Abstract
The spread of the new coronavirus SARS-CoV-2 has substantial social, health, and economic impacts. High viral load in the air in hospitals poses a risk to medical personnel. Cold atmospheric plasma (CAP) is a new technology based on the emission in the air of reactive species, neutral particles, UV radiation, and electromagnetic field. CAP has the potential as an antiviral agent. In this study, an 80-day clinical trial took place at Nicosia General Hospital to evaluate the application of CAP devices for lowering the viral load in the COVID rooms. A total of 284 indoor environment samples were tested by RT-PCR, for which 9 were positive (~3% Positive Rate). After analyzing the initial results, an ion emitter was paired with each patient, and the results showed that the method could eliminate the virus from the COVID wards up to 100%. The number of patients discharged from the hospital in the ionizer group was 4.8% higher than in the non-ionizer group, and 45% fewer patients in the ionizer group who remained in the rooms required oxygen support. The clinical trial shows evidence that composite CAP can decrease coronavirus spread in hospital environments and potentially prevent virus transmission.
1. Introduction
On 30 January 2020, the World Health Organization (WHO) declared a public health emergency of international concern over the global outbreak of SARS-CoV-2 and the disease it causes, COVID-19 [1]. Numerous in-hospital outbreaks of COVID-19 have been associated with aerosol-generating medical procedures. Clinicians in COVID-19 wards have expressed concerns about aerosol-generating procedures during the treatment of patients that could expose healthcare workers to the risk of nosocomial transmission [2,3,4].
What makes SARS-CoV-2 stand out is its unique feature of spreading, even from asymptomatic patients, through aerosol, in addition to direct contact and cough droplets. The actual size of SARS-CoV-2 is about 120 nm in diameter (CDC, Atlanta, GA, USA, 2020) but is usually emitted as a liquid particle whose half-life in the air is estimated to be 2.7 h with upper and lower limits equal to 1.65 and 7.24 h, respectively [5,6]. Liu et al. investigated the aerodynamic nature of SARS-CoV-2 in different areas of two Wuhan hospitals and concluded that SARS-CoV-2 may have the potential to be transmitted through aerosols in rooms without ventilation [7]. All species of known coronaviruses have common characteristics: they have a negatively charged envelope double layer, in which the structural proteins of the membrane (M), envelope (E), and spike (S) are incorporated. Viral proteins can form the same ion channels (viroporins) or can regulate the ion channel function of the host cell. The coronavirus envelope consists mainly of M protein, making it unique among the enveloped viruses [8,9,10,11,12].
In recent years, cold atmospheric plasma (CAP), a new powerful physical-chemical technology to produce ionized gas with temperatures relatively close to room temperature, has been tested in vitro and showed proven antimicrobial, antitumor, and even antiviral properties, but the underlying mechanism is rarely studied [13,14,15,16]. In physics, plasma is the fourth state of matter, where part of the gas contains atoms, radicals, ions, and molecules in the ground and excited states [17]. The antiviral and antimicrobial capacity of CAP has been recently investigated showing a promising result [18,19,20]. Depending on its application, CAP can be classified as direct or indirect plasma. Direct plasma employs the direct application of the CAP device to surfaces or tissues [18,19]. Indirect plasma, or plasma-activated liquid, involves treating a liquid proceeding with CAP, and the liquid is then applied to surfaces or tissues [20,21,22,23].
Joshi et al. demonstrated that normal atmospheric non-thermal plasma treatments were able to effectively sterilize surfaces in less than 120 s when applied to glass surfaces that carried heavy bacteria (Escherichia coli, S. aureus) and methicillin-resistant S. aureus (MRSA) loads [24]. Klaempfl et al. observed damage to spore proteins, especially the inner membrane by reactive oxygen species and suggested that it may be the main mechanism of cold plasma inactivation [25]. Jin et al., in their study, demonstrated a self-made dielectric barrier discharge (DBD) plasma device’s efficiency to treat a SARS-CoV-2 pseudo virus on a stainless-steel disk [26]. The bactericidal activity of cold plasma obtained from air or gases was mainly attributed to combinations of physical (UV and shock waves), chemical (H2O2 and reactive species), and electrical effects [27,28].
