permeability. The increased migration of immune cells in the lung tissue leads ultimately to decreased lung function [35]. The production of the chemokine CXCL10 is also stimulated by IL-6 [35]. As for the cytokine IL-6, CXCL10 is correlated with disease severity [6,42]. IL-6 is a proinflammatory and profibrotic cytokine [2]. Together with elevated serum levels of other proinflammatory cytokines, IL-6 level can predict the severity of lung injury [15]. A systematic review and meta-analysis of 22 studies showed a favorable effect on mortality when treating patients with the IL-6 receptor antagonist tocilizumab [43]. Another study showed that in critically ill COVID-19 ICU patients, a combined treatment with tocilizumab or sarilumab and glucocorticoids resulted in an even greater treatment effect than tocilizumab alone [44]. There is not enough evidence on how tocilizumab affects the risk for fibrosis. Although IL-6 is implicated in pulmonary fibrosis development, according to some studies, it may be that anti-IL-6 treatment puts patients at a higher risk of fibrosis [2]. In a bleomycin (BLM)-induced lung fibrosis model in mice, IL-6 was shown to have pro- and antifibrotic properties. The administration of an IL-6 neutralizing antibody in the early inflammatory phase resulted in more pulmonary fibrosis; thereafter, the IL-6 antibody had a mitigating effect [45].
In PCR results, CXCL10 was significantly upregulated under the nucleoprotein (p = 0.034) and the positive control condition (p < 0.001). In our study, the nucleoprotein was added exogenously. However, in virus-infected patients, the nucleoprotein would be expected to be intracellularly expressed, and it is not known whether it is secreted onto the surface of infected cells. The immune-regulatory effects of SARS-CoV-2 nucleoprotein (also called nucleocapsid protein) have been well reviewed by Wang et al. (2022) [46]. However, it remains unclear how an intracellular viral protein may trigger a cellular signal and induce the expression of certain cytokines when added onto the cell surface. Therefore, this mechanism needs further investigation. Finally, CXCL10 mRNA levels for the condition VLP SARS (p = 0.112) and spike trimer (p = 0.062) were elevated, but not to a significant level. The secretion of CXCL10 is stimulated by IL-6 [35], and it is considered to be a key chemokine involved in the COVID-19 cytokine storm [9]. Furthermore, high levels of this chemokine are associated with a poor clinical prognosis, with severe disease progression and ARDS [17,42]. Thus, CXCL10 represents a potential therapeutic target [42]. This chemokine is suspected to play a role in neurological symptoms, such as taste loss. CXCL10 may contribute to liver and myocardial fibrosis [17]. Regarding pulmonary fibrosis, the role of CXCL10 is not fully understood, even though there are some human studies and animal models existing that investigate the role of CXCL10 in pulmonary fibrosis. For example, in a BLM-induced lung fibrosis model in mice, by inhibiting a CXCL10-mediated fibroblast response, an antifibrotic effect was observed [28], whereas, in human lung transplants, a profibrotic effect was observed. Pulmonary fibrosis and subsequent organ failure are causal in patients with chronic graft rejection. During this process, an upregulation of CXCL10 has been detected in patients [29]. In an ARDS mouse model induced by influenza virus, CXCL10-deficient mice developed less lung damage compared to the wild type [47]. Targeting this chemokine could have a beneficial effect on disease outcomes due to its role in the COVID-19 cytokine storm and tissue damage. Interestingly, the antidiabetic drug Sitagliptin of the dipeptidyl peptidase-4 (DPP4) inhibitor class reduces CXCL10 secretion. It has been suggested that this drug can also be used in COVID-19 patients [17].
TGF-β, together with metalloproteinase 1 and 7, is a major stimulus for pulmonary fibrosis in COVID-19 patients [2]. In our study, no trend for TGF-β was observed either in mRNA expression in RT-qPCR, or at protein levels in ELISA results. The verification of this finding may be achieved by including additional time points, such as after 48 h or 72 h of cell culture.
SARS-CoV-2 has the capacity to inhibit the antiviral response, such as the downregulation of IFN-γ [12]. No trend in IFN-γ expression was detected in our RT-qPCR results. Additionally, the IFN-γ protein concentration measured by ELISA did not allow for any conclusions. As we were looking into the influence of the spike protein, by not using the live virus, we can conclude that the spike protein probably does not have a direct effect on the regulation of IFN-γ and does not in this way suppress the antiviral response.
