Abstract
Increased mortality has been observed in patients who develop acute kidney injury (AKI) in the setting of coronavirus disease 2019 (COVID-19), which has led to the approval of extracorporeal kidney support by the FDA. We analyzed the existing literature to compare the efficacy and therapeutic benefits of various extracorporeal modalities for the oXiris membranes and CytoSorb cartridge in high-flow continuous kidney replacement therapy (HFCKRT). AKI due to COVID-19 is mediated by a state of systemic inflammation (cytokine storm syndrome), leading to multiple organ dysfunction. Although there is no consensus on a protocol for providing kidney support therapy, clinically oriented studies have shown the capacities of oXiris and CytoSorb filters to effectively filter out pro-inflammatory components, leading to improved clinical outcomes in critically ill patients. In this review, we study the development of cytokine storm syndrome, important clinical evidence regarding the roles of various adsorption techniques in kidney support therapy in this setting, and a protocol influenced by FDA recommendations for oXiris and CytoSorb membranes.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus, first identified in December 2019 as the cause of a respiratory illness named coronavirus disease 2019 (COVID-19) [1]. Various therapies (remdesivir, hydroxychloroquine, azithromycin and lopinavir/ritonavir) have been utilized with limited efficacy, and the current focus is on vaccination and preventative measures for COVID-19 over traditional anti-viral treatments. Immunity through convalescent serum and vaccination is still at an experimental stage [2].
COVID-19 has had a severe impact on the overall population, with a total of 349 million global cases and a mortality rate of approximately 1.60% (~5.6 million individuals) (John Hopkins Coronavirus Tracker) as of 24 January 2022. Additionally, COVID infection tends to be more severe in patients with chronic diseases and organ dysfunctions, especially kidney dysfunctions. According to a systematic review by Chen et al., the mortality rate of COVID-19 patients with AKI was found to be 76.5% [3]. Although there is no consensus for attenuating AKI with COVID-19, supportive treatments utilizing continuous kidney replacement therapy (CKRT) with incorporation of devices such as oXiris and CytoSorb have been authorized by the FDA [4,5,6,7]. Therefore, this literature review discusses the research available on the use of various CKRT modes in the COVID-19 population with AKI, with the goal of establishing a comparison of the therapeutic benefits, safety, efficacy and associations with clinical outcomes for high-flow CKRT, oXiris and CytoSorb. In the absence of extensive head-to-head clinical trial data, we leverage several statistical methods to conduct indirect comparisons between various extracorporeal modalities.
2. Incidence
The first case in the United States (US) was detected in January 2020, with the CDC reporting over 31 million cases and 562,296 COVID-19-associated deaths in the US at the time of writing [5,6]. COVID-19 has demonstrated a wide variability in the severity of symptomatic presentation. Individuals may present with symptoms such as cough, fever, loss of smell and taste and/or septic shock leading to AKI and multiple organ failure. Frequency of kidney disease in COVID-19 is negligible; however, AKI among critically ill patients with COVID-19 has an incidence that ranges from 3–23% [8]. In a systematic review by Fabrizi et al., the authors showed that the comorbidities of age and arterial hypertension are correlated with the occurrence of AKI [6]. Other risk factors contributing to AKI include hypovolemia, use of nephrotoxic medications and contrast media [9].
3. Pathophysiology
Envelope-embedded spike proteins on the virus allow for attachment to angiotensin-converting enzyme-2 (ACE 2) receptors on target cells [8]. Transmembrane protease, serine 2 (TMPRSS2) uses endosomal cathepsin L to fuse the virion with the endothelial membrane to enter the cell. Afterwards, the injected replicase transcribes viral mRNA to create pathogenic structural proteins using host-derived ribosomes. Pathogenic proteins then combine with viral RNA to produce a replicated virus, leaving the cell through exocytosis [10]. ACE 2 receptors and TMPRSS2 are both found in high concentrations in alveolar epithelial cells, supporting the conceptual framework that suggests that the respiratory system acts as the gateway to the body [11]. Endothelial barrier disruption, dysfunctional alveolar-capillary oxygen transmission and impaired oxygen diffusion capacity are characteristic features of COVID-19 [12].
The host response to COVID-19 includes standard anti-viral inflammation driven by innate and adaptive immunity, which leads to the clinical findings of impaired lymphopoiesis and lymphocyte apoptosis [13]. In the autopsy report by Xu et al., severe infection leads to diffuse thickening of the alveolar wall with immune cell infiltrates and edema, resulting in bilateral, patchy, ground-glass opacities in CT imaging [14]. Additionally, multiple organ biopsies showed diffuse intravascular coagulation and lymphopenia [11].
4. Cytokine Storm Syndrome
The link between the pathophysiology of COVID-19 and systemic infection is cytokine storms: hyperinflammation due to proinflammatory cytokines and chemokines leading to multiple organ failure [15] and AKI (Table 1, Figure 1).

Table 1.
Interleukins (ILs) and other cytokines implicated in cytokine storm syndrome.
