Does COVID-19 Really Exacerbate Urticaria? A Survey of 166 Patients in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants and Inclusion Criteria
2.2. Survey Content
2.2.1. General Questions
2.2.2. COVID-19 Questions
2.2.3. Urticaria Characteristics and Symptomatic Variation
2.3. Statistical Analysis
3. Results
3.1. General Information Data
3.2. COVID-19 Disease-Related Information
3.3. Urticaria Characteristics and Symptomatic Variation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balp, M.-M.; Lopes da Silva, N.; Vietri, J.; Tian, H.; Ensina, L.F. The Burden of Chronic Urticaria from Brazilian Patients’ Perspective. Dermatol. Ther. 2017, 7, 535–545. [Google Scholar] [CrossRef]
- Guillet, G.; Bécherel, P.-A.; Pralong, P.; Delbarre, M.; Outtas, O.; Martin, L.; Pelvet, B.; Gharbi, H.; Giordano-Labadie, F. The Burden of Chronic Urticaria: French Baseline Data from the International Real-Life AWARE Study. Eur. J. Dermatol. 2019, 29, 49–54. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA2LEN/EDF/WAO Guideline for the Definition, Classification, Diagnosis and Management of Urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The International EAACI/GA2LEN/EuroGuiDerm/APAAACI Guideline for the Definition, Classification, Diagnosis, and Management of Urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Deleanu, D.; Nedelea, I.; Petricau, C.; Leru, P.; Dumitrascu, D.; Muntean, A. Clinical Impact of Omalizumab in Refractory Chronic Urticaria: One Centre Experience. Exp. Ther. Med. 2019, 18, 5078–5081. [Google Scholar] [CrossRef]
- Kocatürk, E.; Salman, A.; Cherrez-Ojeda, I.; Criado, P.R.; Peter, J.; Comert-Ozer, E.; Abuzakouk, M.; Agondi, R.C.; Al-Ahmad, M.; Altrichter, S.; et al. The Global Impact of the COVID-19 Pandemic on the Management and Course of Chronic Urticaria. Allergy 2021, 76, 816–830. [Google Scholar] [CrossRef]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.-K.; Maurer, M. Autoimmune Chronic Spontaneous Urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef]