Regarding the ELISA results, no statistical analysis was performed due to the heterogeneity of the data. For IL-1β and IL-6, similar trends could be observed at protein levels measured by ELISA, and mRNA expression measured by RT-qPCR. A CXCL10 mRNA increase was detected by performing RT-qPCR. However, this was not the case regarding the protein concentration, which was not elevated. However, for other cytokines, there was a discrepancy comparing RT-qPCR and ELISA results. It can be assumed that in some cases cytokine synthesis occurs with a time delay from mRNA transcription. In order to obtain a better picture, it would be necessary to have more samples and more temporal measuring points, such as after 24 h, 48 h, and 72 h.
In this study, the effect of different spike protein formulations (VLP SARS, spike trimer) on cytokine expression by PBMC was investigated. The exact mechanism of how the spike protein has an effect on PBMC is not clear and different receptors with an affinity to the spike protein may play a role. A published study showed that the ACE2 receptor expression on T lymphocytes is sufficiently high for the binding of the spike protein and the subsequent viral infection of these cells [48]. Another study discussed a discrepancy between virus tropism for specific cells and their ACE2 expression. In severe COVID-19 disease, there is also a high T cell infection rate. Other receptors, such as CD147, which are expressed on T lymphocytes and have a high affinity for the spike protein, may also play a role in virus binding and cell entry [49].
The VLP SARS formulation of the spike protein seems to induce a much stronger reaction, resulting in significant gene expression of the mentioned fibrogenic cytokines, in comparison with the recombinant spike trimer. The reason for this discrepancy could be the possibility of cross-linking several of the spike protein binding receptors in the case of the VLP SARS formulation, where many spike trimer complexes are close together, integrated in the membrane of the VLP. The cross-linking of membrane receptors at the surface of cells has been shown for various receptors and ligands to induce or enhance intracellular signaling and cellular responses, well known for FcεRI on mast cells, and for other cells and cellular processes [50,51,52], a principle often used by the immune system [53,54]. As VLP SARS mimics the whole virus, and as the surface structure is concerned, our results may also be valid for infectious virus particles, where multiple spike protein trimer complexes are exposed and may cross-link corresponding receptors, not only inducing the uptake of genomic viral RNA, but also triggering additional metabolic and gene regulatory signals into the cell. On the other hand, a high concentration of spike protein circulating in the blood or intercellular fluids may not induce strong inflammatory responses. However, recently published research has indicated that the spike protein interacts with various host cell receptors resulting in corresponding cell signaling [55,56,57].
5. Conclusions
In conclusion, through this in vitro human study, we showed that the expression of fibrogenic cytokines by PBMC in response to SARS-CoV-2 spike protein could be induced. For IL-1β and IL-6, a significant increase in their mRNA expression by spike protein stimulation was observed. CXCL10 mRNA expression was increased by nucleoprotein stimulation. All these cytokines have been previously shown to play an important role in the cytokine storm, in the development of ARDS, and the progression to pulmonary fibrosis. Additionally, these inflammatory mediators represent possible targets for COVID-19 therapy. To study the influence of immune status on cytokine expression, more data are needed. We assume, based on the experimental results, that the spike protein has a direct effect on the expression of cytokines in immune cells. However, the underlying mechanisms need to be studied more in detail. Finally, our results indicate that infectious virus particles or soluble spike protein may activate immune cells in the capillaries and alveolar tissue of the lungs directly through spike protein-dependent signaling, and induce the production of inflammatory and fibrogenic cytokines that contribute to COVID-19-related lung fibrosis.
Author Contributions
Conceptualization, M.A. and L.F.; methodology, L.F.; validation, M.A., I.F., A.O. and L.F.; formal analysis, M.A., I.F., A.O. and L.F.; investigation, M.A., P.B., I.F., A.O. and L.F.; resources, L.F.; data curation, M.A. and L.F.; writing—original draft preparation, M.A.; writing—review and editing, L.F.; visualization, M.A.; supervision, L.F.; project administration, L.F.; funding acquisition, L.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study according to Swiss legislation, due to blood samples being anonymized.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study by the Swiss Red Cross Blood Bank in Bern, Switzerland, allowing the blood donation to be used for in vitro research (project number P_383).