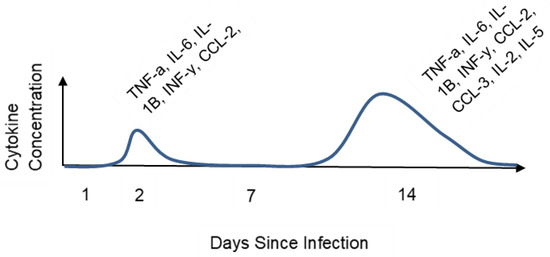
Figure 1.
Cytokine concentration over time in COVID-19 patients. The graph shows an increase in cytokine concentration in serum post-infection. There is an increased modulation of the immune system via additional cytokines.
Extensive data have shown that interleukin (IL)-6 plays a prominent role in the development of cytokine storm syndrome in COVID-19 infection. Several studies suggest that serum levels of IL-6 can be used as a prognostic tool for disease severity [15,18,19]. Animal models with inhibited transcription of IL-6 (through IL-1B and TNF-a) exhibit decreased mortality with SARS-CoV infection [20]. The chemokine CXCL10 has also been considered a satisfactory prognostic marker for SARS disease progression after extensive vivo trials [21,22]. CXCL10 and CXCL8 knockout mice demonstrated higher survivability with the COVID-19 infection, suggesting that these cytokines may play a role in the mechanism of development of AKI in these patients [23].
Clinical markers of cytokine storm syndrome and macrophage-activation syndrome (MAS) often include elevated serum ferritin, increased triglycerides, fever, pancytopenia, coagulopathy, splenomegaly and liver dysfunction [24]. In COVID-19, poor viral clearance due to production of a double membrane without pattern-recognition receptors decreases the anti-viral immune response, exacerbating cytokine release [25]. Decreased type 1 interferon activity, traditionally crucial for blocking viral replication cycle, can further extend the lifetime of viral particles in the host [26]. Barns et al. postulated that neutrophil extracellular traps (NETs), composed of DNA that physiologically opsonize pathogens, may induce plasma membrane disruption of epithelial cells, increasing susceptibility to COVID-19 [27]. This combination of typical coronavirus immune evasion techniques further aggravates the cytokine storm in patients with severe COVID-19 infection.
5. AKI and COVID-19
In general, viral infections predispose patients to the risk of AKI. Furthermore, fluid loss, fever and clinical signs of prerenal azotemia can cause cardiorenal syndrome, leading to kidney injury [28]. Nephrotoxic medication also contributes to kidney injury. For example, remdesivir may induce mitochondrial toxicity, leading to accumulated oxalate nephropathy [29]. Management modalities such as mechanical ventilation and administration of intravenous fluids also contribute to the development of AKI [30,31]. Glomerular diseases (membranous nephropathy, minimal change disease, glomerulonephritides) have been found in kidney biopsies of severely ill patients [31,32].
Sixty percent of AKI cases in COVID-19 are attributed to acute tubular injury, pointing to the mediation of cytokine storm syndrome as an important pathway for intervention [33]. Native kidney biopsies and autopsies illustrate acute tubular injury in the setting of prolonged volume depletion through cytokine-influenced circulatory collapse [34]. MAS characterized by sustained fever, cytopenia and increased ferritin (associated with hyperinflammation; i.e., hemophagocytic lymphohistiocytosis) has been associated with COVID-19 through the interplay of IL-2, IL-6, granulocyte colony stimulating factor (G-CSF), interferon gamma (IFN-g), macrophage inflammatory protein-1a (MIP-1a) and tumor necrosis factor α (TNF-α) [35]. Cytokine storms due to COVID-19 can also lead to hypermetabolism-induced rhabdomyolysis (with increased phosphorus, potassium, uric acid and metabolic acidosis and lowered serum albumin), which is another potential cause of prerenal azotemia [36]. Hypercoagulation in the form of thrombotic microangiopathy may result in cortical necrosis, either due to complement activation or sepsis.
6. Extracorporeal Therapy
Renal support for patients with COVID-19 is often provided to address the systemic inflammatory response syndrome (SIRS). Immune response in sepsis is directed towards ubiquitous pathogens ranging from pathogen-associated recognition patterns (PAMPs) and damage-associated recognition patterns (DAMPs) to bacterial cell-wall components [37]. Kidney replacement therapy (KRT) for critically ill COVID-19 patients with AKI may be used as a potential adjunctive therapy to remove pro-inflammatory mediators with the goal of reducing mortality rates. Continuous kidney replacement therapy (CKRT) is the therapy with the most efficacy in the treatment of severely ill COVID-19 patients, removing endotoxins and cytokines and normalizing the hemodynamic status of the patient. Different modes of CKRT can be used in these subsets of patients. The most commonly used modes are continuous venovenous hemodiafiltration (CVVHDF), continuous venovenous hemodialysis (CVVHD), prolonged intermittent renal replacement therapy (PIRRT), hemoperfusion (HP) and therapeutic plasma exchange (TPE). According to an ADQI workgroup report, CVVHDF and CVVHD are the modes of choice to reduce filtration fraction and decrease clotting risk [38].