- Guillén-Aguinaga, S.; Jáuregui Presa, I.; Aguinaga-Ontoso, E.; Guillén-Grima, F.; Ferrer, M. Updosing Nonsedating Antihistamines in Patients with Chronic Spontaneous Urticaria: A Systematic Review and Meta-Analysis. Br. J. Dermatol. 2016, 175, 1153–1165. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential Association of Mast Cells with Coronavirus Disease 2019. Ann. Allergy Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Dong, X.; Cao, Y.-Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical Characteristics of 140 Patients Infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, E.; Kim, H.-K.; Song, W.-J.; Kwon, H.-S.; Kim, T.-B.; Cho, Y.S. Exacerbation of Chronic Spontaneous Urticaria Following Coronavirus Disease 2019 (COVID-19) Vaccination in Omalizumab-Treated Patients. J. Allergy Clin. Immunol. Pract. 2023, 11, 2403–2410. [Google Scholar] [CrossRef]
- Heudorf, U.; Gottschalk, R. The Risk of Asymptomatic and Symptomatic COVID-19 Infec Tion Among Schoolteachers and Day-Care Workers Compared to Hospital and Nursing-Home Staff. Dtsch. Arztebl. Int. 2021, 118, 213–214. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Kempuraj, D.; Selvakumar, G.P.; Ahmed, M.E.; Raikwar, S.P.; Thangavel, R.; Khan, A.; Zaheer, S.A.; Iyer, S.S.; Burton, C.; James, D.; et al. COVID-19, Mast Cells, Cytokine Storm, Psychological Stress, and Neuroinflammation. Neuroscientist 2020, 26, 402–414. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Sun, Y.-L.; Wang, Y.-F.; Yazici, D.; Azkur, D.; Ogulur, I.; Azkur, A.K.; Yang, Z.-W.; Chen, X.-X.; Zhang, A.