Abstract
A complete response to the challenge of COVID-19 requires diagnosis, prevention, and treatment strategies. Until recently, the treatment arm has included largely ineffective, often unproven medications with minimal impact on disease outcomes. The earlier experimental therapies are now giving way to approved antiviral drugs with a demonstrated capacity for SARS-CoV-2 suppression, and more are on the way. New oral antiviral drugs will expand treatment options for persons with COVID-19 and, if used early, become the first line of defense for reducing hospitalization, mortality, and virus spread. Several oral medications have been approved for treating COVID-19 on an emergency use basis in the United States (US), European Union (EU), United Kingdom (UK), China, Russia, and India, with other countries now facilitating regulatory reviews and approvals. Here, we compare the risk/benefit profiles of three leading oral antiviral drugs: Favipiravir, Molnupiravir, and Paxlovid. These compounds have distinct features supporting their targeted use by persons with COVID-19 disease.
1. Introduction
The 2019 worldwide outbreak of respiratory disease caused by a novel coronavirus, SARS-CoV-2, originated in the city of Wuhan, Hubei Province, China, and spread rapidly to cause a global pandemic. The causative agent of COVID-19 disease is the novel coronavirus SARS-CoV-2. Variants of the original Wuhan strain of the SARS-CoV-2 virus have higher levels of infectivity due to changes in the viral envelope S glycoprotein that reduce the effectiveness of vaccines for prevention and monoclonal antibodies for therapy. Thus, there is an urgent need for ongoing vaccine development, better compliance programs, continued diagnostic surveillance, and efficacious drug therapies. Antiviral therapy can slow or prevent disease progression among persons who are unvaccinated or experience break-through infections and limit the spread of virus among close contacts of infected individuals.
Since January 2020, there have been more than 520,000,000 confirmed cases of COVID-19 and more than 6,270,000 deaths reported to the World Health Organization (WHO). Current estimates for the United States are more than 147,000,000 cases of COVID-19 and nearly 1,000,000 deaths [1]. Multiple vaccine development and distribution programs collectively administered > 10,000,000,000 vaccine doses; more than 500,000,000 vaccine doses were administered in the US alone [2].
Despite efforts to roll out public health awareness and vaccine programs, the COVID-19 pandemic has not been contained and there is a need to supplement vaccination efforts with antiviral therapeutics for treating disease and reducing virus spread. Oral antiviral drugs are particularly valuable options for COVID-19, especially if used early to slow or prevent disease progression. Several antiviral compounds have already been approved for COVID-19 on an emergency use basis in the US, EU, UK, China, Russia, and India, with several countries accelerating reviews for emergency use approval.
The highest levels of SARS-CoV-2 replication occur early in the course of COVID-19 disease [3]. Antiviral therapy delivered soon after diagnosis might be able to prevent progression to the hyperinflammatory state associated with severe COVID-19 illness. Drugs able to suppress viremia may also limit the spread of disease within communities, especially when a large fraction of infected individuals receive home care mainly or exclusively. Once adequate clinical and safety information is available for antiviral drugs, the potential for pre-and post-exposure prophylaxis can be explored as an additional means for reducing community spread of SARS-CoV-2 and protecting healthcare workers or household contacts.
2. Methods
Here, we summarize and compare available information about selected antiviral drugs to provide an integrated view of key aspects impacting their risk/benefit profiles. Our goal is to inform about these agents and support their targeted use in populations at risk for COVID-19 disease. We reviewed publicly available regulatory approval files and published reports for each of three compounds; these reports were identified through a search of available databases (PubMed; Google Scholar).
We focused on nonclinical and clinical risk/benefit profiles for these three compounds because they have received accelerated approvals. We restricted the review to drugs that are approved for oral delivery and demonstrated a direct antiviral mechanism of action. Similarities and differences among these drugs may inform how best to deploy each of them as part of the emerging armamentarium for safe and effective COVID-19 therapy.
3. Regulatory and Clinical Trial Landscape
Three oral antiviral compounds for treating COVID-19 in hospital, outpatient, and community settings include Favipiravir (also known as AVIFAVIR, AVIGAN, and FABIFLU), Molnupiravir (also known as Lagevrio), and the combination of Nirmatrelvir/Ritonavir (also known as Paxlovid). The three compounds (Figure 1) were developed originally for a viral disease other than COVID-19 before being repurposed as a SARS-CoV-2 antiviral drug. Because of prior experience with these drugs, it was possible to develop and provide treatments expeditiously as part of the emergency pandemic response.
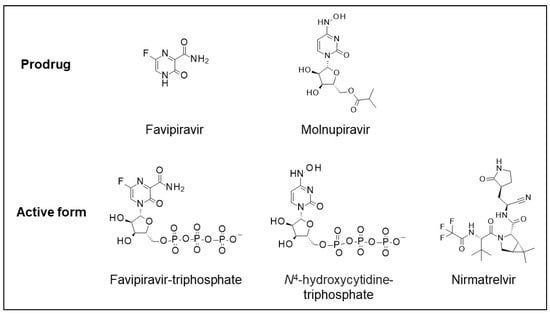
Figure 1.
Structures of Favipirvir and Molnupiravir prodrugs and active forms of Favipiravir-triphosphate, N-hydroxycytidine-triphosphate, and Nimatrelvir.
Favipiravir (T-705) is the oral prodrug of Favipiravir triphosphate and was discovered by Toyama Chemical Co., Ltd. (Tokyo, Japan), during the screening of a chemical library for antiviral activity against influenza virus [4,5]. The prodrug undergoes intracellular phosphoribosylation to the active form Favipiravir Triphosphate (FTP); it is an inhibitor of influenza RNA-dependent RNA polymerase and active against many other RNA viruses.
Favipiravir was approved in Japan in 2014 under the trade name Avigan for the treatment of an outbreak of novel or re-emerging virus infection [5]. Favipiravir was also used successfully for treating patients during an emerging Ebola virus epidemic in West Africa [6] and, on 15 August 2014, the Minister of Health, Welfare and Labor of Japan approved the use of Favipiravir [7]. Favipiravir has also been used for the treatment of Lassa virus, rabies, and severe fever with thrombocytopenia syndrome [8,9,10,11]. Molnupiravir (EIDD2801; MK-4482) is the oral prodrug of N4-hydroxycytidine (NHC; EIDD-1931) and was developed originally by the Drug Innovation Ventures at Emory University [12] as an inhibitor of influenza and respiratory syncytial virus replication. EIDD2801 was subsequently developed by Ridgeback Therapeutics partnering with Merck & Co on Molnupiravir; however, it was never approved in the influenza indication. Nirmatrelvir (PF-00835231), the antiviral component of Paxlovid, is an orally available inhibitor of the SARS-CoV-2 main protease (Mpro; 3CL protease) developed by Pfizer as a therapy for SARS-CoV [13]. Due to susceptibility to Cytochrome (CYP)450 enzyme metabolism resulting in short drug half-life, Nirmatrelvir is taken together with ritonavir (Norvir), an approved (HIV) protease inhibitor used to block CYP450 activity and reduce rates for drug metabolism.
