Assessment of Serum Electrolytes, Biochemical, and Inflammatory Markers in Predicting COVID-19 Severity in COPD Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Preparation and Assay
2.3. Statistical Analysis
2.4. Ethics Approval and Consent to Participate
3. Results
3.1. Sociodemographic Characteristics and Comorbidities of the Study Participants
3.2. Clinical Manifestations among the Study Participants
3.3. Clinical Laboratory Findings
3.3.1. Serum Electrolytes
3.3.2. Serum Biochemical Parameters
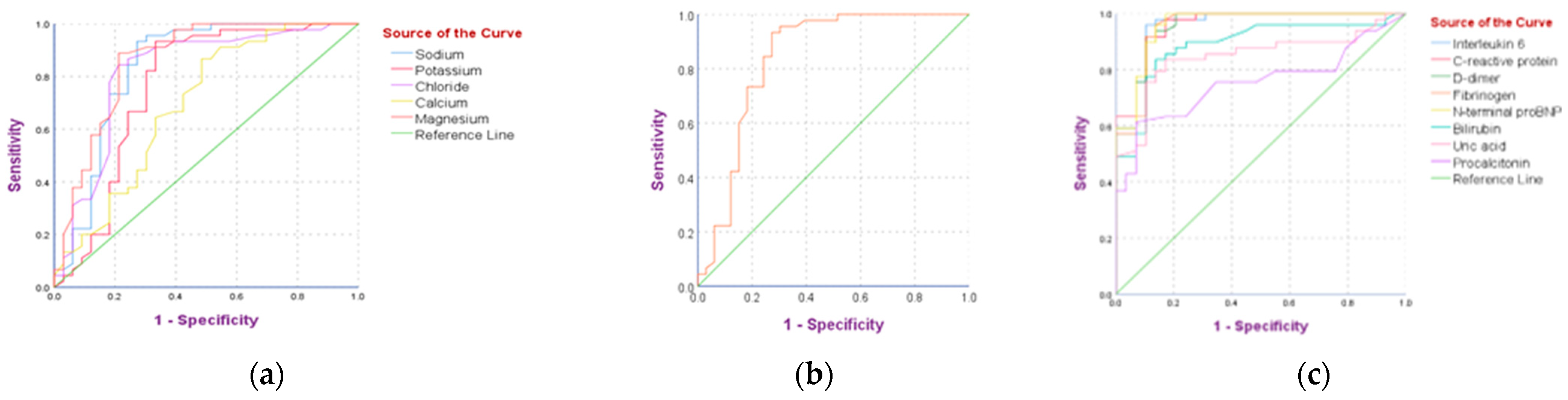
3.3.3. Risk Factors Predicting Disease Severity in Subjects with Concurrent COIVD-19 and COPD
3.3.4. Associations among Clinical Laboratory Markers in COVID-19 Subjects with and without COPD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE). Johns Hopkins University. 2020. Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 9 August 2022).
- COVID-19 Dynamic Dashboard for Bangladesh. Directorate General of Health Services (DGHS). 2022. Available online: http://dashboard.dghs.gov.bd/webportal/pages/covid19.php (accessed on 9 August 2022).
- Gudbjartsson, D.F.; Helgason, A.; Jonsson, H.; Magnusson, O.T.; Melsted, P.; Norddahl, G.L.; Saemundsdottir, J.; Sigurdsson, A.; Sulem, P.; Agustsdottir, A.B.; et al. Spread of SARS-CoV-2 in the Ice-landic population. N. Engl. J. Med. 2020, 382, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Shanjana, Y.; Tushar, M.I.; Mahmud, T.; Rahman, G.M.S.; Milan, Z.H.; Sultana, T.; Chowdhury, A.M.L.H.; Bhuiyan, M.A.; Islam, R.; et al. Hematological abnormalities and comor-bidities are associated with COVID-19 severity among hospitalized patients: Experience from Bangladesh. PLoS ONE 2021, 16, e0255379. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). The top 10 causes of death. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 10 August 2022).