-Z.; et al. Recent Developments in the Immunopathology of COVID-19. Allergy 2023, 78, 369–388. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.-C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune Response to SARS-CoV-2 and Mechanisms of Immunopathological Changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Criado, P.R.; Pagliari, C.; Criado, R.F.J.; Marques, G.F.; Belda, W. What the Physicians Should Know about Mast Cells, Dendritic Cells, Urticaria, and Omalizumab during COVID-19 or Asymptomatic Infections Due to SARS-CoV-2? Dermatol. Ther. 2020, 33, e14068. [Google Scholar] [CrossRef]
- MacGlashan, D.; Saini, S.; Schroeder, J.T. Response of Peripheral Blood Basophils in Subjects with Chronic Spontaneous Urticaria during Treatment with Omalizumab. J. Allergy Clin. Immunol. 2021, 147, 2295–2304.e12. [Google Scholar] [CrossRef]
- Rodriguez, L.; Pekkarinen, P.T.; Lakshmikanth, T.; Tan, Z.; Consiglio, C.R.; Pou, C.; Chen, Y.; Mugabo, C.H.; Nguyen, N.A.; Nowlan, K.; et al. Systems-Level Immunomonitoring from Acute to Recovery Phase of Severe COVID-19. Cell Rep. Med. 2020, 1, 100078. [Google Scholar] [CrossRef]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the Cutaneous Manifestations of COVID-19: A Rapid Prospective Nationwide Consensus Study in Spain with 375 Cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef]
- Ban, G.-Y.; Ye, Y.-M. Real-World Disease Burden of Chronic Urticaria and Vaccine Hesitancy. Allergy Asthma Immunol. Res. 2023, 15, 1–3. [Google Scholar] [CrossRef]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Walter Canonica, G.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic Rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Ren, J.; Pang, W.; Luo, Y.; Cheng, D.; Qiu, K.; Rao, Y.; Zheng, Y.; Dong, Y.; Peng, J.; Hu, Y.; et al. Impact of Allergic Rhinitis and Asthma on COVID-19 Infection, Hospitalization, and Mortality. J. Allergy Clin. Immunol. Pract. 2022, 10, 124–133. [Google Scholar] [CrossRef]