Favipiravir was first to obtain emergency regulatory approval for treating COVID-19 in early 2020. The drug was approved initially in China followed by approval in the Russian Federation and later in India. New oral formulations of Favipiravir are being evaluated in ongoing clinical trials worldwide. As of 7 March 2022, there were 46 clinical trials of Favipiravir for COVID-19 posted on www.clinicaltrials.gov (accessed on 7 March 2022) of which 21 studies were listed as active. There were eight Molnupiravir trials of which four were listed as actively recruiting, one trial was listed as active but not recruiting yet, and one trial was terminated. For Paxlovid, there were four active trials at the time of writing this manuscript.
4. Drug Mechanisms of Action
FTP, the active form of Favipiravir, is incorporated into nascent RNA chains as Favipiravir monophosphate (FMP) by the action of RNA-dependent RNA polymerases (RdRp) from several RNA viruses, including SARS-CoV-2. FTP is a weak inhibitor of RNA chain elongation and incorporation into the growing chain is the prevalent outcome. FTP binds the RdRp complex in the +1 position and is paired with cytidine (C) or uridine (U) residues in the template strand [14,15]. Misreading of the FMP residue results in the corruption and eventual elimination of the viral genome via a mechanism known as lethal mutagenesis [16]. The active form of Molnupiravir, N4-hydroxycytidine triphosphate (NHCTP), is incorporated into growing RNA chains as NHCMP instead of cytidine or uridine triphosphate and is misread in subsequent RNA replication as guanine (G) or adenine (A), resulting in mutagenesis [17]. In vitro studies of combined exposure to Favipiravir and Molnupiravir showed high potency against SARS-CoV-2 replication [18].
Whether FTP acts as a terminator of RNA chain elongation is controversial. In vitro studies demonstrated chain termination when multiple, consecutive FTP were incorporated into a growing RNA [14,15]. However, consecutive incorporation of FTP is unlikely in the cellular environment where guanosine triphosphate (GTP) and adenosine triphosphate (ATP) are present and compete with FTP [14]. NHCTP incorporation does not lead to chain termination [17,19]. Lethal mutagenesis, also called error catastrophe, is the most critical mechanism of action for both Favipiravir and Molnupiravir.
An alternate approach targets viral enzymes required for processing polyproteins encoded by the large, open reading frames of SARS-CoV-2. The main protease is a chymotrypsin-like 67.6 kDa dimer that cleaves SARS-CoV-2 polyproteins in at least 11 sites, using a recognition sequence with a Glycine (Gln) residue at position 1 [20,21]. The Main protease has a Cysteine-Histidine (Cys-His) catalytic dyad first recognized during studies on SARS-CoV [22] that is amenable to targeting by covalent and non-covalent inhibitors. Paxlovid’s Nirmatrelvir is a peptidomimetic inhibitor of the Main protease and is bound covalently to the active site Cys residue. Ritonavir’s role in Paxlovid is to maintain active levels of Nirmatrelvir, mainly by inhibiting drug metabolism via CYP3A4 [23] and inhibiting cellular mechanisms for drug efflux, including the ABCB5 P-glycoprotein (P-gp) transporter [24].
5. Preclinical Potency against SARS-CoV-2 Virus
All three compounds were shown to be effective in vitro against the SARS-CoV-2 virus and its variants. All three compounds were effective in experimental animal models of COVID-19 as inhibitors of infection and disease.
Compared to Molnupiravir and Paxlovid’s Nirmatrelvir, Favipiravir is less potent against the SARS-CoV-2 virus in vitro [15,25]. Despite a lower antiviral potency in vitro, Favipiravir, like Molnupiravir [18,26] and Paxlovid’ s Nirmatrelvir [27], exhibited potent antiviral effects in animal models of SARS-CoV-2 infection (Syrian hamster), using exposures relevant to those observed in clinical trials [28,29]. More importantly, all three compounds were shown to be active, irrespective of the particular SARS-CoV-2 variant used, including being active against the omicron variant [30,31,32].
These drugs are also amenable to working in combination with each other and with other antiviral agents against SARS-CoV-2. Abdelnabi et al. demonstrated that the combination of Favipiravir and Molnupiravir, each at a suboptimal dose, was synergistic and potently reduced the total viral load and infectious virus when treatment was administered before or soon after infection in an experimental model of COVID-19 [18]. This has important implications for safety, potentially minimizing risks that are common among these compounds. A combination of Molnupiravir and Nirmatrelvir was also reported to be active against SARS-CoV-2 in vitro [31].
6. Pharmacokinetics
The animal pharmacokinetics (PK) of all three compounds distinguish their nonclinical profiles and may impact their clinical use for COVID-19. Although both Favipiravir and Molnupiravir are oral prodrugs, Favipiravir has superior bioavailability across all animal species tested, i.e., 100% in mice and rats and 73% in dogs [33]. NHC and its prodrug Molnupiravir are subject to first-pass metabolism via intestinal and hepatic esterases, resulting in an oral bioavailability of 37–45% in mice and 52 to ~77% in dogs and rats, respectively [34].
Paxlovid’s Nirmatrelvir has poor bioavailability due to an extensive CYP3-mediated metabolism and it was necessary to include the CYP3 inhibitor Ritonavir to boost and maintain the drug levels. Ritonavir impacts multiple members of the CYP450 system by inhibiting CYP3A4, CYPD6, CYP2C19, CYP2C28, and CYP2C9 but is an inducer of CYP1A2, CYP2B6, CYP2C9, and CYP2C19 (reviewed by [24]). Ritonavir also exhibits a time-dependent effect on the ABCB5 P-gp transporter with initial inhibition followed by induction [35]. Rapid drug efflux through the ABCB5 P-gp transporter was noted during preclinical studies [27]. Ritonavir effects on P-gp will impact Nirmatrelvir exposure [24], but the clinical relevance is not known.
Nirmatrelvir has a similar protein binding rate to Favipiravir (approximately 69%), while Ritonavir is highly bound to plasma proteins (98–99%) [36]. Molnupiravir is unstable in plasma, while the unbound fraction of NHC was approximately 1 across the animal species and concentrations tested [34].
Biosynthesis of the Favipiravir triphosphorylated form (FVP RTP) in cells was a subject of saturation with an approximate intracellular half-life of about 6–6.5 h. FVP RTP distributes to and is retained in the respiratory system in larger concentrations than in plasma with a half-life of approximately 4.5 h. [33]. Molnupiravir’s metabolite NHC distributes to the lungs and spleen with a similar half-life [34].
FVP-RTP is converted into an inactive M1 metabolite in the liver, primarily via the aldehyde oxidase (AO) [33]. Molnupiravir/NHC is ultimately catabolized to uridine/cytidine which then mixes with the endogenous nucleoside pool [34]. In contrast, Nirmatrelvir is metabolized primarily by CYP3A4 [36].
Favipiravir is mainly eliminated via urine as an inactive M1 metabolite compared with Molnupiravir that is catabolized to endogenous nucleosides. Published reports indicated that Favipiravir can be used safely in COVID-19 patients with end-stage renal disease on dialysis [37]. The available data regarding the influenza indication in Japan suggested that serious adverse events attributable to M1 are unlikely to occur in patients with mild-to-moderate renal impairment if Favipiravir is administered in accordance with the proposed dose regimen. Like Favipiravir, Paxlovid’s Nirmatrelvir is also eliminated through the renal system.