7. High-Flow Continuous Kidney Replacement Therapy
High-flow CKRT is designed for critically ill patients, who often cannot tolerate the rapid changes in fluid and electrolyte shifts. In contrast to dialysis, which is administered for 3–4 h a day, CKRT is often administered continuously for 24 h a day. CKRT plays a role in aiding the kidney’s function, helps remove toxins and excess fluid and balances electrolytes. Sustained low-efficiency dialysis (SLED) is another option if CKRT is unavailable for these patients and offers a similar therapeutic benefit for patients mildly intolerant of rapid changes in fluid balances and electrolytes. SLED is performed in sessions of about 6–8 h duration. Patients with a high blood urea nitrogen-to-creatinine ratio, toxins, fluid overload, severe electrolyte imbalance, acid/base imbalances, sepsis, rhabdomyolysis, congestive heart failure and even open-heart surgery may need CKRT. Currently, there are three main machines used for CKRT: Prismaflex/Prismax, Aquarius and NxStage. Prismaflex is the most commonly used, with the newer Prismax slowly replacing them. The machines work according to the basic principle of drawing blood out of the patient; running it through a filter, which can be selected based on the outcome desired; and then returning the filtered blood to the patient. The three most common access points for the catheter placement include the internal jugular, subclavian and femoral veins [39]. The machine has a blood pump that serves to create negative pressure to draw blood from the patient and positive pressure past the pump to return the blood through the filter [40]. The pump is usually set at 150 to 300 mL/min blood flow. A mechanism is set in place in the circuit after the filter to stop the blood if air is detected. To pull the plasma fluid out of the patient, dialysate is pumped in the opposite direction of the blood in the filter. Although this dialysate is separated by a membrane barrier, the concentration gradient drives the plasma fluid from the patient’s blood to the dialysate, which pools in an effluent bag. A pre-blood replacement fluid line is connected to the blood circuit prior to the filter and a post-filter replacement fluid line is connected to the circuit after the filter to ensure optimal electrolyte and fluid balance [41]. Different types of blood filters are available and used in KRT depending upon the desired therapeutic outcome. The choice of the filter depends upon what one is trying to filter; for removing cytokines, CytoSorb, HCO/MCO and HA330 are used; for removing endotoxins, Toraymyxin and coupled plasma filtration adsorption (CPFA) are used; for removing both cytokines and endotoxins, oXiris is used.
8. oXiris
oXiris is a filter device that can remove cytokines and other inflammatory mediators through its membrane properties. Of the various extracorporeal modalities that exist, the oXiris membrane is unique in that it removes both cytokines and endotoxins through ionic interactions. oXiris is made of an acrylonitrile and sodium methallyl sulfonate copolymer (AN69 copolymer), covered with polyethyleneimine and heparin grafted at a mean concentration of 4500 + 1500 IU/m2 with a standard deviation of 1500 IU/m2. Each layer of oXiris plays a certain role in its filtering capabilities. In the innermost layer, the AN69 copolymer is negatively charged due to the sulfonate, which aids in attracting the positively charged cytokines to separate them from the blood. The second layer is positively charged via polyethyleneimine, which aids in attracting the negatively charged endotoxins. The third layer of heparin addresses the hypercoagulability of the patient and aids in clot prevention (Table 2). oXiris’s mechanism of action is depicted in Figure 2.

Table 2.
Composition of oXiris.
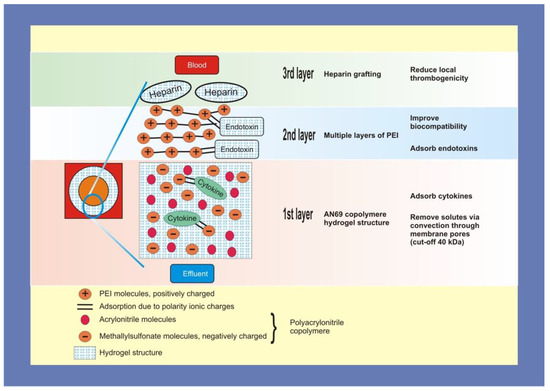
Figure 2.
oXiris’s mechanism of interaction.
On 23 April 2020, oXiris was authorized by the FDA for emergency use to treat AKI in COVID-19 patients. Per protocol, the oXiris Filter Set should be used only with the Prismaflex or Prismax CKRT machines. Additionally, patients must be 18 years or older with confirmed COVID-19 and require blood purification to reduce cytokine levels. oXiris is contraindicated in patients with a known or speculated allergy to heparin or the AN-69 filter. ACE inhibitor use is contraindicated due to the probability of anaphylactic reaction when they are used with AN-69 filter. While the patient is receiving CKRT therapy with oXiris, inflammatory markers should be monitored (Table 3).

Table 3.
oXiris Filter Set indications and contraindications.