- Sutradhar, I.; Das Gupta, R.; Hasan, M.; Wazib, A.; Sarker, M. Prevalence and risk factors of chronic obstructive pulmonary disease in Bangladesh: A systematic review. Cureus 2019, 11, e3970. [Google Scholar] [CrossRef]
- Leung, J.M.; Niikura, M.; Yang, C.W.T.; Sin, D.D. COVID-19 and COPD. Eur. Respir. J. 2020, 56, 2002108. [Google Scholar] [CrossRef] [PubMed]
- Higham, A.; Mathioudakis, A.; Vestbo, J.; Singh, D. COVID-19 and COPD: A narrative review of the basic science and clinical outcomes. Eur. Respir. Rev. 2020, 29, 200199. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Ting, Y.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; The Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Lian, N.; Deng, Y.; Lin, S. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J. Med. Virol. 2020, 92, 1915–1921. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Wang, Z.; Xie, M.; Shi, Z.; Tang, Z.; Li, X.; Li, X.; Lei, C.; Li, U.; et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: A multicenter, retrospective, observational study. J. Thorac. Dis. 2020, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Gemicioglu, B.; Uzun, H.; Borekci, S.; Karaali, R.; Kurugoglu, S.; Atukeren, P.; Sirolu, S.; Durmus, S.; Dirican, A.; Kuskucu, M.A.; et al. Focusing on Asthma and Chronic Obstructive Pulmonary Disease with COVID-19. J. Infect. Dev. Ctries. 2021, 15, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Ceriello, A.; Misra, A.; Aschner, P.; McDonnell, M.; Hassanein, M.; Ji, L.; Mbanya, J.; Fonseca, V. COVID-19 in people living with diabetes: An international consensus. J. Diabetes Its Complicat. 2020, 34, 107671. [Google Scholar] [CrossRef]
- Pauwels, R.A.; Buist, A.S.; Calverley, P.M.; Jenkins, C.R.; Hurd, S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 2001, 163, 1256–1276. [Google Scholar] [CrossRef]
- Patel, A.; Jernigan, D.B. Initial public health response and interim clinical guidance for the 2019 novel coronavirus out-break—United States, December 31, 2019–February 4, 2020. Am. J. Transplant. 2020, 69, 140. [Google Scholar]
- Clinical Spectrum of SARS-CoV-2 Infection. National Institutes of Health. 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ (accessed on 10 August 2022).
- Association, W.M. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- National Institutess of Health. Clinical Spectrum of SARS-CoV-2 Infection; National Institutes of Health: Bethesda, MD, USA, 2022. [Google Scholar]
- Mannino, D.M.; Ford, E.S.; Redd, S.C. Obstructive and restrictive lung disease and markers of inflammation: Data from the third national health and nutrition examination. Am. J. Med. 2003, 114, 758–762. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Wang, D.; Li, R.; Wang, J.; Jiang, Q.; Gao, C.; Yang, J.; Ge, L.; Hu, Q. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: A descriptive study. BMC Infect. Dis. 2020, 20, 519. [Google Scholar] [CrossRef]