Favipiravir’s potential for clinically relevant or significant drug interactions (DDI) is considered low as CYP and transporter involvement in its metabolism are negligible [33,38]. While Favipiravir is not metabolized by the CYP system, Favipiravir was shown to inhibit CYP2C8; however, its clinical relevance is not clear. Regardless, caution should be used when administering drugs that are metabolized by the CYP2C8 system.
7. Clinical Pharmacology
All three compounds were evaluated in standard clinical pharmacology Phase 1 programs that tested single and multiple ascending doses (SAD and MAD studies, all three compounds), food effects, and the impact on intrinsic and extrinsic factors (primarily for Favipiravir). These trials used dose levels that were subsequently shown to be efficacious for COVID-19 disease and the doses were well-tolerated.
The doses evaluated in Favipiravir (Avigan) clinical pharmacology studies (up to 292 healthy subjects of Asian or Caucasian ancestry) were relevant to those evaluated in prior trials of Favipiravir for the treatment of serious viral infections, such as influenza and Ebola (up to 6000 mg/day), as well as those used in COVID-19 clinical trials. The range of single doses evaluated in these studies was 40 to 2400 mg one time or 800 to 1200 mg daily for the repeated doses. Studies from healthy Japanese volunteers showed that Favipiravir was absorbed rapidly with the maximum plasma concentration reached by 2 h after oral administration followed by a rapid decrease with a half-life of 2–5.5 h. Favipiravir exposure exhibited a dose- and time-dependent profile. Exposures in Japanese subjects were somewhat lower compared to those observed in Caucasian subjects, although when normalized by body weight, these differences were minimal [33].
Like Favipiravir, Molnupiravir also displayed rapid absorption in healthy volunteers with a median time to maximum plasma concentration at 1.00 to 1.75 h and a rapid decline over approximately 1 h that was followed by a slower elimination phase after multiple doses or higher single doses [34,39].
No accumulation was observed with Favipiravir or Molnupiravir compared to Paxlovid. Lower exposures of Molnupiravir were observed in obese patients without an impact on antiviral efficacy. In contrast, the antiviral response of Favipiravir in COVID-19 patients was body weight dependent which provided for an original dosing regimen using certain body weight cutoffs [40].
One peculiarity in the clinical pharmacology profile of Favipiravir is a decrease in Ctrough concentrations in the context of viral infection, including COVID-19, indicating that viral infection impacts Favipiravir exposure. Specifically, animals infected with virus, i.e., guinea pigs or hamsters infected with Pichindé virus (PICV; models of acute Arenavirus hemorrhagic fever), were shown to exhibit dramatically lower concentrations of favipiravir; the favipiravir end metabolite, M1, was more prominently found in the infected animals and much higher ratios of M1:Favipiravir were observed in infected animals compared to the non-infected hamsters. These findings suggest that the metabolism of favipiravir may be more pronounced in viral infection [41,42]. It should be also noted that a similar PK pattern was observed in critically ill COVID-19 patients during a trial conducted by Irie et al. who observed that favipiravir trough concentrations in critically ill patients were much lower than that observed in healthy subjects. These authors noted that changes in drug distribution volume and clearance rates are known for patients in intensive care [43]. Additional studies are needed to understand the pharmacodynamics of Favipiravir in critically ill patients [41,43,44].
Food does not appear to have significant effects on the PK profile for any of the three compounds. It should be noted that Favipiravir oral tablet formulations (Avigan Avifavir) have a higher pill burden compared to Molnupiravir or Paxlovid, which does not appear to be of particular concern in outpatient practice.
Favipiravir is not associated with clinically relevant DDI, potentially due to the low involvement of CYP 450 in its drug metabolism and the negligible interactions with several transporters except those involved in the elimination and reabsorption of uric acid. Specifically, Favipiravir was shown to inhibit hURAT1 cells-mediated urate uptake in a concentration-independent manner, while M1 enhanced the uptake in a concentration-dependent manner [33]. Furthermore, Favipiravir and M1 were both shown to inhibit organic anion transporters 1 and 3 (OAT1 and OAT3), which facilitate kidney excretion of uric acid. Furthermore, M1 increases uric acid re-uptake in the proximal renal tubules via urate transporter 1 (URAT1) [45]. As a consequence, the secretion of uric is decreased and its reabsorption via urate transporter is increased [33].
Another clinically relevant DDI may be that with AO inhibitors; however, published literature about this is sparse.
Like Favipiravir, Molnupiravir is also devoid of significant DDI potential. In contrast, Paxlovid metabolism is heavily CYP3A-mediated which poses limits to its clinical use and impacts its risk/benefit profile [46]. The DDI potential of Paxlovid is high and, more importantly, clinically relevant, even within the context of short (5-day) oral treatment for COVID-19 [47,48]. The US EUA label for Paxlovid warns and advises precautions to healthcare professionals on the concomitant use of Paxlovid and certain other drugs that may result in potentially significant drug interactions. Healthcare professionals are advised to consult the full prescribing information prior to and during treatment for potential drug interactions [37]. This poses real-practice challenges for healthcare providers who must identify individuals at risk, implement appropriate monitoring for potentially harmful interactions, and adjust the dose as a part of DDI risk mitigation for Paxlovid. The most common classes of drugs that may have clinically significant DDI with Paxlovid include drugs that are most likely to be used with Paxlovid, either in the context of recommended COVID-19 supportive treatment per clinical practice guidelines or as a part of the patient re-existing polypharmacy due to co-morbidities (Table 1). Among these drugs, CYP3A4 agents predominate this list, especially those with a narrow therapeutic index and strong interaction with CYP3A4 as substrates, inducers, or inhibitors; the use of CYP2D6 agents should also be considered carefully for co-administration with Paxlovid (Table 1).

Table 1.
Therapeutic classes of interest for clinically significant DDI with Paxlovid.
8. Toxicology
Nonclinical safety studies indicated that all three antiviral compounds were well-tolerated. Animal safety pharmacology profiles for all three compounds were devoid of notable adverse effects on major organ system functions [33,34,36]. A few safety concerns common to all three compounds were identified during animal safety studies and may impact their clinical use for treating COVID-19. These mainly include hepatotoxicity and risk to reproduction. Hepatotoxicity is relevant to the clinical use of all three compounds in the context of COVID-19 disease, for which underlying liver disease is a recognized risk factor and SARS-CoV-2 infection is associated with liver damage. Up to 50% of severe COVID-19 patients have liver dysfunction which may be potentiated by the hepatotoxic side effects of medications given for managing the disease [52]. Advanced hepatic impairment is a contraindication for clinical use of all three compounds.
Although the hepatotoxic changes due to Nirmatrelvir were demonstrated as reversible and without histopathological correlates, it is uncertain whether there is a potentiation of hepatoxicity when Nirmatrelvir and Ritonavir are administered in combination; the results of co-administration were reported from the nonclinical program. Liver changes associated with Nirmatrelvir were considered secondary to the CYP3A-mediated enzyme induction, but this finding was limited to rats. Clinical relevance of these changes is unknown in the context of a 5-day treatment duration, but they do occur at 4-fold higher exposure compared to the clinical exposure, indicating relatively low safety margins. The animal studies of Ritonavir identified liver as one of the target organ toxicities, with primary manifestations being hepatocellular, biliary, and phagocytic changes; these changes were accompanied by increases in hepatic enzymes [36]. The approved label of Ritonavir requires monitoring of liver function [53].