In a series of three patients, Padala et al. found that CVVHDF with oXiris filter removed inflammatory markers and cytokines in patients with AKI [40]. Additionally, a prospective case-controlled study in Hong Kong analyzed the application of an endotoxin and cytokine adsorption hemofilter in seven patients with septicemia-associated AKI [41]. The authors support the use of CVVHD and a hemofilter with endotoxin- and cytotoxin-adsorptive capacity in AKI patients over the use of CVVH with a Gambro AK200 Ultra S and a polysulfone high-flux hemofilter. Furthermore, Shum et al. calculated a significantly lower sequential organ failure assessment (SOFA) score by 37% in six patients treated with CVVHDF and oXiris compared to historical controls treated with CVVH [42]. In a study on 16 patients requiring CKRT for septic shock-associated AKI, Broman et al. highlighted the ability of the oXiris filter to effectively remove endotoxins and IL-6. Seven of nine patients had decreased endotoxin levels on oXiris while only one of six showed similar results on a standard filter [43]. Ma et al. reported similar findings in three critically ill COVID-19 patients treated with plasma exchange and oXiris [44]. A multicenter retrospective study showed that use of the oXiris hemofilter in patients with the most severe cases of AKI resulted in better survival than predicted by SOFA scores [45,46]. Additional studies support these same findings. A retrospective study conducted from September 2017 to June 2020 assessed SOFA scores, inflammatory markers and mortality in 70 septic shock patients with AKI treated with oXiris. oXiris-treated patients were shown to have a lower early mortality (47.1 vs. 74.2%) when compared to those treated with an ST150 hemofilter. The oXiris treatment group also resulted in a quicker reduction in SOFA scores at 24, 48 and 72 h marks and a greater decrease in inflammatory markers, such as procalcitonin [47]. This was especially true in a study with 76 patients in septic shock, where the use of oXiris–CVVH demonstrated a 50% decrease in NE demand in 72 h compared to a 25% decrease for the AN69–CVVH control. Additionally, there was a decreased 28 day mortality (47% vs. 73.3%) when using oXiris [48].
In another study, the use of the oXiris Set with CVVH in patients with septic AKI was associated with a greater drop in SOFA score after 48 h compared to control; no significant differences were seen in vasopressor dose, length of hospital stay or mortality [49]. In a larger recent study on 60 septic patients, CKRT with the oXiris Set led researchers to highlight the safety of oXiris and its clinical benefits, including reduced noradrenaline need and reduced endotoxin and cytokines levels [50]. These studies are summarized in Table 4. Furthermore, oXiris has been shown to be useful in the early treatment of abdominal septic shock. CRRT with oXiris performed for 80 h with a 50 year old male with septic shock resulted in a decreased SOFA score from 15 to 11, while blood lactate decreased from 12.5 mmol/L to 4.2 mmol/L and procalcitonin decreased from >100 ng/mL to 14.52 ng/mL [51]. While many case reports and cohort studies seem to suggest the usefulness of oXiris, large, double-blinded, placebo-controlled randomized studies are needed to fully validate and support the use of oXiris.

Table 4.
Summary of recent oXiris trials.
9. CytoSorb
CytoSorb is a filter device consisting of absorbent polymer beads that can remove cytokines and other inflammatory mediators. Unlike oXiris, CytoSorb uses hydrophobic interactions to remove cytokines. On 10 April 2020, CytoSorb was authorized by the FDA for emergency use to reduce pro-inflammatory cytokine levels in patients 18 years of age or older with confirmed COVID-19 in the intensive care unit with respiratory failure (Table 5). Per protocol, CytoSorb is used in a blood pump circuit in a venovenous configuration only. The blood flow rate should be set at 150–500 mL/min and is intended to be changed every 24 h, with the exception of the first day, when it must be changed every 12 h [52]. CytoSorb is contraindicated for patients with platelet counts less than 20,000/μL. Any known allergies to extracorporeal circuit components or heparin-induced thrombocytopenia should be assessed. Morbid obesity with BMI ≥ 40 kg/m2 and pregnancy are also contraindicated for CytoSorb therapy. While the patient is receiving CKRT, inflammatory markers should be routinely assessed and the extracorporeal circuit should be monitored to prevent blood leaks. An important consideration with CytoSorb is that hydrophobic drugs should be filtered out as well, so dosages of these drugs may have to be adjusted.

Table 5.
CytoSorb indications and contraindications.