Most sources producing CAP use air or gas ionization. Meanwhile, many metallic and halogen ions play a critical role in the biochemical processes in living organisms. Te Velthuis et al. discovered that the combination of Zn2+ and PT at low concentrations inhibits the replication of SARS-coronavirus (SARS-CoV) and equine arteritis virus (EAV) in cell culture by blocking RNA-synthesizing activity [29,30,31]. The aim of conducting the study in Nicosia General Hospital was to limit the number of COVID-19 super-spread events within the hospital. This study is using an innovative device that emits a specific group of ions aimed to reduce the spreading of SARS-CoV-2 particles in the air.
The scope of the clinical trials was to evaluate the effectiveness of Ionic Shield by Perenio in COVID-19 wards related to the prevention of the illness spreading.
2. Materials and Methods
All experiments at the General Hospital of Nicosia were performed after receiving a Bioethics Approval from the National Bioethics Committee of Cyprus (ΕΕΒΚ ΕΠ 2021.01.183; Bioethics Date: 7 September 2021). Study name: “Intensive Care Unit Research to Unravel SARS-CoV-2 dispersion ability”.
Inclusion criteria: Patients were eligible to participate in the study if:
- a.
- They have laboratory-confirmed cases of SARS-CoV-2 infection;
- b.
- They were admitted to the COVID ward according to Nicosia General Hospital’s protocol for hospitalization of COVID-19 patients;
- c.
- They received the standard care of treatment including remdensivir, dexamethasone, enoxaparin, and oxygen supplementation.
Patients were admitted to the wards randomly. The patients were either intubated and sent to the ICU or discharged home or to other rehabilitation wards/centers (Table 1).

Table 1.
Room details.
2.1. Air Collection Methodology
Air quality analysis for the presence of SARS-CoV-2 was performed by air collection devices placed in the corridor of the COVID ward and in the rooms near patients. The Ionic Shields by Perenio were placed inside the rooms and were operated 24/7 (Figure 1).

Figure 1.
Installation into COVID-19 wards Ionic Shield by Perenio.
Three types of air sampling devices were used: (a) Pump sampler with filter, (b) Dry sampler, and (c) Air to Liquid samplers. Gelatine filters were placed inside the air samplers. Daily samples from air samplers (Figure 2A,B) from wards and corridors as well as surface sampling and passive sampling were collected (Figure 2C,D). Each sample was tested with RT-PCR for SARS-CoV-2. Different details for the rooms were noted (Table 1) and updated daily.

Figure 2.
Air samplers (A,B) and surface samplers (C,D).
2.2. RT-PCR SARS-CoV-2 Testing
To identify the spread of SARS-CoV-2, the combined techniques of air filtration (Figure 2) and the RT-PCR method were used. Daily samples from rooms and corridors were analyzed individually by RT-PCR using the VIASURE SARS-CoV-2 Real-Time PCR Detection Kit (Certest Biotec S.L., Zaragoza, Spain) in Medifos Center of Laboratory Medicine and Molecular Diagnosis, Nicosia, Cyprus. The Rotor-Gene Q MDx real-time PCR cycler (Qiagen, Hilden, Germany) with 6 channels was used for the experiments.