- Sadiq, A.; Khurram, M.; Malik, J.; Chaudhary, N.A.; Khan, M.M.; Yasmeen, T.; Bhatti, H.W. Correlation of biochemical profile at admission with severity and outcome of COVID-19. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-K.; Zhang, W.-H.; Zou, L.; Liu, Y.; Li, J.-J.; Kan, X.-H.; Dai, L.; Shi, Q.-K.; Yuan, S.-T.; Yu, W.-K.; et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging 2020, 12, 11287–11295. [Google Scholar] [CrossRef]
- Chalela, R.; González-García, J.G.; Chillarón, J.J.; Valera-Hernández, L.; Montoya-Rangel, C.; Badenes, D.; Mojal, S.; Gea, J. Impact of hypo-natremia on mortality and morbidity in patients with COPD exacerbations. Respir. Med. 2016, 117, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Gumus, A.; Haziroglu, M.; Gunes, Y. Association of Serum Magnesium Levels with Frequency of Acute Exacerbations in Chronic Obstructive Pulmonary Disease: A Prospective Study. Pulm. Med. 2014, 2014, 329476. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Grewal, P.; Hotelling, J.; Papamanoli, A.; Cao, K.; Dhaliwal, S.; Jacob, R.; Mojahedi, A.; Bloom, M.E.; Marcos, L.A.; et al. Admission NT-proBNP and outcomes in patients without history of heart failure hospitalized with COVID-19. ESC Hear. Fail. 2021, 8, 4278–4287. [Google Scholar] [CrossRef]
- Bansal, A.; Kumar, A.; Patel, D.; Puri, R.; Kalra, A.; Kapadia, S.R.; Reed, G.W. Meta-analysis Comparing Outcomes in Patients with and Without Cardiac Injury and Coronavirus Disease 2019 (COVID-19). Am. J. Cardiol. 2020, 141, 140–146. [Google Scholar] [CrossRef]
- Medina, A.M.; Marteles, M.S.; Sáiz, E.B.; Martínez, S.S.; Laiglesia, F.R.; Rodríguez, J.A.N.; Pérez-Calvo, J.I. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur. J. Intern. Med. 2011, 22, 167–171. [Google Scholar] [CrossRef]
- Høiseth, A.D.; Omland, T.; Hagve, T.A.; Brekke, P.H.; Søyseth, V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD–a prospective cohort study. Respir. Res. 2012, 13, 97. [Google Scholar] [CrossRef]
- Zwaenepoel, B.; Dhont, S.; Hoste, E.; Gevaert, S.; Schaubroeck, H. The Prognostic Value of Cardiac Biomarkers and Echocardiography in Critical COVID-19. Front. Cardiovasc. Med. 2021, 8, 752237. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, D.; Wen, X.-S.; Cheng, X.-C.; Sun, M.; He, B.; You, L.-N.; Lei, P.; Tan, X.-W.; Qin, S.; et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir. Res. 2020, 21, 83. [Google Scholar] [CrossRef]
- Wang, L.; Chen, F.; Bai, L.; Bai, L.; Huang, Z.; Peng, Y. Association between NT-proBNP Level and the Severity of COVID-19 Pneumonia. Cardiol. Res. Pract. 2021, 2021, 5537275. [Google Scholar] [CrossRef]
- Wang, Q.P.; Cao, X.Z.; Wang, X.D.; Gu, J.; Wen, L.M.; Mao, L.M.; Shan, P.N.; Tang, A.G. Utility of NT-proBNP for identifying LV failure in patients with acute exacerbation of chronic bronchitis. PLoS ONE 2013, 8, e52553. [Google Scholar]
- AboEl-Magd, G.H.; Hassan, T.; Aly, M.H.; Mabrouk, M.M. Echocardiography and N-terminal-pro-brain natriuretic peptide in as-sessment of left ventricular diastolic dysfunction in stable COPD in relation to disease severity. Egypt J. Chest Dis. Tuberc. 2017, 66, 75–80. [Google Scholar] [CrossRef]