- Yao, Y.; Huang, A.; Deng, Y.-K.; Liu, Y.; Zhu, H.-Y.; Wang, N.; Wang, Z.-Z.; Zhu, R.-F.; Yu, D.; Liu, Z. Allergen Immunotherapy Reverses Immune Response to SARS-CoV-2 Vaccine in Patients with Allergic Rhinitis: A Prospective Observational Trial. Am. J. Respir. Crit. Care Med. 2022, 206, 780–783. [Google Scholar] [CrossRef]
- Iinuma, T.; Kiuchi, M.; Hirahara, K.; Kurita, J.; Kokubo, K.; Yagyu, H.; Yoneda, R.; Arai, T.; Sonobe, Y.; Fukuyo, M.; et al. Single-Cell Immunoprofiling after Immunotherapy for Allergic Rhinitis Reveals Functional Suppression of Pathogenic TH2 Cells and Clonal Conversion. J. Allergy Clin. Immunol. 2022, 150, 850–860.e5. [Google Scholar] [CrossRef]
- Hein, R. Chronic Urticaria: Impact of Allergic Inflammation. Allergy 2002, 57 (Suppl. S75), 19–24. [Google Scholar] [CrossRef]
- Chen, Q.; Zhong, H.; Chen, W.C.; Zhai, Z.; Zhou, Z.; Song, Z.; Hao, F. Different Expression Patterns of Plasma Th1-, Th2-, Th17- and Th22-Related Cytokines Correlate with Serum Autoreactivity and Allergen Sensitivity in Chronic Spontaneous Urticaria. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 441–448. [Google Scholar] [CrossRef]
- Kim, H.S.; Noh, G. Effects of the Immunoglobulin/Histamine Complex in Chronic Spontaneous Urticaria Focusing on Remission Induction. Allergy 2022, 77, 2846–2848. [Google Scholar] [CrossRef]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, W.G.; Gimenez-Arnau, A.; Kowalski, M.L.; Martí-Guadaño, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in Allergic Rhinitis and Chronic Urticaria. Allergy 2008, 63 (Suppl. S87), 5–28. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Akin, C.; Valent, P.; Metcalfe, D.D. Mast Cell Activation Syndrome: Proposed Diagnostic Criteria. J. Allergy Clin. Immunol. 2010, 126, 1099–1104.e4. [Google Scholar] [CrossRef]