2.3. Device Characterization Electrical Characteristics
The cold plasma emitter Perenio Ionic Shield™ (Joule Production, SIA, Riga, Latvia) (Figure 3A) is comprised of: A replaceable capsule with electrodes connected to two trays with stable ionic solution preserved in pores of a polymer intact to the ionic solution. Briefly, a cold dielectric barrier discharge (DBD) plasma source was constructed using two parallel hemisphere plastic trays, with the ionic solution serving as electrodes connected to the source of 14 kV and frequency 16 kHz (Figure 3B). Ionic substances selected to combat known coronaviruses including SARS-CoV-2 (CoV type of capsule) composed of liquid ionic solution of salts of AuCl3, AgCl, AgNO3, PtCl4, KCl, MgCl2, ZnCl2, and NaCl.
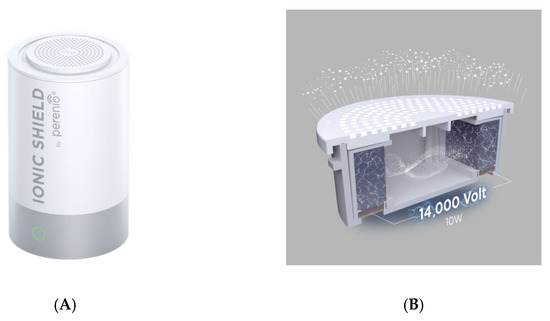
Figure 3.
(A) Cold plasma emitter PERENIO IONIC SHIELD™ (Joule Production, SIA, Riga, Latvia); (B) replaceable capsule with electrodes connected to two trays with a stable ionic solution preserved in pores of a polymer intact for the ionic solution.
The actuator was made from hybrid polymer electrodes. Inside the container with capsules, a bipolar rectangular-waveform voltage with a frequency of 16 kHz was generated to one electrode while the other electrode was grounded. Plasma was generated inside the container with capsules with an input power of 10 W. The device is not generating ozone.
The composite ions are emitted to the air through round pores in the capsule lid. The concentration of negative ions is 36 ± 3 × 103 ions/cm3. Inactivation of SARS-CoV-2 is caused by plasma-generated reactive ions inducing virus leakage and functionality loss [32].
3. Results
Indoor Monitoring of COVID-19 Rooms
Indoor environmental monitoring of COVID-19 was performed in Nicosia hospital with ion emitter and without ion emitter, through sample collection and testing by combined air filtration and RT-PCR techniques. Air samples were collected daily during the 80-day trial. A total of 284 samples were tested by RT-PCR. The results showed that 9 were positive (~3% Positive Rate) samples. The 4 positive tests 1.7% (4/238) observed during the first 2 weeks were samples from group wards (4 patients), where the cold plasma emitter was activated. During this period, only one device was operating per chamber, and the results showed a low virus concentration of Ct > 30 (Figure 4).
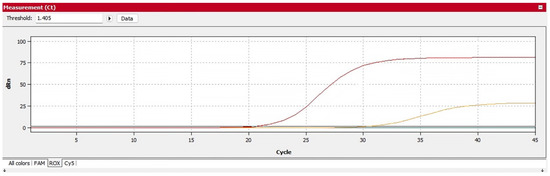
Figure 4.
RT-PCR result with positive curve ORF gene at 33.20 Ct values and N gene at 33.41 Ct values (red curve is positive control; yellow sample from the room without Perenio Ionic Shield).
The size of the rooms was 35 m2 and, according to the manufacturer’s recommendations, the Ionic Shield can ionize rooms up to 20 m2. At the same time, through the sampling, it was seen that the distance of the device from the infected person is primarily an efficiency factor, as the positive samples were found at a far distance from the device. Thus, for the continuation of the tests, one Ion Shield was placed at approximately 1 m from each patient in the large rooms where up to 4 patients can be treated. Afterward and while positive patients entered the rooms, no positive sample was detected in the following days. To avoid inconsistent results, data from week 3 onwards and a single sampling method were selected for analysis. In the following weeks, in the rooms with SARS-CoV-2, positive patients and an active ion emitter, no positive samples were detected. In COVID wards, without ion emitters, 10.9% (5/46) positive samples were detected. Our study showed that Perenio Ionic Shield can eliminate the virus from COVID wards up to 100%. The rooms with the Ionic Shield device had 83 patients hospitalized: 65 (78.3%) of them returned home safely, and three (3) of the remaining eighteen patients still needed oxygen support (16.6%) (Table 2). The percentage of patients discharged from the hospital in the ionizer group was 4.8% higher than in the group without ionizers; in addition, in the ionizer group, 45% fewer patients needed oxygen support.

