COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis
Abstract
:1. Introduction
1.1. Rationale
1.1.1. The Importance of the Issue
1.1.2. How Will the Study Address the Issue?
- This body of research will help us comprehend the increased incidence of COVID-19 and related mortality among COVID-19 patients who have pre-existing or newly acquired cardiovascular diseases.
- This study intends to provide more knowledge on the patients hospitalised with COVID-19 and acute myocardial infarction using pooled Hazard Ratio (HR) and 95% Confidence Interval (CI).
- Regardless of aetiology, it becomes pivotal for the clinicians to understand the standard protocol and other clinical interventions for the treating COVID-19 patients with cardiac complications. Therefore, our systematic review and meta-analysis could provide a better insight into survival outcomes of COVID-19 patients hospitalised with AMI.
- The combined information and data from different studies to be used in our analysis may provide a complete picture of how COVID-19 patients’ prognosis connects with cardio-vascular comorbidities, particularly AMI. This will aid scientists, healthcare workers, and other concerned professionals to acquire a deeper understanding on this subject.
2. Materials and Methods
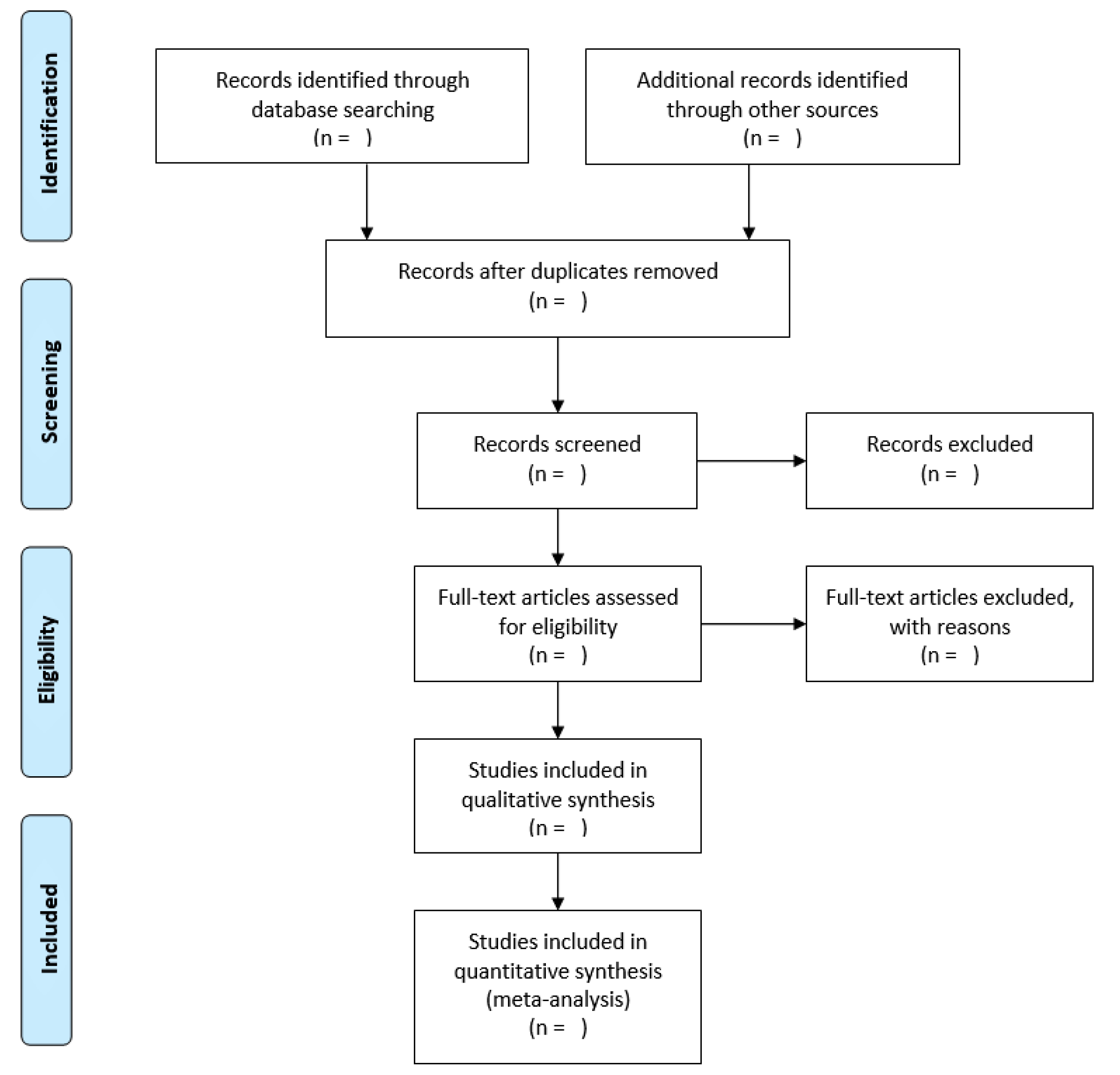
2.1. Search Methods
2.2. Search Terms
2.3. Study Selection
2.3.1. Inclusion Criteria
- (1)
- Studies reporting patients with COVID-19 and other cardiac complications.