- Muntean, I.A.; Pintea, I.; Bocsan, I.C.; Dobrican, C.T.; Deleanu, D. COVID-19 Disease Leading to Chronic Spontaneous Urticaria Exacerbation: A Romanian Retrospective Study. Healthcare 2021, 9, 1144. [Google Scholar] [CrossRef]
- Shelley, W.B.; Shelley, E.D. Acyclovir Therapy for Angioedema and Chronic Urticaria. Cutis 1997, 59, 185–188. [Google Scholar]
- Dreyfus, D.H. Autoimmune Disease: A Role for New Anti-Viral Therapies? Autoimmun. Rev. 2011, 11, 88–97. [Google Scholar] [CrossRef]
- Dreyfus, D.H. Paleo-Immunology: Evidence Consistent with Insertion of a Primordial Herpes Virus-like Element in the Origins of Acquired Immunity. PLoS ONE 2009, 4, e5778. [Google Scholar] [CrossRef]
- Dreyfus, D.H. The DDE Recombinases: Diverse Roles in Acquired and Innate Immunity. Ann. Allergy Asthma Immunol. 2006, 97, 567–576. [Google Scholar] [CrossRef]

| Parameter | n (%) | |
|---|---|---|
| Sex | Male | 49 (29.5%) |
| Female | 117 (70.5%) | |
| Age * | 33.45 ± 12.72 | |
| Past basic diseases | No basic disease | 123 (71.9%) |
| Hypertension | 4 (2.3%) | |
| Diabetes mellitus | 1 (0.6%) | |
| Allergic diseases | 31 (18.1%) | |
| Other | 12 (7%) | |
| COVID-19 vaccine | Yes | 152 (91.6%) |
| No | 14 (8.4%) | |
| Several doses of the COVID-19 vaccine | One dose of New Crown Vaccine (adenovirus vector vaccine) | 4 (2.4%) |
| Two doses of New Crown Vaccine (inactivated) | 60 (36.1%) | |
| Three doses of New Crown Vaccine (recombinant protein vaccine) | 88 (53%) | |
| Diagnostic modalities | Nucleic acid testing | 40 (24.1%) |
| Antigen detection | 76 (45.78%) | |