Increased liver weight was observed in the 28-day rodent (rat) study of Molnupiravir with exposure margins of 7.8- and 4.2-fold for males and females, respectively, when compared to exposure associated with the human clinical dose of 800 mg BID. No histopathological correlates were observed, and these findings were not observed in the 13-week study. However, it should be noted that increased liver enzymes were reported in Molnupiravir clinical trials [34].
Favipiravir hepatotoxicity has been interpreted mainly in the context of its predominant hepatic metabolism via aldehyde oxidase. Favipiravir exposure in the animal repeat-dose toxicity studies at NOAEL levels compared favorably in humans using the approved influenza dosing regimen but indicated no safety margins for liver toxicity. However, due to the lack of histopathological changes in liver tissue in the animal studies combined with liver safety findings in the clinical studies of influenza therapy, the risk of liver toxicity is considered low for clinical use of favipiravir at the approved influenza doses [33]. It also appears that its clinical use is associated with a low incidence of increased liver enzymes [40,54,55]. Hematopoietic toxicity was observed in animal safety studies with both Favipiravir and Molnupiravir; however, it appears that these changes did not translate to the clinical use of either compound.
Bone and cartilage toxicity were identified as risks uniquely associated with Molnupiravir. In animal studies, Molnupiravir was associated with bone and cartilage toxicity with no or very low margins of safety (0.7- to 3.3-fold in male and female rats, respectively); this was based on the NOAEL exposure of a 150 mg/kg/day dose. Because of these findings, Molnupiravir use in people younger than 18 years of age is contraindicated [34]. It is unknown whether these changes are relevant to menopausal and/or post-menopausal women who commonly suffer from osteoporosis.
Hyperuricemia is a mechanism-based toxicity unique to Favipiravir due to the inhibition of organic anion transporters (OAT) 1 and 3 by both the parental compound and its metabolite M1. It is recognized that this is the main mechanism utilized by favipiravir to reduce uric acid excretion via urine and cause elevated blood uric acid levels. In patients, elevated levels of uric acid usually return to values within the reference ranges upon discontinuing the drug. Nevertheless, it should be kept in mind that this action of favipiravir may have clinical significance in patients with histories of gout, renal dysfunction (increased blood concentrations of M1), or hyperuricemia as well as in patients simultaneously using other drugs that trigger elevated levels of blood uric acid. This risk is adequately managed by the currently approved Favipiravir labels [6], although the outpatient experience to date indicates that this risk is relatively low in the context of COVID-19.
With regard for risks to reproduction, and because both male and female individuals of childbearing potential are eligible for treatment with any of the three compounds, risk to reproduction is an important safety aspect. Paxlovid, as a drug combination, was not evaluated in a standard reproductive toxicity program. Nirmatrelvir was tolerable in standard fertility and embryofetal animal studies at doses up to 1000 mg/kg [56]. Ritonavir is devoid of clinically relevant risks to reproduction, although it exhibited toxicity to embryofetal development at doses associated with maternal toxicity, hepatotoxicity, elevated total cholesterol and triglycerides, and diabetes mellitus [57]. It should be emphasized that Paxlovid is authorized currently for use in the US by individuals 12 years of age and older weighing at least 40 kg. However, safety among the younger population was not supported by data as no animal studies in juvenile animals were conducted nor were clinical studies initiated in children 12–18 years of age. A popPK modeling of the adult data was used to justify the use of the drug in this population. Only recently, the sponsor initiated clinical trials in younger individuals. Nevertheless, the safety profile of Paxlovid in this population is confused by uncertainties and missing information.
Favipiravir was considered to be teratogenic in animal reproductive toxicity studies without safety margins from the exposures associated with clinical doses. Favipiravir also distributes in male sperm and breast milk. Avigan’s Japanese label requires informed consent related to strict adherence for contraception and negative pregnancy tests for females of childbearing potential [33]. Due to its distribution in human milk, lactation is also contraindicated and use of the drug is limited to individuals 18 years and older. Favipiravir has been found to have adverse effects in juvenile animal studies [33].
Molnupiravir was associated with adverse embryofetal effects in the preliminary embryofetal toxicity studies at exposures 7.5-fold of those associated with the clinical dose. These findings, combined with the toxicity to bone and cartilage, exclude prescribing Molnupiravir to individuals younger than 18 years of age [34]. However, the respective labels/instructions for use of both Favipiravir and Molnupiravir are, so far, managing these risks in the clinical setting, depending on strict compliance with requirements for contraception and interruption of lactation.
9. Clinical Safety
Favipiravir, Molnupiravir, and Paxlovid are reasonably well-tolerated by COVID-19 patients and observed differences in their safety profiles reflect different dosing regimens and variation in the populations of COVID-19 patients. Major safety concerns were effectively mitigated by the approved labels (Favipiravir) or fact sheet instructions for use (Molnupiravir/Paxlovid). The relatively short treatment duration of 5–10 days further mitigates most of the safety concerns. The safety profile of Favipiravir in COVID-19 treatment was mostly associated with hyperuricemia, increased liver enzymes, diarrhea, and nausea. Molnupiravir use commonly included adverse events (AE) in the central nervous system (CNS) category, such as dizziness and headache, as well as gastrointestinal (GI) tract AE, such as diarrhea and nausea. Vomiting, rash, and urticaria were uncommon.
Common adverse drug reactions (ADR) to Paxlovid included GI tract disturbances such as nausea, diarrhea (including severe electrolyte imbalance), vomiting, dyspepsia, dysgeusia, oral and peripheral paresthesia. CNS ADR included headache, dizziness, peripheral neuropathy, seizure, syncope, and others. Decreased hematology parameters, increased liver enzymes, and parameters of renal function were also noted.
The safety attributes of relevance to COVID-19 and related to the mechanism of action for all three compounds include hepatotoxicity and risk to reproduction. Safety attributes uniquely attributable to individual compounds included hyperuricemia associated with Favipiravir, hematopoietic toxicity and significant DDI related to the use of Paxlovid, and bone and cartilage toxicity reported during Molnupiravir use.
Hepatotoxicity was consistent with abnormal liver parameters. Increased levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and blood bilirubin, including jaundice, were listed as common (≥1/100 to <1/10) ADR in clinical trials of Paxlovid. Additional ADR indicative of disturbed hepatic function, although uncommon (1 to 0.1% of exposed individuals), included increased alkaline phosphatase [36]. Favipiravir hepatotoxicity manifested as an increase in liver enzymes [40,54,58]. The available clinical data on Molnupiravir do not list details on specific ADR out of the most common ADR which were related to GI disturbances. The incidence rate of hepatotoxicity in clinical trials was relatively low, i.e., 1–2 vs. 4% in placebo [34,59].
Hyperuricemia is an attribute unique to Favipiravir and is based on its interference with the metabolic pathways of uric acid in the body because its metabolism involves aldehyde oxidase. Patients with gout or a history of gout and patients with hyperuricemia are at risk when using Favipiravir [33,45,54].