- (2)
- Studies reporting cardiovascular comorbidities.
- (3)
- Outcomes of patients with STEMI.
- (4)
- Clinical data of patients with COVID-19 and AMI.
- (5)
- Studies that complied with the PRISMA guidelines for systematic review and meta-analysis.
2.3.2. Exclusion Criteria
- (1)
- Studies published in languages other than English.
- (2)
- Letter to the editor, case studies, review articles, fact sheets and non-human studies.
- (3)
- Unpublished studies, uninterpretable data, conference proceedings or thesis.
- (4)
- Studies using patient’s Information from datasets.
- (5)
- Duplicates will be removed, and the study will be excluded if it falls within the exclusion criteria.
2.4. Data Collection and Management
- (1)
- Authors details
- (2)
- Year of publication
- (3)
- Study location
- (4)
- Patients’ details (Age, Gender, Ethnicity)
- (5)
- Cardiovascular risk profile (Diabetes, Smoking history, Hypertension, Percutaneous coronary intervention (PCI), Dyslipidemia, Coronary heart disease (CAD), Chronic obstructive pulmonary disease (COPD))
- (6)
- In the case of MI, it will be categorised into ST-elevation MI (STEMI) or Non-STEMI
- (7)
- Troponin level
- (8)
- Left ventricle function
- (9)
- Cardiogenic shock status
- (10)
- Mortality
2.5. Publication Bias
2.6. Heterogeneity Assessment
2.7. Reporting of the Review and Ethics
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Hu, X.; Song, J.; Du, C.; Xu, J.; Yang, D.; Chen, D.; Zhong, M.; Jiang, J.; Xiong, W. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19). MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; He, W.-T.; Wang, L.; Lai, A.; Ji, X.; Zhai, X.; Li, G.; Suchard, M.A.; Tian, J.; Zhou, J. COVID-19: Epidemiology, evolution, and cross-disciplinary perspectives. Trends Mol. Med. 2020, 26, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L. COVID-19 and cardiovascular disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wu, Q.; Zhang, Z. Pangolin homology associated with 2019-nCoV. BioRxiv 2020. [Google Scholar]
- Booth, C.M.; Matukas, L.M.; Tomlinson, G.A.; Rachlis, A.R.; Rose, D.B.; Dwosh, H.A.; Walmsley, S.L.; Mazzulli, T.; Avendano, M.; Derkach, P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawi, A.; Ryoo, S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016, 49, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ma, Y.; Zhang, J.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, K.; Hirsch, M.; Bloom, A. Coronavirus disease 2019 (COVID-19): Epidemiology, virology, and prevention. Lancet. Infect. Dis 2020, 1, 2019–2020. [Google Scholar]
- Xu, X.-W.; Wu, X.-X.; Jiang, X.-G.; Xu, K.-J.; Ying, L.-J.; Ma, C.-L.; Li, S.-B.; Wang, H.-Y.; Zhang, S.; Gao, H.-N.; et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: Retrospective case series. BMJ 2020, 368, m606. [Google Scholar] [CrossRef] [Green Version]
- Belani, P.; Schefflein, J.; Kihira, S.; Rigney, B.; Delman, B.; Mahmoudi, K.; Mocco, J.; Majidi, S.; Yeckley, J.; Aggarwal, A.; et al. COVID-19 is an independent risk factor for acute ischemic stroke. Am. J. Neuroradiol. 2020, 41, 1361–1364. [Google Scholar] [CrossRef]
- Nishiga, M.; Wang, D.W.; Han, Y.; Lewis, D.B.; Wu, J.C. COVID-19 and cardiovascular disease: From basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020, 17, 543–558. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Lindmark, K.; Connolly, A.-M.F. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet 2021, 398, 599–607. [Google Scholar] [CrossRef]
- Lai, C.-C.; Liu, Y.H.; Wang, C.-Y.; Wang, Y.-H.; Hsueh, S.-C.; Yen, M.-Y.; Ko, W.-C.; Hsueh, P.-R. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J. Microbiol. Immunol. Infect. 2020, 53, 404–412. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [Green Version]
- Momtazmanesh, S.; Shobeiri, P.; Hanaei, S.; Mahmoud-Elsayed, H.; Dalvi, B.; Rad, E.M. Cardiovascular disease in COVID-19: A systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt. Heart J. 2020, 72, 41. [Google Scholar] [CrossRef]
- Libby, P. The heart in COVID-19: Primary target or secondary bystander? Basic Transl. Sci. 2020, 5, 537–542. [Google Scholar]