Table 2.
Clinical observation details.
No side effect was observed through the use of the Ionic Shield from patients and medical personnel reported that they feel safer with the devices operating on the champers.
4. Discussion
The pandemic of COVID-19 has extended to the second year and showed that new effective methods of prevention of spreading the virus are needed to protect people and medical staff from spreading. Different technologies are commonly used for air cleanings, such as air filters or UV lamps, but they have safety limitations due to their harmful impact on the human body. The CAP technology is a promising scientifically proven safe alternative to chemical disinfectants and UV.
In terms of possible mechanisms involved in the observed inactivation, SARS-CoV-2 is a single-stranded RNA-positive virus that resembles other coronaviruses in that it responds to CAP therapies. The charged particles that accumulate on the surface of the virus destroy the cell membranes through electrostatics disruptions. The electrostatic forces from such an accumulation can exceed the tensile strength of the membrane, leading to its rupture. Plasma active species can cause oxidation of amino acids, nucleic acids, and unsaturated fatty acid peroxides by interacting with membrane lipids, leading to changes in membrane function. The CAP for inactivation of SARS-CoV-2 should be induced by reactive plasma-generated species that cause virus leakage and loss of function. Levels of these active species can be adjusted by plasma source design, ionic feed types, operating conditions, nature of the product/substrate, and the microorganism itself. Previous studies have identified the breaking of structurally important bonds, such as C-C, C-O, and C-N [32]. Recent studies showed that cold atmospheric plasma treatment can cause the deactivation of proteins, complete inactivation of SARS-CoV-2 spike protein binding to ACE2 protein, and the RNA deactivation was observed in vitro in the presence of the He–air mixed feed gas plasma [33].
The ability to control and modify the composition and physical-chemical properties of cold plasma produced by CAP sources opens a new prospect for its use as a specifically targeted antibacterial, antiviral, and antifungal agent.
Plasma air treatment with Perenio Ionic Shield has been shown to inactivate aerosol-transmitted SARS-CoV-2 rapidly and effectively in rooms for coronavirus and therefore has great potential as a safe and effective means of preventing the transmission of virus and infections.
In our study, we found that composite ion plasma disinfection was effective in reducing viral contamination in big COVID wards. The high treatment effectiveness was observed also after increasing the amount of ion diffusion per room for big COVID wards and showed total elimination of SARS-CoV-2 in the air.
Composite ions’ cold plasma disinfection is a better alternative compared to the current chemical-based decontamination and can be utilized for different purposes like surfaces and in air purification and could be safe and an energy-efficient and dry method for SARS-CoV-2 virus inactivation. Our results confirmed that this cold plasma technology does not replace the traditional disinfection protocol, but it can improve it in big rooms with limited devices installed.
The pioneer method of the emission of specific groups of ions used in the Perenio Ionic Shield device in combination with bipolar ionization showed promising effects not only for inactivation of the virus but for a higher percentage of recovery from COVID-19.
Composite ions’ cold plasma disinfection is a safe and energy-efficient and dry method for SARS-CoV-2 virus inactivation which can be used together with the traditional disinfection protocol. In this regard, our proposal is to use this cold plasma air disinfection approach of implementing anti-COVID-19 measurements in hospital rooms.