The risk for hematopoietic toxicity in Favipiravir-treated patients appears to be low. The Molnupiravir nonclinical safety profile indicated a risk for bone marrow toxicity and was devoid of significant hematopoietic toxicity in clinical trials [34,59].
10. Clinical Efficacy
Direct comparisons of clinical efficacy for Favipiravir, Molnupiravir, and Paxlovid are not possible because of differences regarding when the efficacy studies were conducted (earlier in the pandemic with Favipiravir and later with Molnupiravir and Paxlovid), study designs, endpoints, duration of treatment, concomitant supportive therapies, doses, and more importantly, patient populations, to name the most important differences (Table 2). However, all three compounds were effective for clearing the virus and improving clinical symptoms of COVID-19. These successful antiviral agents significantly reduced progression to severe illness, hospitalization, and mortality.

Table 2.
Completed Phase 2 and 3 randomized clinical trials of antivirals in non-hospitalized subjects with mild-to-moderate COVID-19.
Favipiravir is one of the most clinically used antiviral drugs for COVID-19 due to early regulatory approvals worldwide, while the clinical experiences with Molnupiravir and Paxlovid are more limited with Paxlovid use increasing rapidly especially in the United States. In addition, there was substantial exposure to Favipiravir when used to treat influenza and other severe RNA viral infections, i.e., Ebola. Thus, the medical community has a more comprehensive overall clinical experience with Favipiravir compared to the limited but increasing experience with clinical use of Molnupiravir or Paxlovid.
Favipiravir is effective for treating SARS-CoV-2 infection both in hospitalized patients and outpatients [40,60], while Molnupiravir was not effective in hospitalized patients [34,59]. Paxlovid was evaluated only in outpatients [36,61].
Only Paxlovid is authorized for use in patients younger than 18 years of age; however, this was not supported with real-world data but was approved on the basis of popPK modeling. A trial in pediatric patients younger than 18 years of age has been posted on Clinical.trials.gov (accessed on 7 March 2022) [62].
Patients in a Favipiravir (AVIFAVIR) trial [40] had confirmed PCR diagnosis of SARS-CoV-2 infection compared to patients enrolled in Molnupiravir and Paxlovid trials that accepted a variety of diagnostic tests depending on availability of tests in countries where studies were conducted and confirmed diagnoses at study entry were not required. The relevance of these factors to variability in the results is not known.
The duration of treatment with Favipiravir (AVIFAVIR) in clinical trials was up to 10–14 days or until viral clearance was confirmed by two negative PCR tests. The duration of Molnupiravir treatment is 5 days, similar to Paxlovid.
Favipiravir (Avigan, AVIFAVIR) dosing employs a loading dose on Day 1 followed by daily maintenance doses compared to Molnupiravir and Paxlovid that do not utilize a loading dose but employ a flat dosing schedule for 5 days. It is uncertain how the necessary duration of treatment with Molnupiravir was defined because the median time to viral clearance in the Phase 2 trial with virologic endpoint was approximately 14 days.
The Favipiravir (AVIFAVIR) dosing regimen is based on body weight cutoffs, which provides for more accurate exposure in patients, i.e., those who are heavier can get adequate doses while avoiding potential overdosing in smaller patients. Both Molnupiravir and Paxlovid do not take this factor into account. Molnupiravir exposure was observed to be 20% lower in obese patients but without apparent impact on antiviral efficacy [34].
Median time to viral clearance with Favipiravir in hospitalized patients in clinical trials was 4 days [63], and in early post-approval use, 6 days of treatment compared with standard supportive therapy provided statistically significant outcome differences [64]. Both Molnupiravir and Paxlovid used placebo for comparison in a double-blind design setting; the median time to viral clearance with Molnupiravir was 14 days, while PAXLOVID caused a significant reduction in viral load within 3–5 days of treatment initiation [61]. The best results were observed for each of these compounds when patients were enrolled within 3 days after symptom onset. This is consistent with the mechanism of antiviral action and justifies the use of antivirals for treatment of COVID-19 at the earliest possible time. The median time to clinical improvement followed similar patterns. More importantly, these effects were associated with a significant reduction in 28-day mortality for all three drugs.
Drugs with proven activity against SARS-CoV-2 might be combined to achieve higher than expected potency. Favipiravir combinations with other non-antiviral agents were clinically effective in COVID-19 patients, including those who were critically ill. A combination of Favipiravir and steroids was reported to be beneficial for preventing severe COVID-19 pneumonia when drugs were administered in the early stage of disease [65,66,67]. Similar benefits in COVID-19 patients were reported with the combination of Favipiravir and Tocilizumab [68] and Favipiravir plus Nafamostat mesylate [69]. Favipiravir plus the serine protease inhibitor Aprotinin accelerated viral clearance and prevented the transfer of patients to intensive care [63].
Molnupiravir is also attractive for combination drug therapy. Brequinar, a dihydroorotate dehydrogenase inhibitor (DHOD), is being evaluated in non-hospitalized patients with COVD-19 (NCT04575038) and was proposed for combination therapy with Molnupiravir after encouraging preclinical results [70]. Molnupiravir combined with Nirmatrelvir showed additive effects when tested against the beta and delta strains of the SARS-CoV-2 virus [71].
12. Summary of the Clinical Risk/Benefit Profiles
All three compounds provide potent virus suppression and clinical benefits when administered early after SARS-CoV-2 infection. However, their clinical risks need to be understood in the context of their potential for DDI and, to some extent, to their mechanism of action. Caution must be used when prescribing Paxlovid due to its complex impact on metabolism and transporter functions. The extent to which risk is mitigated by the short treatment course remains uncertain.
Molnupiravir is not a subject of relevant DDI, and other risks are tolerable within the doses and regimens used for COVID-19 therapy; however, Molnupiravir is a DNA mutagen with uncertain potential for long-term damage. Again, this risk is mitigated by using a short treatment interval, although the clinical impact of this decision is uncertain.
Favipiravir’s potential for clinically relevant or significant drug interactions is also considered low However, Favipiravir was shown to inhibit CYP2C8 and also interferes with the uric acid pathways. Therefore, caution should be used when administering drugs in patients with pre-existing uric acid disorders as well as with agents that are metabolized by the CYP2C8 system. These risks appear to be low in the context of the current outpatient experience with COVID-19.
13. Discussion
Our review compared the risk/benefit profiles of three oral antiviral drugs approved currently for treating COVID-19. This is not an exhaustive evaluation of the differences among these compounds as there is substantial variation among clinical study methods and differences in the availability of data from nonclinical studies. Further, information about COVID-19 therapy is a fast-evolving landscape while public health programs begin to comprehend the vital role for antiviral therapy in combating the pandemic. We endeavored to provide a snapshot of critical issues impacting the potential for success with the currently available, approved oral antiviral drugs. This perspective may facilitate a comparative evaluation of new drugs that are or will begin clinical trials. The clinical use of oral antiviral therapies for COVID-19 disease is expected to undergo substantial growth with the expanded use of existing drugs and new compounds entering the market. A detailed understanding of drug properties and careful comparisons among available therapeutic options is especially important for treating a complex disease such as COVID-19 and where pandemic virus spread has created the highest level of urgency.