The first study to test the effectiveness and potential risks of CytoSorb use in 20 patients with septic shock found that therapy was safe. CytoSorb was beneficial in reducing norepinephrine needs and blood levels of procalcitonin and endothelin-1 when compared to controls [16]. A randomized, controlled, open-label trial on critically ill patients with septic shock and acute respiratory distress syndrome showed that CytoSorb, when used for 1 hr/day for 7 days, enabled significantly higher removal of IL-6 compared to controls without affecting plasma levels [53]. This study set the stage for future research to investigate the safety of the device and test clinical outcomes, such as mortality [17]. Other studies have also looked at the utility of CytoSorb therapy, with satisfactory results [54,55]. However, several other studies have not shown such satisfactory outcomes with the use of CytoSorb. A randomized trial [56] and a systematic review [57] found insufficient evidence to support a beneficial effect of CytoSorb on patient outcomes. Schadler et al. did not find that CytoSorb achieved a significant reduction in plasma levels of IL-6 (compared to conventional management), which may be necessary for improving patient outcomes. A single-center RCT by Supady et al. demonstrated an adverse effect of employing CytoSorb on medium-term patient survival [58,59]. While some reports suggest a potential benefit of CytoSorb, further research in the form of double-blind, placebo-controlled trials is needed to establish the benefits of this therapy.
10. Other Modalities
Reports on the use of hemoperfusion with microporous resin cartridge devices, such as the Jafron, have recently been published, showing improved survival in various conditions, such as sepsis. These sets of adsorption cartridges are the HA resin hemoperfusion cartridges initially labeled HA130, HA230 and HA330. While the efficacy of their use requires more research, studies into their main clinical applications are documented. These cartridges can vary in resin pore size to allow for removal of substances with various molecular weights, such as protein-bound uremic toxins, hydrophobic substances, free hemoglobin and cytokines [57]. Each cartridge with a different pore size is best used in certain scenarios, with HA130 (pore size 500 Da–40 kDa) being used best for chronic conditions and HA330 (500 Da–60 kDa) being used best for acute inflammatory conditions [58]. HA330 was initially studied in septic patients with acute lung injury, and it resulted in decreased overall pro-inflammatory cytokines, improved oxygenation, attenuated lung injury and decreased length of ICU stay. An alternative study assessing the use of the cartridge in extra-pulmonary sepsis showed statistically significant reduced levels of IL-1 and TNF-α in bronchoalveolar lavage and plasma, ultimately improving the patient’s hemodynamics and reducing the duration of mechanical ventilation required by three days [59].
Zhou et al. also showed the benefit of blood purification in improving sepsis mortality rates using polymyxin B in Japan as part of the EUPHAS trial, with noted benefits for both hemodynamic and respiratory parameters [59]. These results, however, were not replicated in the ABDOMIX trials with the commercial Toraymyxin product and with CytoSorb [60,61]. A recent in vitro examination showed statistically significant removal of cytokines by oXiris and CytoSorb compared to Toraymyxin [62].
11. Supporting Studies
Patients with severe COVID-19 appear to have greater elevations in IL-6 and C-reactive protein levels than those with moderate COVID-19. The excessive IL-1/IL-6 response to infection with the SARS-CoV-2 virus appears to contribute to patient symptomology and outcomes. Elevated levels of IL-6 have been reported to predict the occurrence of acute respiratory distress syndrome and mortality in patients with COVID-19 [38,63,64,65,66]. Behind other strategies, application of the newly developed technology for extracorporeal blood purification (EBP) might enhance the adequacy of renal support therapies in COVID-19. Inflammatory cytokines, DAMPs and PAMPs, including endotoxins and SARS-CoV-2 particles, are possible contributors to multiple organ failure and mortality in critically ill COVID-19 patients. EBP techniques have been shown to remove cytokines, DAMPs and PAMPS, such as endotoxins and circulating viral particles. Hemoperfusion, therapeutic plasma exchange and CRRT with surface-modified AN69 or PMMA membranes, as well as MCO or HCO membranes, are techniques potentially applicable in removing circulating molecules implicated in the pathophysiology of COVID-19 [38].
Critically ill patients with COVID-19 presenting with systemic inflammation and organ dysfunction may benefit from immunomodulation and RRT. EBP has been shown to mediate immunomodulation in patients with a maladaptive inflammatory response. A strong pathophysiologic rationale thus supports the use of EBP in COVID-19. In particular, EBP may attenuate systemic inflammation, helping to prevent or mitigate multiple organ dysfunction. Moreover, EBP with the oXiris Filter Set was shown to be technically feasible and not associated with major adverse events. Nevertheless, future randomized trials are certainly required to demonstrate the clinical effects of EBP in COVID-19 [5].
12. Conclusions
In conclusion, COVID-19 is an emerging respiratory virus that has severely challenged health care systems around the world. Extracorporeal therapies, as approved by the FDA, have been shown to improve clinical outcomes in critically ill COVID-19 patients requiring ventilatory support in single-center studies. The oXiris filter device removes both cytokines and endotoxins through ionic interactions with its membrane composition, while CytoSorb uses hydrophobic polymer beads to remove cytokines and other inflammatory mediators. Ongoing clinical trials demonstrate the theoretical and clinical importance of extracorporeal therapies to improving health outcomes while highlighting the continued need for solid evidence. Further evaluation of the efficacy of various modalities, with a focus on oXiris and CytoSorb HA, is pivotal for establishing generalizable supportive treatments for AKI in the setting of COVID-19.