- Méndez-Sánchez, N.; Vítek, L.; Aguilar-Olivos, N.E.; Uribe, M. Bilirubin as a Biomarker in Liver Disease. In Biomarkers in Liver Disease; Patel, V.B., Preedy, V.R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 81–304. [Google Scholar] [CrossRef]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis. Markers 2015, 2015, 382918. [Google Scholar] [CrossRef]
- Araç, S.; Özel, M. A new parameter for predict the clinical outcome of patients with COVID-19 pneumonia: The direct/total bilirubin ratio. Int. J. Clin. Pract. 2021, 75, e14557. [Google Scholar] [CrossRef]
- Koseki, T.; Nakajima, K.; Iwasaki, H.; Yamada, S.; Takahashi, K.; Doi, Y.; Mizuno, T. Baseline uric acid levels and steady-state favipiravir concentrations are associated with occurrence of hyperuricemia among COVID-19 patients. Int. J. Infect. Dis. 2021, 115, 218–223. [Google Scholar] [CrossRef]
- Tian, F.; Song, W.; Wang, L.; Zeng, Q.; Zhao, Z.; Feng, N.; Fan, J.; Wang, Y.; Wang, J.; Ma, X. NT-pro BNP in AECOPD-PH: Old biomarker, new insights-based on a large retrospective case-controlled study. Respir. Res. 2021, 22, 321. [Google Scholar] [CrossRef]
- Bartziokas, K.; Papaioannou, A.I.; Loukides, S.; Papadopoulos, A.; Haniotou, A.; Papiris, S.; Kostikas, K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2013, 43, 43–53. [Google Scholar] [CrossRef]
- Mannino, D.M.; Thorn, D.; Swensen, A.; Holguin, F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur. Respir. J. 2008, 32, 962–969. [Google Scholar] [CrossRef]
- Chan, N.C.; Weitz, J.I. COVID-19 coagulopathy, thrombosis, and bleeding. Blood 2020, 136, 381. [Google Scholar] [CrossRef]
- Di Micco, P.; Russo, V.; Carannante, N.; Imparato, M.; Cardillo, G.; Lodigiani, C. Prognostic Value of Fibrinogen among COVID-19 Patients Admitted to an Emergency Department: An Italian Cohort Study. J. Clin. Med. 2020, 9, 4134. [Google Scholar] [CrossRef]
- Sui, J.; Noubouossie, D.F.; Gandotra, S.; Cao, L. Elevated Plasma Fibrinogen Is Associated with Excessive Inflammation and Disease Severity in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2021, 11, 734005. [Google Scholar] [CrossRef]
- Murat, S.; Murat, B.; Dural, M.; Mert, G.O.; Cavusoglu, Y. Prognostic value of D-dimer/fibrinogen ratio on in-hospital outcomes of patients with heart failure and COVID-19. Biomarkers Med. 2021, 15, 1519–1528. [Google Scholar] [CrossRef]
- Mohan, M.; Parthasarathi, A.; Chaya, S.K.; Siddaiah, J.B.; Mahesh, P.A. Fibrinogen: A Feasible Biomarker in Identifying the Severity and Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Cureus 2021, 13, e16864. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Tian, F.; Ge, L.; Shang, X.; Zeng, Q.; Feng, N.; Fan, J.; Wang, J.; Ma, X. Clinical Significance of Procalcitonin, C-Reactive Protein, and Interleukin-6 in Helping Guide the Antibiotic Use for Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Dis. Markers 2021, 2021, 8879401. [Google Scholar] [CrossRef]