14. Conclusions
Based on the available preclinical and clinical data, all three compounds exhibit positive risk/benefit profiles in COVID-19 patients. Each drug has provided life-saving intervention in COVID-19 disease progression with acceptable risks, and their use is impacting the epidemic of SARS-CoV-2 infection. Favipiravir has the most extensive clinical experience and is an effective COVID-19 therapy in many parts of the world. Molnupiravir and Paxlovid have more limited clinical experience and are less widely used due to their recent introduction as COVID-19 therapies. These drugs manifest a spectrum of antiviral activity, resulting in the accelerated clearance of virus and improvement of clinical symptoms with reduced progression to severe illness and mortality. Accelerated drug approvals are already changing the landscape of treatment of COVID-19, supplementing vaccination efforts and making a positive difference in regions where vaccines may not be widely available. Due to the direct and potent impacts on viral burden, these drugs have potential for curbing further spreading of the infection in affected communities and households.
Although some attributes are shared among these drugs, they also have distinct properties due to their medicinal chemistries, mechanisms of action, PK behavior, dosing regimens, DDI, and clinical performance. While Favipiravir is acknowledged to have a high barrier to resistance mutations, less is known about Molnupiravir and Paxlovid regarding the potential for drug-resistant SARS-CoV-2 variants to emerge. The potential for drug resistance encourages a continuing effort to develop new drugs for multiple SARS-CoV-2 targets and perform surveillance for resistant variants. It remains uncertain whether distinct populations of COVID-19 patients may respond to one or to all three of the drugs. An analysis of the available and emerging data and the continuous analysis of their similarities and differences will contribute to a better understanding of COVID-19 treatment strategies.
Author Contributions
S.C., C.D.P. and N.S. conceptualized the paper; S.C. developed the methodology; S.C., N.S. and C.D.P. wrote and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded privately by Viriom, Inc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All sources were cited in references.
Conflicts of Interest
S.C. is a regulatory affairs consultant to Viriom, Inc. N.S. and C.D.P. are employees in and stockholders of Viriom, Inc. Viriom has a research and development interest in antiviral medications but no competing commercial interest that would cause a conflict.
References
- US Centers for Disease Control and Prevention. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 20 May 2022).
- World Health Organization. Available online: https://covid19.who.int/ (accessed on 8 March 2022).
- Paules, C.I.; Fauci, A.S. COVID-19: The therapeutic landscape. Med 2021, 2, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FUJIFILM Toyama Chemical Co., Ltd. Avigan Tablets 200 mg. Available online: https://www.sukl.cz (accessed on 14 June 2022).
- Mentre, F.; Taburet, A.M.; Guedj, J.; Anglaret, J.; Keita, S.; de Lamballerie, X. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect. Diseases 2015, 15, 150–151. [Google Scholar] [CrossRef]
- Nagata, T.; Lefor, A.K.; Hasegawa, M.; Ishii, M. Favipiravir: A new medication for the Ebola virus disease pandemic. Disaster Med. Public Health Prep. 2015, 9, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.M.; Cui, N.; Yuan, C.; Zhang, S.F.; Lu, Q.B.; Yang, Z.D.; Xin, Q.L.; Song, Y.B.; Zhang, X.A.; et al. Clinical effect and antiviral mechanism of T-705 in treating severe fever with thrombocytopenia syndrome. Signal Transduct. Target. Ther. 2021, 6, 145. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Lu, Q.B.; Yao, W.S.; Zhao, J.; Zhang, X.A.; Cui, N.; Yuan, C.; Yang, T.; Peng, X.F.; Lv, S.M.; et al. Clinical efficacy and safety evaluation of favipiravir in treating patients with severe fever with thrombocytopenia syndrome. EBioMedicine 2021, 72, 103591. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Jain, M.; Bahrate, S.D. Favipiravir has been investigated for teh treatment of life-threatening pathogens such as Ebola virus, Lassa virus, and now COVID-19: A Review. Asian J. Parmacy Res. 2021, 11, 39–42. [Google Scholar] [CrossRef]
- Yoon, J.J.; Toots, M.; Lee, S.; Lee, M.E.; Ludeke, B.; Luczo, J.M.; Ganti, K.; Cox, R.M.; Sticher, Z.M.; Edpuganti, V.; et al. Orally Efficacious Broad-Spectrum Ribonucleoside Analog Inhibitor of Influenza and Respiratory Syncytial Viruses. Antimicrob. Agents Chemother. 2018, 62, e00766-18. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.L.; Kania, R.S.; Brothers, M.A.; Davies, J.F.; Ferre, R.A.; Gajiwala, K.S.; He, M.; Hogan, R.J.; Kozminski, K.; Li, L.Y.; et al. Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19. J. Med. Chem. 2020, 63, 12725–12747. [Google Scholar] [CrossRef]
- Peng, Q.; Peng, R.; Yuan, B.; Wang, M.; Zhao, J.; Fu, L.; Qi, J.; Shi, Y. Structural Basis of SARS-CoV-2 Polymerase Inhibition by Favipiravir. Innovation 2021, 2, 100080. [Google Scholar] [CrossRef]
- Shannon, A.; Selisko, B.; Le, N.T.; Huchting, J.; Touret, F.; Piorkowski, G.; Fattorini, V.; Ferron, F.; Decroly, E.; Meier, C.; et al. Rapid incorporation of Favipiravir by the fast and permissive viral RNA polymerase complex results in SARS-CoV-2 lethal mutagenesis. Nat. Commun. 2020, 11, 4682. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Daifuku, R.; Loeb, L.A. Viral error catastrophe by mutagenic nucleosides. Annu. Rev. Microbiol. 2004, 58, 183–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabinger, F.; Stiller, C.; Schmitzova, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Hobartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; Kaptein, S.J.F.; Zhang, X.; Do, T.N.D.; Langendries, L.; Vangeel, L.; Breuer, J.; Pang, J.; Williams, R.; et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine 2021, 72, 103595. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Gotte, M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Rut, W.; Groborz, K.; Zhang, L.; Sun, X.; Zmudzinski, M.; Pawlik, B.; Wang, X.; Jochmans, D.; Neyts, J.; Mlynarski, W.; et al. SARS-CoV-2 M(pro) inhibitors and activity-based probes for patient-sample imaging. Nat. Chem. Biol. 2021, 17, 222–228. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.H. An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef]
- Waters, L.; Marra, F.; Pozniak, A.; Cockburn, J.; Boffito, M. Ritonavir and COVID-19: Pragmatic guidance is important. Lancet 2022, 399, 1464–1465. [Google Scholar] [CrossRef]
- Heskin, J.; Pallett, S.J.C.; Mughal, N.; Davies, G.W.; Moore, L.S.P.; Rayment, M.; Jones, R. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet 2022, 399, 21–22. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, C.; Xu, X.; Chong, T.H.; Zhang, L.; Cheung, P.P.; Huang, X. The mechanism of action of T-705 as a unique delayed chain terminator on influenza viral polymerase transcription. Biophys. Chem. 2021, 277, 106652. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.M.; Wolf, J.D.; Plemper, R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021, 6, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Driouich, J.S.; Cochin, M.; Lingas, G.; Moureau, G.; Touret, F.; Petit, P.R.; Piorkowski, G.; Barthelemy, K.; Laprie, C.; Coutard, B.; et al. Favipiravir antiviral efficacy against SARS-CoV-2 in a hamster model. Nat. Commun. 2021, 12, 1735. [Google Scholar] [CrossRef]
- Kaptein, S.J.F.; Jacobs, S.; Langendries, L.; Seldeslachts, L.; Ter Horst, S.; Liesenborghs, L.; Hens, B.; Vergote, V.; Heylen, E.; Barthelemy, K.; et al. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2-infected hamsters, whereas hydroxychloroquine lacks activity. Proc. Natl. Acad. Sci. USA 2020, 117, 26955–26965. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.E.; Noell, S.; Plotnikova, O.; Ferre, R.; Liu, W.; Bolanos, B.; Fennell, K.; Nicki, J.; Craig, T.; Zhu, Y.; et al. Structural basis for the in vitro efficacy of nirmatrelvir against SARS-CoV-2 variants. J. Biol. Chem. 2022, 298, 101972. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Lavrijsen, M.; Lamers, M.M.; de Vries, A.C.; Rottier, R.J.; Bruno, M.J.; Peppelenbosch, M.P.; Haagmans, B.L.; Pan, Q. SARS-CoV-2 Omicron variant is highly sensitive to molnupiravir, nirmatrelvir, and the combination. Cell Res. 2022, 32, 322–324. [Google Scholar] [CrossRef]
- Standard, B. COVID-19: Russia’s Avifavir Pill Shows Promise against Omicron, Delta. Available online: https://www.business-standard.com/article/current-affairs/COVID-19-russia-s-avifavir-pill-shows-promise-against-omicron-delta-121122800498_1.html (accessed on 28 December 2021).