| Suspected symptoms, not tested | 50 (30.12%) |
| Parameter | n (%) | |
|---|---|---|
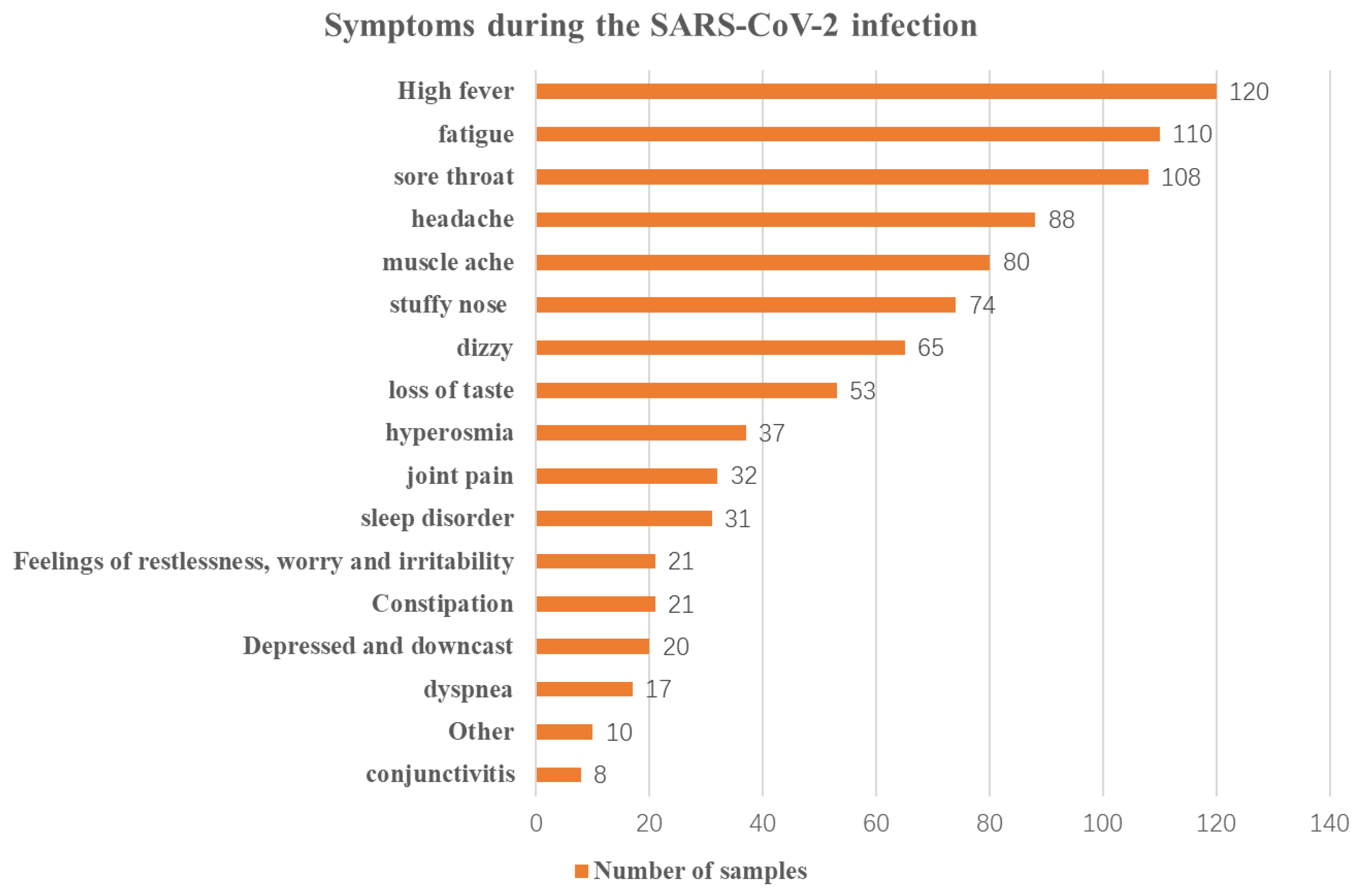
| Main symptoms of SARS-CoV-2 infection | High fever | 120 (13.4%) |
| Constipation | 21 (2.3%) | |
| Stuffy nose | 74 (8.3%) | |
| Sore throat | 108 (12.1%) | |
| Headache | 88 (9.8%) | |
| Dizziness | 65 (7.3%) | |
| Fatigue | 110 (12.3%) | |
| Conjunctivitis | 8 (0.9%) | |
| Joint pain | 32 (3.6%) | |
| Muscle ache | 80 (8.9%) | |
| Dyspnea | 17 (1.9%) | |
| Loss of taste | 53 (5.9%) | |
| Hyperosmia | 37 (4.1%) | |
| Depresion and feeling downcast | 20 (2.2%) | |
| Feelings of restlessness, worry, and irritability | 21 (2.3%) | |
| Sleep disorder | 31 (3.5%) | |
| Other | 10 (1.1%) | |
| Symptoms of SARS-CoV-2 infection—overall self-perception | Mild symptoms | 40 (24.1%) |
| Moderate symptoms | 90 (54.2%) | |
| Severe symptoms | 36 (21.7%) | |
| Whether SARS-CoV-2 infection was treated | Yes | 95 (57.2%) |
| No | 71 (42.8%) | |
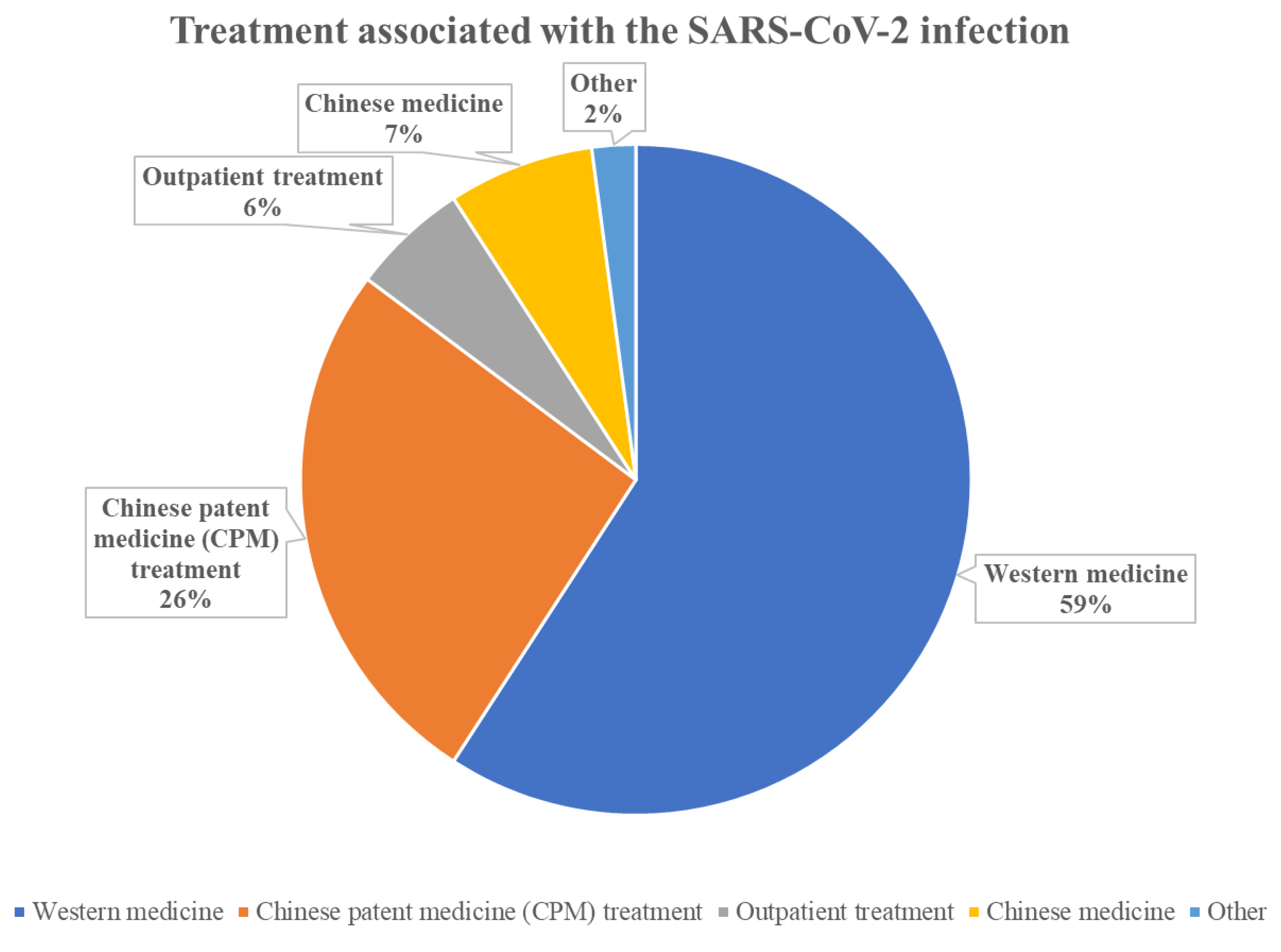
| What treatment was given for SARS-CoV-2 infection | Western medicine | 84 (88.4%) |
| Chinese patent medicine (CPM) treatment | 37 (39.0%) | |
| Outpatient treatment | 10 (10.5%) | |
| Chinese medicine | 8 (8.4%) | |
| Other | 3 (3.2%) | |
| Onset to recovery * | 11.54 ± 16.027 | |
| Course of disease | Less than 6 weeks | 25 (15.1%) |
| 6~2 years | 51 (30.7%) | |
| 2~5 years | 44 (26.5%) | |
| Over 5 years | 46 (27.7%) | |
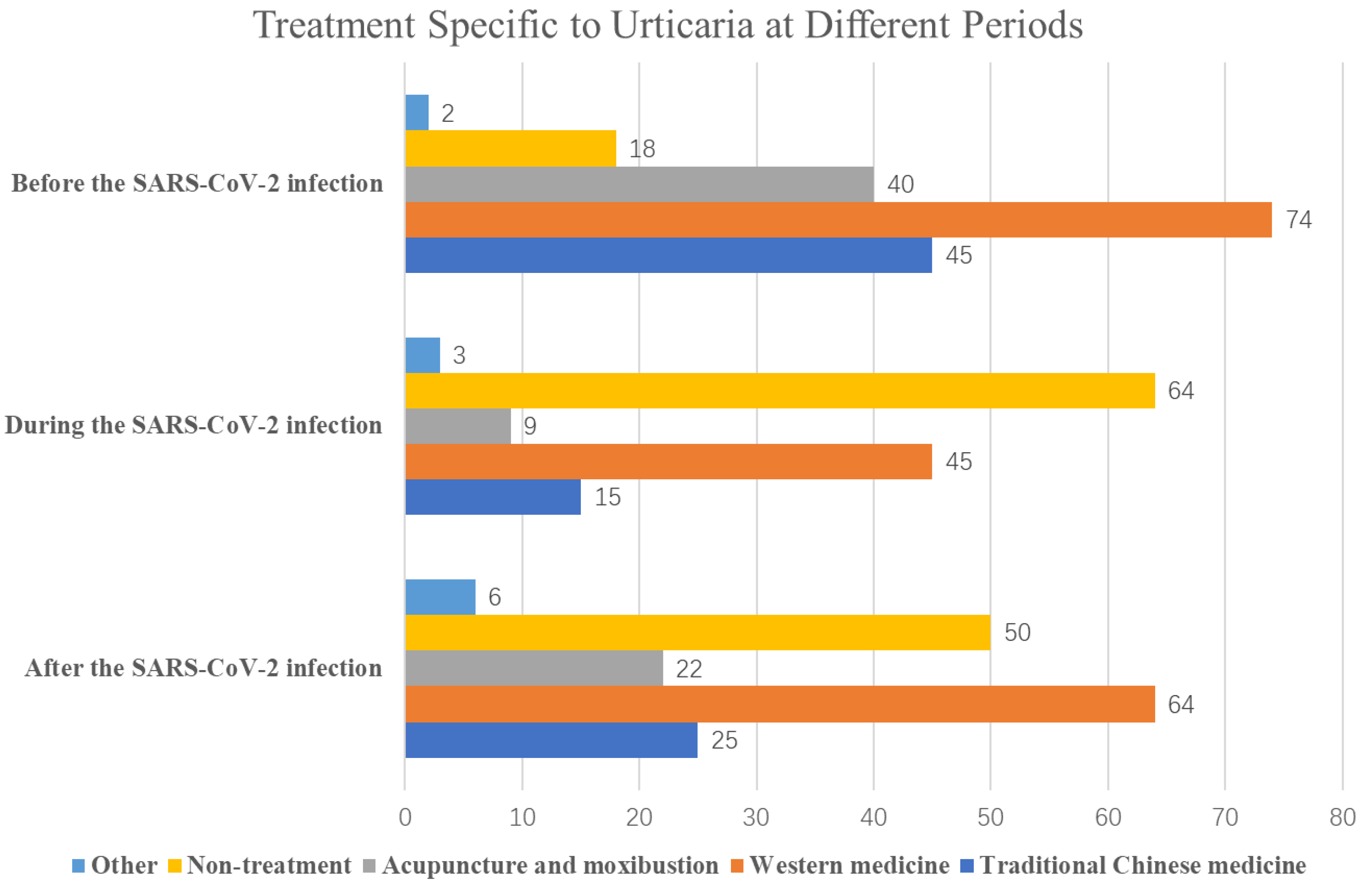
| When did the hives first flare up | Before SARS-CoV-2 infection | 115 (69.3%) |
| During SARS-CoV-2 infection | 3 (1.8%) | |
| After SARS-CoV-2 infection | 15 (9%) | |
| Resolved before SARS-CoV-2 infection | 33 (19.9%) | |
| Flare-ups of urticaria after administration of medication to alleviate the symptoms of SARS-CoV-2 infection | Aggravating | 9 (7.6%) |