- Aziz, M.; Fatima, R.; Assaly, R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020, 92, 2283. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, B.; Qu, Y.; Chen, Y.; Xiong, J.; Feng, Y.; Men, D.; Huang, Q.; Liu, Y.; Yang, B.; et al. Detectable Serum Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load (RNAemia) Is Closely Correlated with Drastically Elevated Interleukin 6 Level in Critically Ill Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2020, 71, 1937–1942. [Google Scholar] [CrossRef]
- Garcia-Rio, F.; Miravitlles, M.; Soriano, J.B.; Muñoz, L.; Duran-Tauleria, E.; Sánchez, G.; Sobradillo, V.; Ancochea, J.; EPI-SCAN Steering Committee. Systemic inflammation in chronic ob-structive pulmonary disease: A population-based study. Respir Res. 2010, 11, 63. [Google Scholar] [CrossRef]
- Yasuda, N.; Gotoh, K.; Minatoguchi, S.; Asano, K.; Nishigaki, K.; Nomura, M.; Ohno, A.; Watanabe, M.; Sano, M.; Kumada, M. An increase of soluble Fas, an inhibitor of apoptosis, associated with progression of COPD. Respir. Med. 1998, 92, 993–999. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D. Association between chronic obstructive pulmonary disease and systemic in-flammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef]
- Herold, T.; Jurinovic, V.; Arnreich, C.; Lipworth, B.J.; Hellmuth, J.C.; von Bergwelt-Baildon, M.; Klein, M.; Weinberger, T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020, 146, 128–136.e4. [Google Scholar] [CrossRef]
- Luo, X.; Zhou, W.; Yan, X.; Guo, T.; Wang, B.; Xia, H.; Ye, L.; Xiong, J.; Jiang, Z.; Liu, Y.; et al. Prognostic Value of C-Reactive Protein in Patients with Coronavirus 2019. Clin. Infect. Dis. 2020, 71, 2174–2179. [Google Scholar] [CrossRef]
- Prasetya, I.B.; Cucunawangsih; Lorens, J.O.; Sungono, V.; El-Khobar, K.E.; Wijaya, R.S.O.; Sungono, V.; El-Khobar, K.E.; Wijaya, R.S. Prognostic value of inflammatory markers in patients with COVID-19 in Indonesia. Clin. Epidemiol. Glob. Health 2021, 11, 100803. [Google Scholar] [CrossRef]
- Demirtaş, E.; Demirtaş, E. Diagnostic value of neutrophil to lymphocyte ratio to rule out chronic obstructive pulmonary disease exacerbation from acute heart failure in the emergency department. Disaster Emerg. Med. J. 2019, 4, 102–108. [Google Scholar] [CrossRef]
- Pancirov, D.; Radišić Biljak, V.; Stjepanović, G.; Čepelak, I. Hematological markers of anemia and C-reactive protein in patients with stable chronic obstructive pulmonary disease. Biochem. Med. 2009, 19, 266–276. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Zeng, K.; Deng, F.; Lin, C.; Huang, W. Interleukin-6 is a Strong Predictor of the Frequency of COPD Exac-erbation Within 1 Year. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2945. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Z.; Zhao, X.; Guo, F.; Wang, T.; Zhu, F. Determinants of increased fibrinogen in COVID-19 patients with and without diabetes and impaired fasting glucose. Clin. Appl. Thromb. 2021, 27, 1076029621996445. [Google Scholar] [CrossRef]