- Japanese Pharmaceuticals and Medical Devices Agency. Avigan, Japanese Pharmaceuticals and Medical Devices Agency (PMDA) Report on the Deliberation Results. Available online: https://www.pmda.go.jp/files/000210319.pdf (accessed on 7 March 2022).
- European Medicines Agency. Molnupiravir Article 5(3) Opinions. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/referral-procedures/article-53-opinions (accessed on 19 November 2021).
- Foisy, M.M.; Yakiwchuk, E.M.; Hughes, C.A. Induction effects of ritonavir: Implications for drug interactions. Ann. Pharmacother. 2008, 42, 1048–1059. [Google Scholar] [CrossRef]
- European Medicines Agency. Paxlovid for the Treatment of COVID-19—EMEA/H/A-5(3)/1513. Available online: https://www.ema.europa.eu/en/documents/referral/paxlovid-pf-07321332-ritonavir-COVID-19-article-53-procedure-conditions-use-conditions-distribution_en.pdf (accessed on 16 December 2021).
- Koshi, E.; Saito, S.; Okazaki, M.; Toyama, Y.; Ishimoto, T.; Kosugi, T.; Hiraiwa, H.; Jingushi, N.; Yamamoto, T.; Ozaki, M.; et al. Efficacy of favipiravir for an end stage renal disease patient on maintenance hemodialysis infected with novel coronavirus disease 2019. CEN Case Rep. 2021, 10, 126–131. [Google Scholar] [CrossRef]
- Telbisz, A.; Ambrus, C.; Mozner, O.; Szabo, E.; Varady, G.; Bakos, E.; Sarkadi, B.; Ozvegy-Laczka, C. Interactions of Potential Anti-COVID-19 Compounds with Multispecific ABC and OATP Drug Transporters. Pharmaceutics 2021, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Painter, W.P.; Holman, W.; Bush, J.A.; Almazedi, F.; Malik, H.; Eraut, N.; Morin, M.J.; Szewczyk, L.J.; Painter, G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e02428-20. [Google Scholar] [CrossRef]
- Ivashchenko, A.A.; Dmitriev, K.A.; Vostokova, N.V.; Azarova, V.N.; Blinow, A.A.; Egorova, A.N.; Gordeev, I.G.; Ilin, A.P.; Karapetian, R.N.; Kravchenko, D.V.; et al. AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin. Infect. Dis. 2021, 73, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Madelain, V.; Mentre, F.; Baize, S.; Anglaret, X.; Laouenan, C.; Oestereich, L.; Nguyen, T.H.T.; Malvy, D.; Piorkowski, G.; Graw, F.; et al. Modeling Favipiravir Antiviral Efficacy Against Emerging Viruses: From Animal Studies to Clinical Trials. CPT Pharmacomet. Syst. Pharmacol. 2020, 9, 258–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendenhall, M.; Russell, A.; Smee, D.F.; Hall, J.O.; Skirpstunas, R.; Furuta, Y.; Gowen, B.B. Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic Fever. PloS Negl. Trop. Dis. 2011, 5, e1342. [Google Scholar] [CrossRef] [PubMed]
- Irie, K.; Nakagawa, A.; Fujita, H.; Tamura, R.; Eto, M.; Ikesue, H.; Muroi, N.; Tomii, K.; Hashida, T. Pharmacokinetics of Favipiravir in Critically Ill Patients With COVID-19. Clin. Transl. Sci. 2020, 13, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Sissoko, D.; Laouenan, C.; Folkesson, E.; M’Lebing, A.B.; Beavogui, A.H.; Baize, S.; Camara, A.M.; Maes, P.; Shepherd, S.; Danel, C.; et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PloS Med. 2016, 13, e1001967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishima, E.; Anzai, N.; Miyazaki, M.; Abe, T. Uric Acid Elevation by Favipiravir, an Antiviral Drug. Tohoku J. Exp. Med. 2020, 251, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. Paxlovid Fact Sheet For Healthcare Providers. Available online: https://www.covid19oralrx-hcp.com/?source=google&HBX_PK=s_paxlovid+healthcare&skwid=43700068281648831&gclid=Cj0KCQjw-JyUBhCuARIsANUqQ_LsoVyWK8Yyq8Ox7nmhOZV6BhZafwaQJkhAuN92ClzxO1Je9mAEz8IaAr8yEALw_wcB&gclsrc=aw.ds (accessed on 22 December 2021).
- Marzolini, C.; Kuritzkes, D.R.; Marra, F.; Boyle, A.; Gibbons, S.; Flexner, C.; Pozniak, A.; Boffito, M.; Waters, L.; Burger, D.; et al. Prescribing Nirmatrelvir-Ritonavir: How to Recognize and Manage Drug-Drug Interactions. Ann. Intern. Med. 2022, 175, 744–746. [Google Scholar] [CrossRef]
- Marzolini, C.; Kuritzkes, D.R.; Marra, F.; Boyle, A.; Gibbons, S.; Flexner, C.; Pozniak, A.; Boffito, M.; Waters, L.; Burger, D.; et al. Recommendations for the Management of Drug-Drug Interactions Between the COVID-19 Antiviral Nirmatrelvir/Ritonavir (Paxlovid) and Comedications. Clin. Pharmacol. Ther. 2022. [Google Scholar] [CrossRef]
- Outpatient Guidance for Treatment of COVID-19 in Adults and Children. Available online: https://www.med.umich.edu/asp/pdf/outpatient_guidelines/COVID-19-amb-treatment.pdf (accessed on 14 June 2022).
- COVID-19 Drug Interactions. Available online: https://www.covid19-druginteractions.org/ (accessed on 14 June 2022).