Author Contributions
Conceptualization, S.S., V.E., D.P. and R.R.; formal analysis, S.S., V.E., S.B., T.Z., A.D. and R.R.; Search Criteria, S.S., T.Z., A.D. and R.R.; writing—original draft preparation, S.S., V.E., D.P., B.A. and V.B.; writing—review and editing, S.S., D.P., B.A., S.B. and R.R.; supervision, R.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This was literature review and all the data can be found from the references we used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Boregowda, U.; Gandhi, D.; Jain, N.; Khanna, K.; Gupta, N. Comprehension Literature Review and Evidence evaluation of Experimental Treatment in COVID 19 Contagion. Clin. Med. Insights: Circ. Respir. Pulm. Med. 2020, 14, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.T.; Shao, S.C.; Lai, E.C.C.; Hung, M.J.; Chen, Y.C. Mortality rate of acute kidney injury in SARS, MERS, and COVID-19 infection: A systematic review and meta-analysis. Crit. Care 2020, 24, 439. [Google Scholar] [CrossRef] [PubMed]
- COVID Data Tracker. Center for Disease Control and Prevention. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 16 April 2021).
- Villa, G.; Romagnoli, S.; De Rosa, S.; Greco, M.; Resta, M.; Pomarè Montin, D.; Prato, F.; Patera, F.; Ferrari, F.; Rotondo, G.; et al. Blood purification therapy with a hemodiafilter featuring enhanced adsorptive properties for cytokine removal in patients presenting COVID-19: A pilot study. Crit. Care 2020, 24, 605. [Google Scholar] [CrossRef]
- Supady, A.; Duerschmied, D.; Bode, C.; Rieder, M.; Lother, A. Extracorporeal cytokine adsorption as an alternative to pharmacological inhibition of IL-6 in COVID-19. Crit. Care 2020, 24, 51. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7439244/ (accessed on 28 August 2022). [CrossRef]
- Rabi, F.A.; Al Zoubi, M.S.; Kasasbeh, G.A.; Salameh, D.M.; Al-Nasser, A.D. SARS-CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens 2020, 9, 231. [Google Scholar] [CrossRef]
- Raina, R.; Chakraborty, R.; Sethi, S.K.; Bunchman, T. Kidney Replacement Therapy in COVID-19 Induced Kidney Failure and Septic Shock: A Pediatric Continuous Renal Replacement Therapy [PCRRT] Position on Emergency Preparedness with Resource Allocation. Front. Pediatr. 2020, 8, 413. [Google Scholar] [CrossRef]
- Fabrizi, F.; Alfieri, C.M.; Cerutti, R.; Lunghi, G.; Messa, P. COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Pathogens 2020, 9, 1052. [Google Scholar] [CrossRef]
- Neerukonda, S.N.; Katneni, U. A Review on SARS-CoV-2 Virology, Pathophysiology, Animal Models, and Anti-Viral Interventions. Pathogens 2020, 9, 426. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Sun, H.; Guo, P.; Zhang, L.; Wang, F. Serum Interleukin-6 Concentrations and the Severity of COVID-19 Pneumonia: A Retrospective Study at a Single Center in Bengbu City, Anhui Province, China, in January and February 2020. Med. Sci. Monit. 2020, 26, e926941. [Google Scholar] [CrossRef]
- Słomka, A.; Kowalewski, M.; Żekanowska, E. Coronavirus Disease 2019 (COVID–19): A Short Review on Hematological Manifestations. Pathogens 2020, 9, 493. [Google Scholar] [CrossRef]
- Singh, Y.P.; Chhabra, S.C.; Lashkari, K.; Taneja, A.; Garg, A.; Chandra, A.; Chhabra, M.; Singh, G.P.; Jain, S. Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb®) in the management of septic shock: A retrospective observational study. Int. J. Artif. Organs. 2020, 43, 372–378. [Google Scholar] [CrossRef]
- Min, C.-K.; Cheon, S.; Ha, N.-Y.; Sohn, K.M.; Kim, Y.; Aigerim, A.; Shin, H.M.; Choi, J.-Y.; Inn, K.-S.; Kim, J.-H.; et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016, 6, 25359. [Google Scholar] [CrossRef]
- Liu, F.; Li, L.; Xu, M.; Wu, J.; Luo, D.; Zhu, Y.; Li, B.; Song, X.; Zhou, X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020, 127, 104370. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Y.; Ou, W.; Ming, F.; Liang, G.; Qian, Y.; Cai, Q.; Dong, S.; Hu, S.; Wang, W.; et al. Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study. J. Transl. Med. 2020, 18, 406. [Google Scholar] [CrossRef]
- DeDiego, M.L.; Nieto-Torres, J.L.; Regla-Nava, J.A.; Jimenez-Guardeño, J.M.; Fernandez-Delgado, R.; Fett, C.; Castaño-Rodriguez, C.; Perlman, S.; Enjuanes, L. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 2014, 88, 913–924. [Google Scholar] [CrossRef]