- Debi, H.; Itu, Z.T.; Amin, M.T.; Hussain, F.; Hossain, M.S. Association of serum C-reactive protein (CRP) and D-dimer concentration on the severity of COVID-19 cases with or without diabetes: A systematic review and meta-analysis. Expert Rev. Endocrinol. Metab. 2022, 17, 83–93. [Google Scholar] [CrossRef]
- Caro-Codón, J.; Rey, J.R.; Buño, A.; Iniesta, A.M.; Rosillo, S.O.; Castrejon-Castrejon, S.; Rodriguez-Sotelo, L.; Martinez, L.A.; Marco, I.; Merino, C.; et al. Characterization of NT-proBNP in a large cohort of COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 456–464. [Google Scholar] [CrossRef]
- Kesmez Can, F.; Özkurt, Z.; Öztürk, N.; Sezen, S. Effect of IL-6, IL-8/CXCL8, IP-10/CXCL 10 levels on the severity in COVID-19 infection. Int. J. Clin. Pract. 2021, 75, e14970. [Google Scholar] [CrossRef] [PubMed]
- Avila-Nava, A.; Cortes-Telles, A.; Torres-Erazo, D.; López-Romero, S.; Aké, R.C.; Solis, A.L.G. Serum IL-6: A potential biomarker of mortality among SARS-CoV-2 infected patients in Mexico. Cytokine 2021, 143, 155543. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef]
- Jøntvedt Jørgensen, M.; Holter, J.C.; Christensen, E.E.; Schjalm, C.; Tonby, K.; Pischke, S.E.; Jenum, S.; Skeie, L.G.; Nur, S.; Lind, A.; et al. Increased interleukin-6 and macro-phage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci. Rep. 2020, 10, 21697. [Google Scholar] [CrossRef]
- Ergan, B.; Şahin, A.A.; Topeli, A. Serum Procalcitonin as a Biomarker for the Prediction of Bacterial Exacerbation and Mortality in Severe COPD Exacerbations Requiring Mechanical Ventilation. Respiration 2016, 91, 316–324. [Google Scholar] [CrossRef]
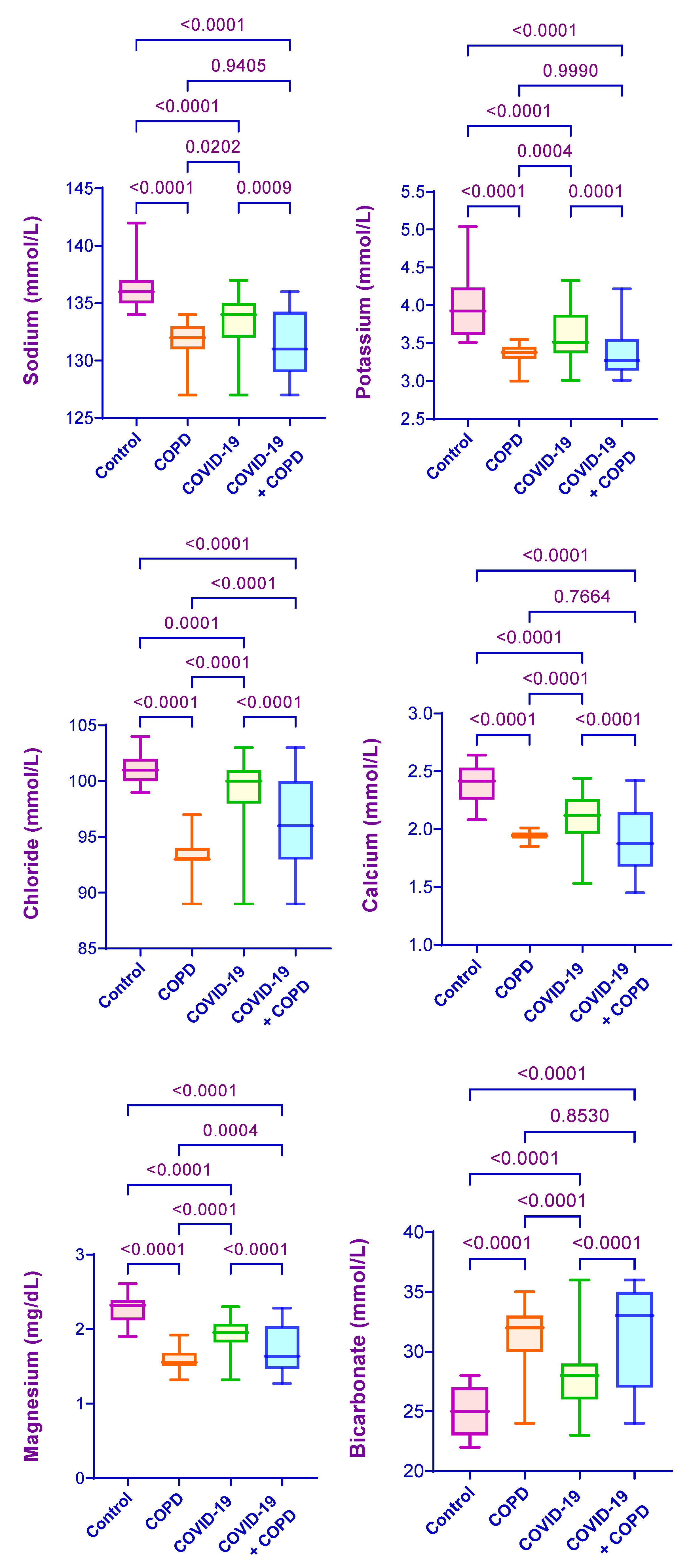
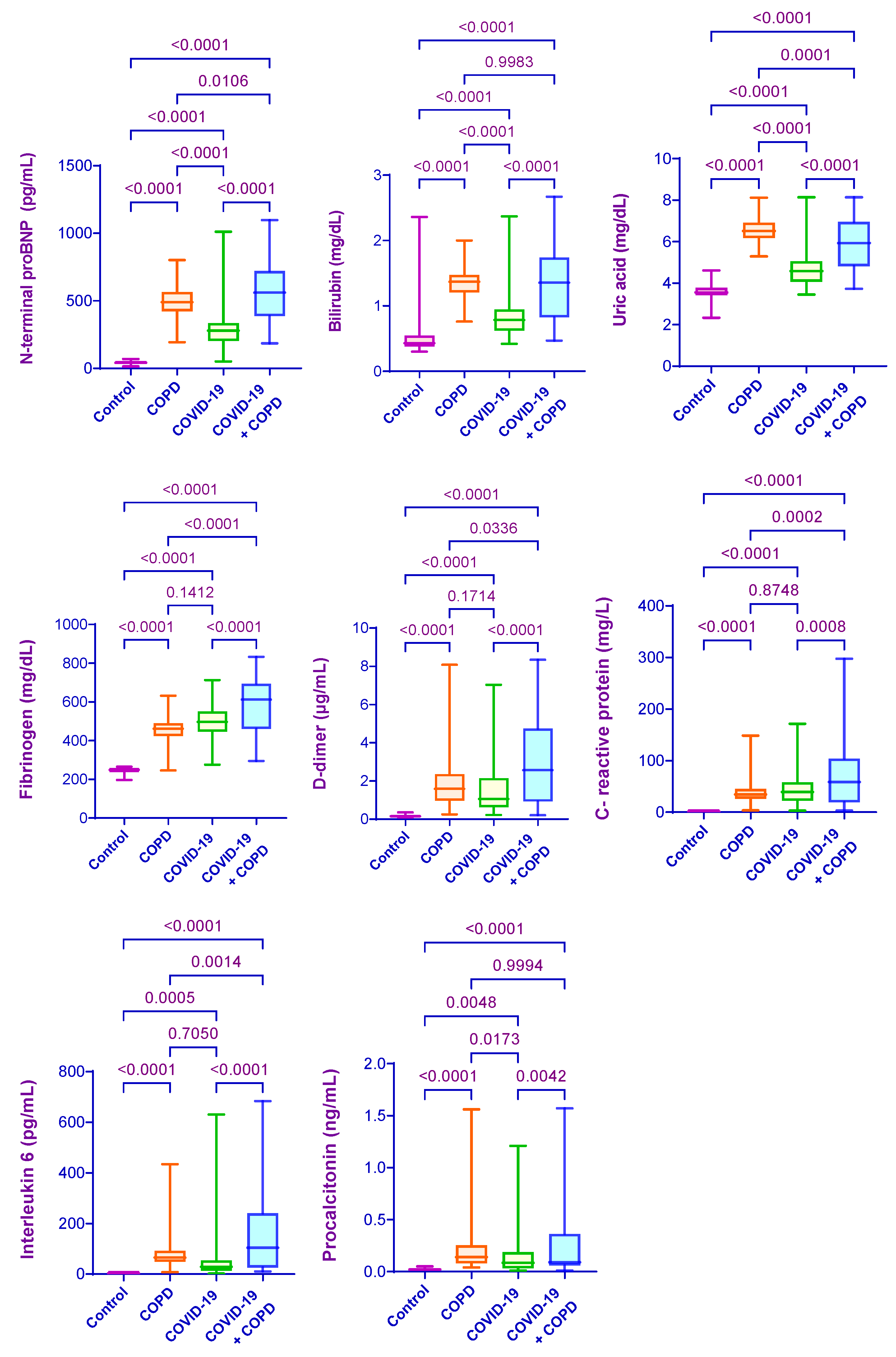
| Variables | Control (n = 208) | COPD (n = 392) | COVID-19 (n = 410) | COVID-19 + COPD (n = 562) | F/χ2 | p Value |
|---|---|---|---|---|---|---|
| Age (years) | 48.40 ± 9.30 | 50.35 ± 9.87 | 49.09 ± 9.12 | 51.94 ± 9.78 | 2.247 | 0.0828 |
| BMI (kg/m2) | 24.02 ± 2.01 | 21.74 ± 2.51 a | 24.26 ± 1.94 b | 21.97 ± 2.59 a,c | 25.53 | <0.0001 |
| Gender | ||||||
| Male | 113 (54.3%) | 239 (61.0%) | 226 (55.1%) | 323 (57.5%) | 3.711 | 0.2944 |
| Female | 95 (45.7%) | 153 (39.0%) | 184 (44.9%) | 239 (42.5%) | ||
| Residency status | ||||||
| Rural | 74 (35.6%) | 246 (62.8%) | 167 (40.7%) | 360 (64.1%) | 92.41 | <0.0001 |
| Urban | 134 (64.4%) | 146 (37.2%) | 243 (59.3%) | 202 (35.9%) | ||
| Smoking status | ||||||
| Never | 149 (71.6%) | 158 (40.3%) | 281 (68.5%) | 266 (47.3%) | 261.2 | <0.0001 |
| Current | 43 (20.7%) | 44 (11.2%) | 87 (21.2%) | 36 (6.4%) | ||
| Former | 16 (7.7%) | 190 (48.5%) | 42 (10.3%) | 260 (46.3%) | ||
| Comorbidities | ||||||
| Hypertension | 23 (11.1%) | 124 (31.6%) | 107 (26.1%) | 224 (39.9%) | 64.40 | <0.0001 |
| Diabetes | 14 (6.7%) | 66 (16.8%) | 58 (14.1%) | 129 (23.0%) | 32.06 | <0.0001 |
| CAD | 3 (1.4%) | 44 (11.2%) | 27 (6.6%) | 71 (12.6%) | 28.13 | <0.0001 |
| Stroke | 0 (0%) | 22 (5.6%) | 11 (2.7%) | 36 (6.4%) | 19.24 | 0.0002 |
| Symptoms | COPD (n = 392) | COVID-19 (n = 410) | COVID-19 + COPD (n = 562) | χ2 | p Value |
|---|---|---|---|---|---|
| Fever (≥38.0 ℃) | 87 (22.2%) | 398 (97.1%) | 558 (99.3%) | 901.0 | <0.0001 |