- Drug-Drug Interactions between Ritonavir-Boosted Nirmatrelvir (Paxlovid) and Concomitant Medications. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir—paxlovid-/paxlovid-drug-drug-interactions/ (accessed on 14 June 2022).
- Jothimani, D.; Venugopal, R.; Abedin, M.F.; Kaliamoorthy, I.; Rela, M. COVID-19 and the liver. J. Hepatol. 2020, 73, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Abbott Inc. Norvir Prescribing Information. 2012. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020945s034lbl.pdf (accessed on 7 March 2022).
- Kaur, R.J.; Charan, J.; Dutta, S.; Sharma, P.; Bhardwaj, P.; Sharma, P.; Lugova, H.; Krishnapillai, A.; Islam, S.; Haque, M.; et al. Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect. Drug Resist. 2020, 13, 4427–4438. [Google Scholar] [CrossRef]
- Pilkington, V.; Pepperrell, T.; Hill, A. A review of the safety of favipiravir—A potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020, 6, 45–51. [Google Scholar] [CrossRef]
- Catlin, N.R.; Bowman, C.J.; Campion, S.N.; Cheung, J.R.; Nowland, W.S.; Sathish, J.G.; Stethem, C.M.; Updyke, L.; Cappon, G.D. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 M(pro) inhibitor in animal models. Reprod. Toxicol. 2022, 108, 56–61. [Google Scholar] [CrossRef]
- NORVIR Product Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209512lbl.pdf (accessed on 13 June 2022).
- Ruzhentsova, T.A.; Oseshnyuk, R.A.; Soluyanova, T.N.; Dmitrikova, E.P.; Mustafaev, D.M.; Pokrovskiy, K.A.; Markova, T.N.; Rusanova, M.G.; Kostina, N.E.; Agafina, A.S.; et al. Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19. Am. J. Transl. Res. 2021, 13, 12575–12587. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simon-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Pfizer Inc. General Investigation for PAXLOVID PACK NCT05263908. Available online: https://clinicaltrials.gov/ct2/show/NCT05263908 (accessed on 23 April 2022).
- Ivashchenko, A.A.; Azarova, V.N.; Egorova, A.N.; Karapetian, R.N.; Kravchenko, D.V.; Krivonos, N.V.; Loginov, V.G.; Poyarkov, S.V.; Merkulova, E.A.; Rosinkova, O.S.; et al. Effect of Aprotinin and Avifavir((R)) Combination Therapy for Moderate COVID-19 Patients. Viruses 2021, 13, 1253. [Google Scholar] [CrossRef]
- Gunaratne, S.H.; Tieu, H.V.; Wilkin, T.J.; Taylor, B.S. CROI 2021: Advances in Antiretroviral Therapy for HIV and Antiviral Therapy for COVID-19. Top. Antivir. Med. 2021, 29, 361–378. [Google Scholar] [PubMed]
- Inoue, H.; Jinno, M.; Ohta, S.; Kishino, Y.; Kawahara, T.; Mikuni, H.; Sato, H.; Yamamoto, M.; Sato, Y.; Onitsuka, C.; et al. Combination treatment of short-course systemic corticosteroid and favipiravir in a successfully treated case of critically ill COVID-19 pneumonia with COPD. Respir. Med. Case Rep. 2020, 31, 101200. [Google Scholar] [CrossRef]
- Murohashi, K.; Hagiwara, E.; Kitayama, T.; Yamaya, T.; Higa, K.; Sato, Y.; Otoshi, R.; Shintani, R.; Okabayashi, H.; Ikeda, S.; et al. Outcome of early-stage combination treatment with favipiravir and methylprednisolone for severe COVID-19 pneumonia: A report of 11 cases. Respir. Investig. 2020, 58, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Kondoh, Y.; Kada, A.; Doi, Y.; Tomii, K.; Mukae, H.; Murata, N.; Imai, R.; Okamoto, M.; Yamano, Y.; et al. Phase II Clinical Trial of Combination Therapy with Favipiravir and Methylprednisolone for COVID-19 with Non-Critical Respiratory Failure. Infect. Dis. Ther. 2021, 10, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, Q.; Zhang, C.; Li, J.; Wei, M.; Qin, Y.; Chen, G.; Wang, K.; Yu, J.; Wu, Z.; et al. Tocilizumab combined with favipiravir in the treatment of COVID-19: A multicenter trial in a small sample size. Biomed. Pharmacother. 2021, 133, 110825. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Hibino, M.; Hase, R.; Yamamoto, M.; Kasamatsu, Y.; Hirose, M.; Mutoh, Y.; Homma, Y.; Terada, M.; Ogawa, T.; et al. A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19. Antimicrob. Agents Chemother. 2020, 64, e01897-20. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine biosynthesis inhibitors synergize with nucleoside analogs to block SARS-CoV-2 infection. bioRxiv 2021. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 2022, 604, 134–140. [Google Scholar] [CrossRef]
- Padhi, A.K.; Shukla, R.; Saudagar, P.; Tripathi, T. High-throughput rational design of the remdesivir binding site in the RdRp of SARS-CoV-2: Implications for potential resistance. iScience 2021, 24, 101992. [Google Scholar] [CrossRef]
- Baranovich, T.; Wong, S.S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef] [Green Version]
- Perales, C.; Domingo, E. Antiviral Strategies Based on Lethal Mutagenesis and Error Threshold. Curr. Top. Microbiol. Immunol. 2016, 392, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Pachetti, M.; Marini, B.; Benedetti, F.; Giudici, F.; Mauro, E.; Storici, P.; Masciovecchio, C.; Angeletti, S.; Ciccozzi, M.; Gallo, R.C.; et al. Emerging SARS-CoV-2 mutation hot spots include a novel RNA-dependent-RNA polymerase variant. J. Transl. Med. 2020, 18, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullrich, S.; Ekanayake, K.B.; Otting, G.; Nitsche, C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg. Med. Chem. Lett. 2022, 62, 128629. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hill, C.S.; Sarkar, S.; Tse, L.V.; Woodburn, B.M.D.; Schinazi, R.F.; Sheahan, T.P.; Baric, R.S.; Heise, M.T.; Swanstrom, R. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 through Lethal Mutagenesis but Is Also Mutagenic to Mammalian Cells. J. Infect. Dis. 2021, 224, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Swanstrom, R.; Schinazi, R.F. Lethal mutagenesis as an antiviral strategy. Science 2022, 375, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H., 3rd; Stevens, L.J.; et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [Green Version]
- Kiso, M.; Takahashi, K.; Sakai-Tagawa, Y.; Shinya, K.; Sakabe, S.; Le, Q.M.; Ozawa, M.; Furuta, Y.; Kawaoka, Y. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc. Natl. Acad. Sci. USA 2010, 107, 882–887. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Du, Y.X.; Chen, X.P. Favipiravir: Pharmacokinetics and Concerns About Clinical Trials for 2019-nCoV Infection. Clin. Pharmacol. Ther. 2020, 108, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Ganter, B.; Meier, C. Improving properties of the nucleobase analogs T-705/T-1105 as potential antiviral. Annu. Rep. Med. Chem. 2021, 57, 1–47. [Google Scholar] [CrossRef]
- Cully, M. A tale of two antiviral targets—And the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2022, 21, 3–5. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).