Data Availability Statement
All data in this study were newly generated and are available from the corresponding authors for further processing.
Acknowledgments
We would like to acknowledge Rudolf Rohr and Laurent Falquet for their advice on data analysis and statistics.
Conflicts of Interest
The authors declare no conflict of interest.
References
- George, P.M.; Wells, A.U.; Jenkins, R.G. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 2020, 8, 807–815. [Google Scholar] [CrossRef]
- Mohammadi, A.; Balan, I.; Yadav, S.; Matos, W.F.; Kharawala, A.; Gaddam, M.; Sarabia, N.; Koneru, S.C.; Suddapalli, S.K.; Marzban, S. Post-COVID-19 Pulmonary Fibrosis. Cureus 2022, 14, e22770. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Confalonieri, P.; Pozzan, R.; Tavano, S.; Mondini, L.; Baratella, E.; Pagnin, A.; Lerda, S.; Geri, P.; Biolo, M.; et al. Severe COVID-19 ARDS treated by bronchoalveolar lavage with diluted exogenous pulmonary surfactant as salvage therapy: In pursuit of the Holy Grail. J. Clin. Med. 2022, 11, 3577. [Google Scholar] [CrossRef]
- Maione, F.; Casillo, G.M.; Raucci, F.; Salvatore, C.; Ambrosini, G.; Costa, L.; Scarpa, R.; Caso, F.; Bucci, M. Interleukin-17A (IL-17A): A silent amplifier of COVID-19. Biomed. Pharmacother. 2021, 142, 111980. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Liu, G.-H.; Gao, Y.-D. Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.-D.Z.; Wang, X.-M. CXCL10 an important chemokine associated with cytokine storm in COVID-19 infected patients. Europ. Rev. Med. Pharmacol. Sci. 2020, 24, 7497–7505. [Google Scholar]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology, 10th ed.; Elsevier Ltd.: Philadephia, PA, USA, 2022. [Google Scholar]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.-C.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.-Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef]
- Geng, H.; Subramanian, S.; Wu, L.; Bu, H.F.; Wang, X.; Du, C.; De Plaen, I.G.; Tan, X.-D. SARS-CoV-2 ORF8 Forms Intracellular Aggregates and Inhibits IFNgamma-Induced Antiviral Gene Expression in Human Lung Epithelial Cells. Front. Immunol. 2021, 12, 679482. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Moore Vofel, J.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendation. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lee, J.-H.; Kim, J.; Xiong, F.; Al Hasani, L.; Shi, Y.; Simpson, E.N.; Zhu, X.; Chen, Y.-T.; Shivshankar, P.; et al. SARS-CoV-2 restructures host chromatin architecture. Nat. Microbiol. 2023, 8, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Basheer, M.; Saad, E.; Kananeh, M.; Asad, L.; Khayat, O.; Badarne, A.; Abdo, Z.; Arraf, N.; Milhem, F.; Bassal, T.; et al. Cytokine Patterns in COVID-19 Patients: Which Cytokines Predict Mortality and Which Protect Against? Curr. Issues Mol. Biol. 2022, 44, 4735–4747. [Google Scholar] [CrossRef]
- Vasarmidi, E.; Tsitoura, E.; Spandidos, D.A.; Tzanakis, N.; Antoniou, K.M. Pulmonary fibrosis in the aftermath of the COVID-19 era (Review). Exp. Ther. Med. 2020, 20, 2557–2560. [Google Scholar] [CrossRef]
- Julian, D.R.; Kazakoff, M.A.; Patel, A.; Jaynes, J.; Willis, M.S.; Yates, C.C. Chemokine-Based Therapeutics for the Treatment of Inflammatory and Fibrotic Convergent Pathways in COVID-19. Curr. Pathobiol. Rep. 2021, 9, 93–105. [Google Scholar] [CrossRef]
- Colarusso, C.; Terlizzi, M.; Maglio, A.; Molino, A.; Candia, C.; Vitale, C.; Hansbro, P.M.; Vatrella, A.; Pinto, A.; Sorrentino, R. Activation of the AIM2 Receptor in Circulating Cells of Post-COVID-19 Patients With Signs of Lung Fibrosis Is Associated With the Release of IL-1alpha, IFN-alpha and TGF-beta. Front. Immunol. 2022, 13, 934264. [Google Scholar] [CrossRef]