| Alleviate | 13 (11%) | |
| Unaffected | 96 (81.4%) |
| Variables | Number of Samples | Mean ± Standard Deviation | F | p ** | LSD |
|---|---|---|---|---|---|
| 1 | 115 | 5.17 ± 1.67 | 8.84 | <0.01 | 1 > 2, 1 > 3 |
| 2 | 118 | 4.23 ± 1.98 | |||
| 3 | 166 | 4.37 ± 1.93 |
| Variables | Number of Samples | M (P25, P75) | H | p * |
|---|---|---|---|---|
| 1 | 115 | 0.464 (0.464, 0.763) | 12.23 | <0.05 |
| 2 | 118 | 0.464 (0.138, 0.763) | ||
| 3 | 166 | 0.464 (0.138, 0.763) |
| Variables | Number of Samples | M (P25, P75) | H | p ** |
|---|---|---|---|---|
| 1 | 115 | 0.695 (0.395, 0.695) | 21.001 | <0.01 |
| 2 | 118 | 0.394 (0.123, 0.695) | ||
| 3 | 166 | 0.394 (0.123, 0.695) |
| Variables | Number of Samples | M (P25, P75) | Z | p * |
|---|---|---|---|---|
| a | 45 | 0.482 (0.482, 0.482) | 3.737 | <0.05 |
| b | 64 | 0.820 (0.248, 0.820) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Zou, Z.; Cao, W.; Shi, Y.; Xiao, X.; Chen, S.; Li, Y. Does COVID-19 Really Exacerbate Urticaria? A Survey of 166 Patients in China. COVID 2023, 3, 1707-1720. https://doi.org/10.3390/covid3120118
Yang Q, Zou Z, Cao W, Shi Y, Xiao X, Chen S, Li Y. Does COVID-19 Really Exacerbate Urticaria? A Survey of 166 Patients in China. COVID. 2023; 3(12):1707-1720. https://doi.org/10.3390/covid3120118
Chicago/Turabian StyleYang, Qian, Zihao Zou, Wei Cao, Yunzhou Shi, Xianjun Xiao, Sijue Chen, and Ying Li. 2023. "Does COVID-19 Really Exacerbate Urticaria? A Survey of 166 Patients in China" COVID 3, no. 12: 1707-1720. https://doi.org/10.3390/covid3120118
APA StyleYang, Q., Zou, Z., Cao, W., Shi, Y., Xiao, X., Chen, S., & Li, Y. (2023). Does COVID-19 Really Exacerbate Urticaria? A Survey of 166 Patients in China. COVID, 3(12), 1707-1720. https://doi.org/10.3390/covid3120118