| Headache | 124 (31.6%) | 108 (26.3%) | 159 (28.3%) | 2.809 | 0.2455 |
| Cough | 225 (57.4%) | 266 (64.9%) | 403 (71.7%) | 21.05 | <0.0001 |
| Fatigue | 312 (79.6%) | 211 (51.5%) | 464 (82.6%) | 129.0 | <0.0001 |
| Dizziness | 269 (68.6%) | 87 (21.2%) | 412 (73.3%) | 295.4 | <0.0001 |
| Nausea | 14 (3.6%) | 75 (18.3%) | 107 (19.0%) | 52.23 | <0.0001 |
| Vomiting | 11 (2.8%) | 43 (10.5%) | 63 (11.2%) | 23.52 | <0.0001 |
| Smell or taste loss | 7 (1.8%) | 275 (67.1%) | 389 (69.2%) | 495.1 | <0.0001 |
| Abdominal pain | 0 (0%) | 81 (19.8%) | 173 (30.8%) | 144.9 | <0.0001 |
| Diarrhea | 0 (0%) | 59 (14.4%) | 158 (28.1%) | 137.4 | <0.0001 |
| Rhinorrhea | 0 (0%) | 162 (39.5%) | 194 (34.5%) | 197.3 | <0.0001 |
| Nasal congestion | 22 (5.6%) | 81 (19.8%) | 102 (18.1%) | 38.68 | <0.0001 |
| Sputum production | 291(74.2%) | 146 (35.6%) | 424 (75.4%) | 190.8 | <0.0001 |
| Sore throat | 49 (12.5%) | 83 (20.2%) | 109 (19.4%) | 10.22 | 0.0060 |
| Hemoptysis | 55 (14.0%) | 11 (2.7%) | 94 (16.7%) | 47.96 | <0.0001 |
| Dyspnea | 348 (88.8%) | 162 (39.5%) | 519 (92.3%) | 410.0 | <0.0001 |
| Chest tightness | 225 (57.4%) | 77 (18.8%) | 361 (64.2%) | 213.1 | <0.0001 |
| Wheeze | 334 (85.2%) | 0 (0%) | 503 (89.5%) | 932.8 | <0.0001 |
| Peripheral edema | 94 (24.0%) | 0 (0%) | 123 (21.9%) | 111.7 | <0.0001 |
| Oxygen saturation (SpO2) < 94% | 218 (55.6%) | 92 (22.4%) | 353 (62.8%) | 165.5 | <0.0001 |
| Clinical Parameters | GOLD-1 (n = 237) | GOLD-2 (n = 185) | GOLD-3 (n = 89) | GOLD-4 (n = 51) | p Value |
|---|---|---|---|---|---|
| Sodium | 134.68 ± 0.81 | 130.39 ± 0.76 a | 129.75 ± 1.32 a,b | 128.44 ± 1.93 a,b,c | <0.0001 |
| Potassium | 3.63 ± 1.09 | 3.19 ± 1.20 a | 3.09 ± 0.68 a | 3.03 ± 0.53 a,b | <0.0001 |
| Chloride | 100.32 ± 2.06 | 95.17 ± 1.97 a | 92.46 ± 4.14 a,b | 90.11 ± 2.32 a,b,c | <0.0001 |
| Calcium | 2.15 ± 0.58 | 1.90 ± 0.23 a | 1.59 ± 0.81 a,b | 1.57 ± 0.70 a,b | <0.0001 |
| Magnesium | 2.05 ± 0.73 | 1.62 ± 0.42 a | 1.41 ± 0.67 a,b | 1.36 ± 0.33 a,b | <0.0001 |
| Bicarbonate | 27.98 ± 1.97 | 33.02 ± 2.35 a | 34.58 ± 1.04 a,b | 35.33 ± 0.17 a,b | <0.0001 |
| NT-proBNP | 352.06 ± 83.40 | 535.58 ± 121.18 a | 774.08 ± 98.27 a,b | 933.68 ± 157.53 a,b,c | <0.0001 |
| Bilirubin | 0.87 ± 0.25 | 1.57 ± 0.38 a | 1.86 ± 0.64 a,b | 2.33 ± 0.15 a,b,c | <0.0001 |
| Uric acid | 4.78 ± 1.15 | 6.20 ± 0.54 a | 7.11 ± 0.85 a,b | 7.84 ± 1.07 a,b,c | <0.0001 |
| Fibrinogen | 434.83 ± 164.24 | 642.03 ± 115.30 a | 720.64 ± 172.92 a,b | 792.01 ± 217.67 a,b | <0.0001 |
| D-dimer | 0.89 ± 1.72 | 3.14 ± 2.05 a | 5.51 ± 1.47 a,b | 7.45 ± 2.96 a,b,c | <0.0001 |
| C-reactive protein | 29.88 ± 26.11 | 74.67 ± 76.09 a | 122.67 ± 75.42 a,b | 218.48 ± 85. 64 a,b,c | <0.0001 |
| Interleukin-6 | 41.83 ± 29.51 | 145.86 ± 78.94 a | 357.38 ± 103.47 a,b | 568.07 ± 142.63 a,b,c | <0.0001 |
| Procalcitonin | 0.04903 ± 0.04 | 0.1779 ± 0.09 a | 0.5233 ± 0.13 a,b | 0.9971 ± 0.32 a,b,c | <0.0001 |
| Clinical Parameters | AUC | Std. Error | p Value | 95% Confidence Interval | Cutoff Value | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||