5. Conclusions
The 80-day trial, which began in September 2021, is the first clear demonstration of effective inactivation of bioaerosol containing SARS-CoV-2 in hospital environments by cold plasma devices emitting composite ions. The effectiveness of inactivation depends on the distance of the device from the infected person. Our study showed that the Perenio ionic shield can eliminate the virus from COVID wards up to 100%. Overall, the effectiveness of cold plasma in inactivating SARS-CoV-2 on a variety of surfaces with a wide range of composition, roughness, and absorbency without damaging surfaces is encouraging and demonstrates the promising application of cold plasma for virus inactivation on surfaces. The results also showed that the number of patients discharged from the hospital in the ionizer group was 4.8% higher than in the non-ionizer group, and 45% fewer patients in the ionizer group who remained in the rooms required oxygen support. These results suggest that cold plasma should be investigated for the rehabilitation effect. Since cold plasma is significantly safer than most other treatment methods such as alcohol, UV radiation, and the like, this work opens a wide range of opportunities for the scientific and medical communities. Nevertheless, Nicosia General Hospital COVID wards are currently operating safer than at any stage since the start of the pandemic. Monitoring and installation of the device are extremely beneficial for the medical personnel as well.
Author Contributions
Conceptualization, C.L., V.K., A.K., S.K.; methodology, C.L., V.K.; validation, T.A., C.L.; formal analysis S.K., A.K.; investigation, T.A.; resources, C.C., L.H. data curation, T.A., C.L., V.K.; writing—original draft preparation, V.K., C.L., T.A.; writing—review and editing, C.C., E.R., L.H.; supervision, C.L.; project administration, V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All experiments at the General Hospital of Nicosia were performed after receiving a Bioethics Approval from the National Bioethics Committee of Cyprus (ΕΕΒΚ ΕΠ 2021.01.183; Bioethics Date: 7/9/2021). Study name: “Intensive Care Unit Research to Unravel SARS-CoV-2 dispersion ability”.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank all doctors of Nicosia General Hospital for their hard and valuable work aimed at resisting the pandemic of COVID-19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Available online: https://www.who.int/publications/m/item/covid-19-public-health-emergency-of-international-concern-(pheic)-global-research-and-innovation-forum (accessed on 3 April 2022).
- European Centre for Disease Prevention and Control an Agency of the European Union. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/transmission (accessed on 3 April 2021).
- Venugopal, U.; Jilani, N.; Rabah, S.; Shariff, M.A.; Jawed, M.; Mendez Batres, A.; Abubacker, M.; Menon, S.; Pillai, A.; Shabarek, N.; et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int. J. Infect. Dis. 2021, 102, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.W.; Jayaraj, V.J.; Ng, C.W.; Sam, I.C.; Said, M.A.; Ahmad Zaki, R.; Hairi, N.N.; Nik Farid, N.D.; Hoe, V.C.; Isahak, M.; et al. Propagation of a hospital-associated cluster of COVID-19 in Malaysia. BMC Infect Dis. 2021, 21, 1238. [Google Scholar] [CrossRef] [PubMed]
- U.S. CDC: Coronaviruses. Updated February 15. Available online: https://www.cdc.gov/coronavirus/types.html (accessed on 1 December 2021).