- Colarusso, C.; Maglio, A.; Terlizzi, M.; Vitale, C.; Molino, A.; Pinto, A.; Vatrella, A.; Sorrentino, R. Post-COVID-19 Patients Who Develop Lung Fibrotic-like Changes Have Lower Circulating Levels of IFN-beta but Higher Levels of IL-1alpha and TGF-beta. Biomedicines 2021, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Delpino, M.V.; Quarleri, J. SARS-CoV-2 Pathogenesis: Imbalance in the Renin-Angiotensin System Favors Lung Fibrosis. Front. Cell. Infect. Microbiol. 2020, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Mehal, W.Z.; Iredale, J.; Friedman, S.L. Scraping fibrosis: Expressway to the core of fibrosis. Nat. Med. 2011, 17, 552–553. [Google Scholar] [CrossRef]
- Ramani, K.; Biswas, P.S. Interleukin-17: Friend or foe in organ fibrosis. Cytokine 2019, 120, 282–288. [Google Scholar] [CrossRef]
- Leask, A. COVID-19: Is fibrosis the killer? J. Cell Commun. Signal. 2020, 14, 255. [Google Scholar] [CrossRef]
- Martinez-Flores, D.; Zepeda-Cervantes, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 Vaccines Based on the Spike Glycoprotein and Implications of New Viral Variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Sziksz, E.; Pap, D.; Lippai, R.; Beres, N.J.; Fekete, A.; Szabo, A.J.; Vannay, A. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediators Inflamm. 2015, 2015, 764641. [Google Scholar] [CrossRef]
- Peter, H.H.; Pichler, W.; Müller-Ladner, U. Klinische Immunologie, 3rd ed.; Urban&Fischer: Munich, Germany, 2012. [Google Scholar]
- Tager, A.M.; Kradin, R.L.; LaCamera, P.; Bercury, S.D.; Campanella, G.S.V.; Leary, C.P.; Polosukhin, V.; Zhao, L.-H.; Sakamoto, H.; Blackwell, T.S.; et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am. J. Respir. Cell Mol. Biol. 2004, 31, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Sacreas, A.; Yang, J.Y.C.; Vanaudenaerde, B.M.; Sigdel, T.K.; Liberto, J.M.; Damm, I.; Verleden, G.M.; Vos, R.; Verleden, S.E.; Sarwal, M.M. The common rejection module in chronic rejection post lung transplantation. PLoS ONE 2018, 13, e0205107. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, A.; Naser, A.Y.; Alwafi, H.; Minshawi, F. Profile of Circulatory Cytokines and Chemokines in Human Coronaviruses: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 666223. [Google Scholar] [CrossRef] [PubMed]
- Gurczynski, S.J.; Moore, B.B. IL-17 in the lung: The good, the bad, and the ugly. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L6–L16. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, I.A.; Ramsay, A.J.; Karupiah, G.; Rolph, M.S.; Mahalingam, S.; Ruby, J.C. Cytokines and immunity to viral infections. Immunol. Rev. 1997, 159, 119–135. [Google Scholar] [CrossRef]
- Keyel, P.A. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine 2014, 69, 136–145. [Google Scholar] [CrossRef]
- Kheradmand, F.; Corry, D.B. Chemokines, CXC / CXCL10. In Encyclopedia of Respiratory Medicine, 1st ed.; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Cambridge, MA, USA, 2006; pp. 402–407. [Google Scholar]
- Coperchini, F.; Chiovato, L.; Rotondi, M. Interleukin-6, CXCL10 and Infiltrating Macrophages in COVID-19-Related Cytokine Storm: Not One for All But All for One! Front. Immunol. 2021, 12, 668507. [Google Scholar] [CrossRef]
- Declercq, J.; De Leeuw, E.; Lambrecht, B.N. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine 2022, 157, 155934. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Kritas, S.K.; Frydas, I.; Younas, A.; Ronconi, G. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: A promising inhibitory strategy. J. Biol. Regul. Homeost. Agents 2020, 34, 1971–1975. [Google Scholar] [PubMed]