- Huang, K.J.; Su, I.J.; Theron, M.; Wu, Y.C.; Lai, S.K.; Liu, C.C.; Lei, H.Y. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005, 75, 185–194. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Schulert, G.S.; Grom, A.A. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res. Clin. Rheumatol. 2014, 28, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; van der Meer, Y.; Zevenhoven-Dobbe, J.; Onderwater, J.J.; van der Meulen, J.; Koerten, H.K.; Mommaas, A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006, 80, 5927–5940. [Google Scholar] [CrossRef]
- Chan, J.F.; Lau, S.K.; To, K.K.; Cheng, V.C.; Woo, P.C.; Yuen, K.Y. Middle East respiratory syndrome coronavirus: Another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015, 28, 465–522. [Google Scholar] [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020, 217, e20200652. [Google Scholar] [CrossRef]
- Apetrii, M.; Enache, S.; Siriopol, D.; Burlacu, A.; Kanbay, A.; Kanbay, M.; Scripcariu, D.; Covic, A. A brand-new cardiorenal syndrome in the COVID-19 setting. Clin. Kidney. J. 2020, 13, 291–296. [Google Scholar] [CrossRef]
- Izzedine, H.; Jhaveri, K.D.; Perazella, M.A. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020, 97, 1297–1298. [Google Scholar] [CrossRef]
- Sebastian, R.; Arunachalam, J.; Rajendran, M. Temporal Clustering of Anti-glomerular Basement Membrane Disease in COVID-19 Pandemic: A Case Series. Int. J. Nephrol. Renovasc. Dis. 2021, 14, 393–398. [Google Scholar] [CrossRef]
- Allez, M.; Denis, B.; Bouaziz, J.D.; Battistella, M.; Zagdanski, A.M.; Bayart, J.; Lazaridou, I.; Gatey, C.; Pillebout, E.; Chaix Baudier, M.L.; et al. COVID-19-Related IgA Vasculitis. Arthritis Rheumatol. 2020, 72, 1952–1953. [Google Scholar] [CrossRef]
- Kolhe, N.V.; Fluck, R.J.; Selby, N.M.; Taal, M.W. Acute kidney injury associated with COVID-19: A retrospective cohort study. PLoS Med. 2020, 17, e1003406. [Google Scholar] [CrossRef]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Correction in Lancet 2020, 395, 496. [Google Scholar] [CrossRef]
- Patel, N.; Rein, J.L.; Sanchez-Russo, L.; Winston, J.; Uribarri, J. COVID-19-Associated Acute Kidney Injury: A Case Series. Kidney Med. 2020, 2, 668–669. [Google Scholar] [CrossRef]
- Jhaveri, K.D.; Meir, L.R.; Chang, B.S.F.; Parikh, R.; Wanchoo, R.; Barilla-LaBarca, M.L.; Bijol, V.; Hajizadeh, N. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020, 98, 509–512. [Google Scholar] [CrossRef]
- Lewis, D.H.; Chan, D.L.; Pinheiro, D.; Armitage-Chan, E.; Garden, O.A. The immunopathology of sepsis: Pathogen recognition, systemic inflammation, the compensatory anti-inflammatory response, and regulatory T cells. J. Vet. Intern. Med. 2012, 26, 457–482. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J., Jr.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Padala, S.A.; Vakiti, A.; White, J.J.; Mulloy, L.; Mohammed, A. First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA. J. Clin. Med. Res. 2020, 12, 454–457. [Google Scholar] [CrossRef]
- Salvatori, G.; Ricci, Z.; Bonello, M.; Ratanarat, R.; D’Intini, V.; Brendolan, A.; Dan, M.; Piccinni, P.; Bellomo, R.; Ronco, C. First clinical trial for a new CRRT machine: The Prismaflex. Int. J. Artif. Organs 2004, 27, 404–409. [Google Scholar] [CrossRef]
- Shum, H.P.; Chan, K.C.; Kwan, M.C.; Yan, W.W. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med. J. 2013, 19, 491–497. [Google Scholar] [CrossRef][Green Version]
- Broman, M.E.; Hansson, F.; Vincent, J.L.; Bodelsson, M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS ONE 2019, 14, e0220444. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xia, P.; Zhou, Y.; Liu, Z.; Zhou, X.; Wang, J.; Li, T.; Yan, X.; Chen, L.; Zhang, S.; et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin. Immunol. 2020, 214, 108408. [Google Scholar] [CrossRef] [PubMed]