- Amato-Lourenço, L.F.; de Souza Xavier Costa, N.; Dantas, K.C.; Lombardi, S.C.F.S.; Júnior, A.M.; Lindoso, J.A.L.; Mauad, T. Quantification of airborne SARS-CoV-2 genomic particles in different hospital settings. Sci. Rep. 2021, 11, 21284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Lan, K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- McClenaghan, C.; Hanson, A.; Lee, S.J.; Nichols, C.G. Coronavirus Proteins as Ion Channels: Current and Potential Research. Front. Immunol. 2020, 11, 573339. [Google Scholar] [CrossRef]
- Breitinger, U.; Farag, N.S.; Sticht, H.; Breitinger, H.G. Viroporins: Structure, function, and their role in the life cycle of SARS-CoV-2. Int. J. Biochem. Cell Biol. 2022, 145, 106185. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, R.; Lee, I.; Zhang, W.; Sun, J.; Wang, W.; Meng, X. Characterization of the SARS-CoV-2 E Protein: Sequence, Structure, Viroporin, and Inhibitors. Protein Sci. 2021, 30, 1114–1130. [Google Scholar] [CrossRef]
- Hassan, S.S.; Choudhury, P.P.; Dayhoff, G.W., II; Aljabali, A.A.; Uhal, B.D.; Lundstrom, K.; Rezaei, N.; Pizzol, D.; Adadi, P.; Lal, A.; et al. The importance of accessory protein variants in the pathogenicity of SARS-CoV-2. Arch Biochem. Biophys. 2022, 717, 109124. [Google Scholar] [CrossRef]
- Yoshimoto, F.K. The Proteins of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2 or n-COV19), the Cause of COVID-19. Protein J. 2020, 39, 198–216. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P.; Trejbal, J.; Demo, P. Applications of Cold Atmospheric Pressure Plasma Technology in Medicine, Agriculture and Food Industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Tabares, F.L.; Junkar, I. Cold Plasma Systems and their Application in Surface Treatments for Medicine. Molecules 2021, 26, 1903. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Sherman, J.H.; Keidar, M. The Application of the Cold Atmospheric Plasma-Activated Solutions in Cancer Treatment. Anticancer Agents Med. Chem. 2018, 18, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Burm, K.T.A.L. Plasma: The Fourth State of Matter. Plasma Chem Plasma Process 2012, 32, 401–407. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxidative Med Cell Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Isbary, G.; Stolz, W.; Morfill, G.; Landthaler, M.; Shimizu, T.; Steffes, B.; Nosenko, T.; Zimmermann, J.L.; Karrer, S. Plasma applications in medicine with a special focus on dermatology. J. Eur. Acad. Derm. Venereol. 2011, 25, 1–11. [Google Scholar] [CrossRef]
- Judée, F.; Fongia, C.; Ducommun, B.; Yousfi, M.; Lobjois, V.; Merbahi, N. Short and long-time effects of low-temperature Plasma Activated Media on 3D multicellular tumor spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar] [CrossRef]
- O’Connor, N.; Cahill, O.; Daniels, S.; Galvin, S.; Humphreys, H. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J. Hosp. Infect. 2014, 88, 59–65. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar] [CrossRef]
- Feizollahi, E.; Misra, N.N.; Roopesh, M.S. Factors influencing the antimicrobial efficacy of Dielectric Barrier Discharge (DBD) Atmospheric Cold Plasma (ACP) in food processing applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 666–689. [Google Scholar] [CrossRef]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control 2010, 38, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Klaempfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.-F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.-U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Env. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Xu, Y.; Dai, C.; Zhou, X.; Xu, Q.; Wu, Z. Cold atmospheric plasma: A non-negligible strategy for viral RNA inactivation to prevent SARS-CoV-2 environmental transmission. AIP Adv. 2021, 11, 085019. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, P.; Zhang, L.; Peng, S.; Wang, X.; Luo, H.; Wu, G. Disinfection of Escherichia coli in ice by surface dielectric barrier discharge plasma. Appl. Phys. Lett. 2021, 119, 090601. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 5. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. (Eds.) Metal Ions in Biological Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity In Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J. Med. Microbiol. 2020, 69, 1228–1234. [Google Scholar] [CrossRef]
- Chen, Z.; Wirz, R. Cold Atmospheric Plasma for COVID-19. Preprints 2020, 2020040126. [Google Scholar] [CrossRef][Green Version]
- Khanikar, R.R.; Kalita, M.; Kalita, P.; Kashyap, B.; Das, S.; Khan, M.R.; Bailung, H.; Sankaranarayanan, K. Cold atmospheric pressure plasma for attenuation of SARS-CoV-2 spike protein binding to ACE2 protein and the RNA deactivation. RSC Adv. 2022, 12, 9466–9472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).