- Karina, K.; Christoffel, L.M.; Novariani, R.; Rosadi, I.; Rosliana, I.; Rosidah, S.; Sobariah, S.; Fatkhurohman, N.; Puspitaningrum, N.; Hertati, Y.; et al. The Effect of Intravenous Autologous Activated Platelet-Rich Plasma Therapy on “Profibrotic Cytokine” IL-1beta Levels in Severe and Critical COVID-19 Patients: A Preliminary Study. Scientifica 2021, 2021, 9427978. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.-M.; Bezie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020, 2, e393–e400. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021, 76, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. What Is Currently Known about the Role of CXCL10 in SARS-CoV-2 Infection? Int. J. Mol. Sci. 2022, 23, 3673. [Google Scholar] [CrossRef] [PubMed]
- Berardicurti, O.; Ruscitti, P.; Ursini, F.; D’Andrea, S.; Ciaffi, J.; Meliconi, R.; Iagnocco, A.; Cipriani, P.; Giacomelli, R. Mortality in tocilizumab-treated patients with COVID-19: A systematic review and meta-analysis. Clin. Exp. Rheumatol. 2020, 38, 1247–1254. [Google Scholar] [PubMed]
- REMAP-GAP Investigators. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tanaka, K.; Fujita, T.; Umezawa, H.; Amano, H.; Yoshioka, K.; Naito, Y.; Hatano, M.; Kimura, S.; Tatsumi, K.; et al. Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respir. Res. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.; Yu, X.; Lan, H.-Y. Signaling mechanisms of SARS-CoV-2 nucleocapsid protein in viral infection, cell death and inflammation. Int. J. Biol. Sci. 2022, 18, 4704–4713. [Google Scholar] [CrossRef]
- Ichikawa, A.; Kuba, K.; Morita, M.; Chida, S.; Tezuka, H.; Hara, H.; Sasaki, T.; Ohteki, T.; Ranieri, V.M.; dos Santos, C.C.; et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am. J. Respir. Crit. Care Med. 2013, 187, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Welch, J.L.; Xiang, J.; Chang, Q.; Houtman, J.C.D.; Stapleton, J.T. T-Cell Expression of Angiotensin-Converting Enzyme 2 and Binding of Severe Acute Respiratory Coronavirus 2. J. Infect. Dis. 2022, 225, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef] [PubMed]
- Oettgen, H.C.J. Mast cells in food allergy: Inducing immediate reactons and shaping long-term immunity. Allergy Clin. Immunol. 2023, 151, 21–25. [Google Scholar] [CrossRef]
- Chen, A.; Moy, V.T. Cross-linking of cell surface receptors enhances cooperiativity of moleuclar adhesion. Biophys. J. 2000, 78, 2814–2820. [Google Scholar] [CrossRef]
- Moody, P.R.; Syers, E.J.; Magnusson, J.P.; Alexander, C.; Borri, P.; Watson, P.; Jones, A.T. Receptor crosslinking: A general method to trigger internalization and lysosomal targeting of therapeutic receptor-ligand comples. Mol. Ther. 2015, 23, 12. [Google Scholar] [CrossRef]
- Mkaddem, S.B.; Benhamou, M.; Monteiro, R.C. Understanding Fc receptor involvement in inflammatory diseases: From mechanisms to new therapeutic tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Garcia, V.E.; Chluyan, H.E. SLAM and CD31: Signaling molecules involved in cytokine secretion druing the development of innate and adaptive immune responses. Cytokine Growth Factor Rev. 2007, 18, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein mediated cell signaling in lung vasular cells. Vascul. Pharmacol. 2021, 137, 106923. [Google Scholar] [CrossRef] [PubMed]
- Palakkott, A.R.; Alneyadi, A.; Muhammad, K.; Eid, A.H.; Amiri, K.M.A.; Ayoub, M.A.; Iratni, R. The SARS-CoV-2 spike protein activates epidermal growth ractor receptor-mediated signaling. Vaccines 2023, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Santos Alves, V.; Silva Santos, S.A.C.; Leite-Aguiar, R.; Rapiva-Pereira, E.; Rodrigues dos Reis, R.; Calazans, M.L.; Gripp Fernandes, G.; Silva Antonio, L.; de Lima, E.V.; Kurtenbach, E.; et al. SARS-CoV-2 spike protein alters microglial purinergic signaling. Front. Immunol. 2023, 14, 1158460. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).