| Na+ | 0.838 | 0.053 | <0.001 | 0.734 | 0.941 | 132.5 | 94.3% | 74.5% |
| K+ | 0.763 | 0.062 | <0.001 | 0.64 | 0.885 | 3.43 | 94.4% | 66.1% |
| Cl− | 0.819 | 0.053 | <0.001 | 0.714 | 0.924 | 96.5 | 84.8% | 78.4% |
| Ca++ | 0.693 | 0.063 | <0.004 | 0.57 | 0.817 | 1.96 | 86.7% | 51.2% |
| Mg++ | 0.861 | 0.046 | <0.001 | 0.77 | 0.951 | 1.67 | 88.6% | 78.6% |
| HCO3− | 0.838 | 0.053 | <0.001 | 0.734 | 0.941 | 29.5 | 93.3% | 72.3% |
| Interleukin-6 | 0.958 | 0.022 | <0.001 | 0.915 | 1 | 51.375 | 95.9% | 89.7% |
| C-reactive protein | 0.954 | 0.023 | <0.001 | 0.909 | 0.999 | 40.2 | 91.8% | 89.7% |
| D-dimer | 0.955 | 0.023 | <0.001 | 0.909 | 1 | 1.645 | 93.9% | 86.2% |
| Fibrinogen | 0.954 | 0.025 | <0.001 | 0.905 | 1 | 510 | 95.9% | 86.2% |
| NT-proBNP | 0.959 | 0.022 | <0.001 | 0.916 | 1 | 511.2 | 89.8% | 89.7% |
| Bilirubin | 0.888 | 0.039 | <0.001 | 0.812 | 0.964 | 1.1 | 83.7% | 86.2% |
| Uric acid | 0.847 | 0.045 | <0.001 | 0.759 | 0.935 | 5.16 | 83.7% | 82.8% |
| Procalcitonin | 0.754 | 0.054 | <0.001 | 0.647 | 0.860 | 0.085 | 75.5% | 65.5% |
| Clinical Parameters | Pearson r | p Value |
|---|---|---|
| Interleukin 6 and NT-proBNP | 0.8692 | <0.0001 |
| Interleukin 6 and bilirubin | 0.9170 | <0.0001 |
| Interleukin 6 and uric acid | 0.9044 | <0.0001 |
| Interleukin 6 and fibrinogen | 0.8601 | <0.0001 |
| Interleukin 6 and D-dimer | 0.9519 | <0.0001 |
| Interleukin 6 and C-reactive protein | 0.9535 | <0.0001 |
| Interleukin 6 and procalcitonin | 0.8494 | <0.0001 |
| C-reactive protein and NT-proBNP | 0.8962 | <0.0001 |
| C-reactive protein and bilirubin | 0.9171 | <0.0001 |
| C-reactive protein and uric acid | 0.9175 | <0.0001 |
| C-reactive protein and fibrinogen | 0.8856 | <0.0001 |
| C-reactive protein and D-dimer | 0.9400 | <0.0001 |
| C-reactive protein and procalcitonin | 0.7836 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mim, F.; Reza, M.S.; Khalil, M.I.; Karim, N.; Shahjalal, H.M.; Hossain, M.I.; Hossain, M.S. Assessment of Serum Electrolytes, Biochemical, and Inflammatory Markers in Predicting COVID-19 Severity in COPD Patients. COVID 2023, 3, 792-806. https://doi.org/10.3390/covid3060059
Mim F, Reza MS, Khalil MI, Karim N, Shahjalal HM, Hossain MI, Hossain MS. Assessment of Serum Electrolytes, Biochemical, and Inflammatory Markers in Predicting COVID-19 Severity in COPD Patients. COVID. 2023; 3(6):792-806. https://doi.org/10.3390/covid3060059
Chicago/Turabian StyleMim, Farzana, Md. Selim Reza, Md. Ibrahim Khalil, Nurul Karim, Hussain Md. Shahjalal, Md. Ibrahim Hossain, and Md. Sabir Hossain. 2023. "Assessment of Serum Electrolytes, Biochemical, and Inflammatory Markers in Predicting COVID-19 Severity in COPD Patients" COVID 3, no. 6: 792-806. https://doi.org/10.3390/covid3060059
APA StyleMim, F., Reza, M. S., Khalil, M. I., Karim, N., Shahjalal, H. M., Hossain, M. I., & Hossain, M. S. (2023). Assessment of Serum Electrolytes, Biochemical, and Inflammatory Markers in Predicting COVID-19 Severity in COPD Patients. COVID, 3(6), 792-806. https://doi.org/10.3390/covid3060059