- Tan, W.; Aboulhosn, J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int. J. Cardiol. 2020, 309, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Orwin, R.G. A fail-safe N for effect size in meta-analysis. J. Educ. Stat. 1983, 8, 157–159. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Cochran, W.G. The combination of estimates from different experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020; Journal Pre-proof. [Google Scholar]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Gonzalez, S.; Vasilakos, A.; Cao, H.; Leung, V.C.M. Body area networks: A survey. Mob. Netw. Appl. 2011, 16, 171–193. [Google Scholar] [CrossRef]
- Hadjem, M.; Salem, O.; Naït-Abdesselam, F. An ECG monitoring system for prediction of cardiac anomalies using WBAN. In Proceedings of the 2014 IEEE 16th International Conference on e-Health Networking, Applications and Services (Healthcom), Natal, Brazil, 15–18 October 2014; pp. 441–446. [Google Scholar]
- Hadjem, M.; Salem, O.; Abdesselam, F.N.; Mehaoua, A. Early detection of Myocardial Infarction using WBAN. In Proceedings of the 2013 IEEE 15th International Conference on e-Health Networking, Applications and Services (Healthcom 2013), Lisbon, Portugal, 9–12 October 2013; pp. 135–139. [Google Scholar]
- Sugano, H.; Hara, S.; Tsujioka, T.; Nakajima, S.; Inoue, T.; Takeuchi, K.; Nakamura, H. Continuous ECG data gathering by a wireless vital sensor—Evaluation of its sensing and transmission capabilities. In Proceedings of the 2010 IEEE 11th International Symposium on Spread Spectrum Techniques and Applications, Taichung, Taiwan, 17–20 October 2010; pp. 98–102. [Google Scholar]
- Delano, M.K.; Sodini, C.G. A long-term wearable electrocardiogram measurement system. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–6. [Google Scholar]
- Gravina, R.; Fortino, G. Automatic methods for the detection of accelerative cardiac defense response. IEEE Trans. Affect. Comput. 2016, 7, 286–298. [Google Scholar] [CrossRef]
- Khan, R.A.; Pathan, A.-S.K. The state-of-the-art wireless body area sensor networks: A survey. Int. J. Distrib. Sens. Netw. 2018, 14, 1550147718768994. [Google Scholar] [CrossRef] [Green Version]
- Hussain, I.; Park, S.J. Big-Ecg: Cardiographic Predictive Cyber-Physical System for Stroke Management. IEEE Access 2021, 9, 123146–123164. [Google Scholar] [CrossRef]
- Hussain, I.; Park, S.J. HealthSOS: Real-Time Health Monitoring System for Stroke Prognostics. IEEE Access 2020, 8, 213574–213586. [Google Scholar] [CrossRef]
| S No. | Search Terms |
|---|---|
| 1. | “Acute myocardial infarction” AND “2019-nCov” OR “SARS-CoV-2” |
| 2. | “Acute myocardial infarctio” OR “AMI” AND “COVID-19” |
| 3. | “Severe acute respiratory syndrome coronavirus 2” OR “COVID-19” AND “Cardiovascular disease” |
| 4. | “2019-nCov” OR “SARS-CoV-2” AND “Cardiovascular disease” OR “CVD” |
| 5. | “COVID-19” OR “SARS-CoV-2” AND “Heart” OR “CVD” |
| 6. | “COVID-19” AND “Cardiovascular risk factors” |
| 7. | “2019-nCoV” OR “COVID-19” AND “STEMI” |
| 8. | “2019-nCoV” OR “COVID-19” AND “Cardiac outcome” |
| 9. | “2019-nCoV” OR “COVID-19” AND “Coronary artery disease” |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaw, P.; Senguttuvan, N.B.; Raymond, G.; Sankar, S.; Mukherjee, A.G.; Kunale, M.; Kodiveri Muthukaliannan, G.; Baxi, S.; Mani, R.R.; Rajagopal, M.; et al. COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis. COVID 2022, 2, 138-147. https://doi.org/10.3390/covid2020010
Shaw P, Senguttuvan NB, Raymond G, Sankar S, Mukherjee AG, Kunale M, Kodiveri Muthukaliannan G, Baxi S, Mani RR, Rajagopal M, et al. COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis. COVID. 2022; 2(2):138-147. https://doi.org/10.3390/covid2020010
Chicago/Turabian StyleShaw, Peter, Nagendra Boopathy Senguttuvan, Greg Raymond, Srivarshini Sankar, Anirban Goutam Mukherjee, Milind Kunale, Gothandam Kodiveri Muthukaliannan, Siddhartha Baxi, Ravishankar Ram Mani, Mogana Rajagopal, and et al. 2022. "COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis" COVID 2, no. 2: 138-147. https://doi.org/10.3390/covid2020010
APA StyleShaw, P., Senguttuvan, N. B., Raymond, G., Sankar, S., Mukherjee, A. G., Kunale, M., Kodiveri Muthukaliannan, G., Baxi, S., Mani, R. R., Rajagopal, M., Samiappan, S., Krishnan, S., & Jayaraj, R. (2022). COVID-19 Outcomes in Patients Hospitalised with Acute Myocardial Infarction (AMI): A Protocol for Systematic Review and Meta-Analysis. COVID, 2(2), 138-147. https://doi.org/10.3390/covid2020010