- Schwindenhammer, V.; Girardot, T.; Chaulier, K.; Grégoire, A.; Monard, C.; Huriaux, L.; Illinger, J.; Leray, V.; Uberti, T.; Crozon-Clauzel, J.; et al. oXiris® Use in Septic Shock: Experience of Two French Centres. Blood Purif. 2019, 47 (Suppl. 3), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ugurov, P.; Popevski, D.; Gramosli, T.; Neziri, D.; Vuckova, D.; Gjorgon, M.; Stoicovski, E.; Marinkovic, S.; Veljanovska-Kiridjievska, L.; Ignevska, K.; et al. Early Initiation of Extracorporeal Blood Purification Using the AN69ST (oXiris®) Hemofilter as a Treatment Modality for COVID-19 Patients: A Single-Centre Case Series. Braz. J. Cardiovasc. Surg. 2020. published online ahead of print. [Google Scholar] [CrossRef]
- Turani, F.; Barchetta, R.; Falco, M.; Busatti, S.; Weltert, L. Continuous Renal Replacement Therapy with the Adsorbing Filter oXiris in Septic Patients: A Case Series. Blood Purif. 2019, 47 (Suppl 3), 1–5. [Google Scholar] [CrossRef]
- Guan, M.; Wang, H.; Tang, X.; Zhao, Y.; Wang, F.; Zhang, L.; Fu, P. Continuous Renal Replacement Therapy with Adsorbing Filter oXiris in Acute Kidney Injury with Septic Shock: A Retrospective Observational Study. Front. Med. 2022, 9, 789623. [Google Scholar] [CrossRef]
- Xie, J.; Xiao, W.; Lin, J. Effect of oXiris-CVVH on the Clinical Outcomes of Patients with Septic Shock: An Inverse Probability of Treatment-Weighted Analysis. Blood Purif. 2022, 1–18. [Google Scholar] [CrossRef]
- Rizvi, S.; Danic, M.; Silver, M.; LaBond, V. Cytosorb filter: An adjunct for survival in the COVID-19 patient in cytokine storm? a case report. Heart Lung. 2021, 50, 44–50. [Google Scholar] [CrossRef]
- Hawchar, F.; László, I.; Öveges, N.; Trásy, D.; Ondrik, Z.; Molnar, Z. Extracorporeal cytokine adsorption in septic shock: A proof of concept randomized, controlled pilot study. J. Crit. Care 2019, 49, 172–178. [Google Scholar] [CrossRef]
- Wei, T.; Chen, Z.; Li, P.; Tang, X.; Marshall, M.R.; Zhang, L.; Fu, P. Early use of endotoxin absorption by oXiris in abdominal septic shock: A case report. Medicine 2020, 99, e19632. [Google Scholar] [CrossRef]
- Schädler, D.; Pausch, C.; Heise, D.; Meier-Hellmann, A.; Brederlau, J.; Weiler, N.; Marx, G.; Putensen, C.; Spies, C.; Jörres, A.; et al. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS ONE 2017, 12, e0187015. [Google Scholar] [CrossRef]
- Supady, A.; Weber, E.; Rieder, M.; Lother, A.; Niklaus, T.; Zahn, T.; Frech, F.; Müller, S.; Kuhl, M.; Benk, C.; et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): A single centre, open-label, randomised, controlled trial. Lancet Respir. Med. 2021. published online ahead of print. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Pomarè Montin, D.; Ankawi, G.; Lorenzin, A.; Neri, M.; Caprara, C.; Ronco, C. Biocompatibility and Cytotoxic Evaluation of New Sorbent Cartridges for Blood Hemoperfusion. Blood Purif. 2018, 46, 187–195. [Google Scholar] [CrossRef]
- Ankawi, G.; Fan, W.; Pomarè Montin, D.; Lorenzin, A.; Neri, M.; Caprara, C.; de Cal, M.; Ronco, C. A New Series of Sorbent Devices for Multiple Clinical Purposes: Current Evidence and Future Directions. Blood Purif. 2019, 47, 94–100. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, S.R.; Yang, Z.L.; Liu, J.Y. Effect on extrapulmonary sepsis-induced acute lung injury by hemoperfusion with neutral microporous resin column. Ther. Apher. Dial. 2013, 17, 454–461. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Z.; Murugan, R.; Kellum, J.A. Blood purification and mortality in sepsis: A meta-analysis of randomized trials. Crit. Care Med. 2013, 41, 2209–2220. [Google Scholar] [CrossRef]
- Payen, D.M.; Guilhot, J.; Launey, Y.; Lukaszewicz, A.C.; Kaaki, M.; Veber, B.; Pottecher, J.; Joannes-Boyau, O.; Martin-Lefevre, L.; Jabaudon, M.; et al. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: A multicenter randomized control trial. Intensive Care Med. 2015, 41, 975–984. [Google Scholar] [CrossRef]
- Poli, E.C.; Alberio, L.; Bauer-Doerries, A.; Marcucci, C.; Roumy, A.; Kirsch, M.; Stefano, E.; Liaudet, L.; Schneider, A. Cytokine clearance with CytoSorb® during cardiac surgery: A pilot randomized controlled trial. Crit. Care 2019, 23, 108. [Google Scholar] [CrossRef]
- Buckley, L.F.; Wohlford, G.F.; Ting, C.; Alahmed, A.; Van Tassell, B.W.; Abbate, A.; Devlin, J.W.; Libby, P. Role for Anti-Cytokine Therapies in Severe Coronavirus Disease 2019. Crit. Care Explor. 2020, 2, e0178. [Google Scholar] [CrossRef]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. 2020, 31, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).