Impact of Hydroxychloroquine Treatment of COVID-19 on Cardiac Conduction: The Beat Goes On
Abstract
:1. Introduction
2. Methods
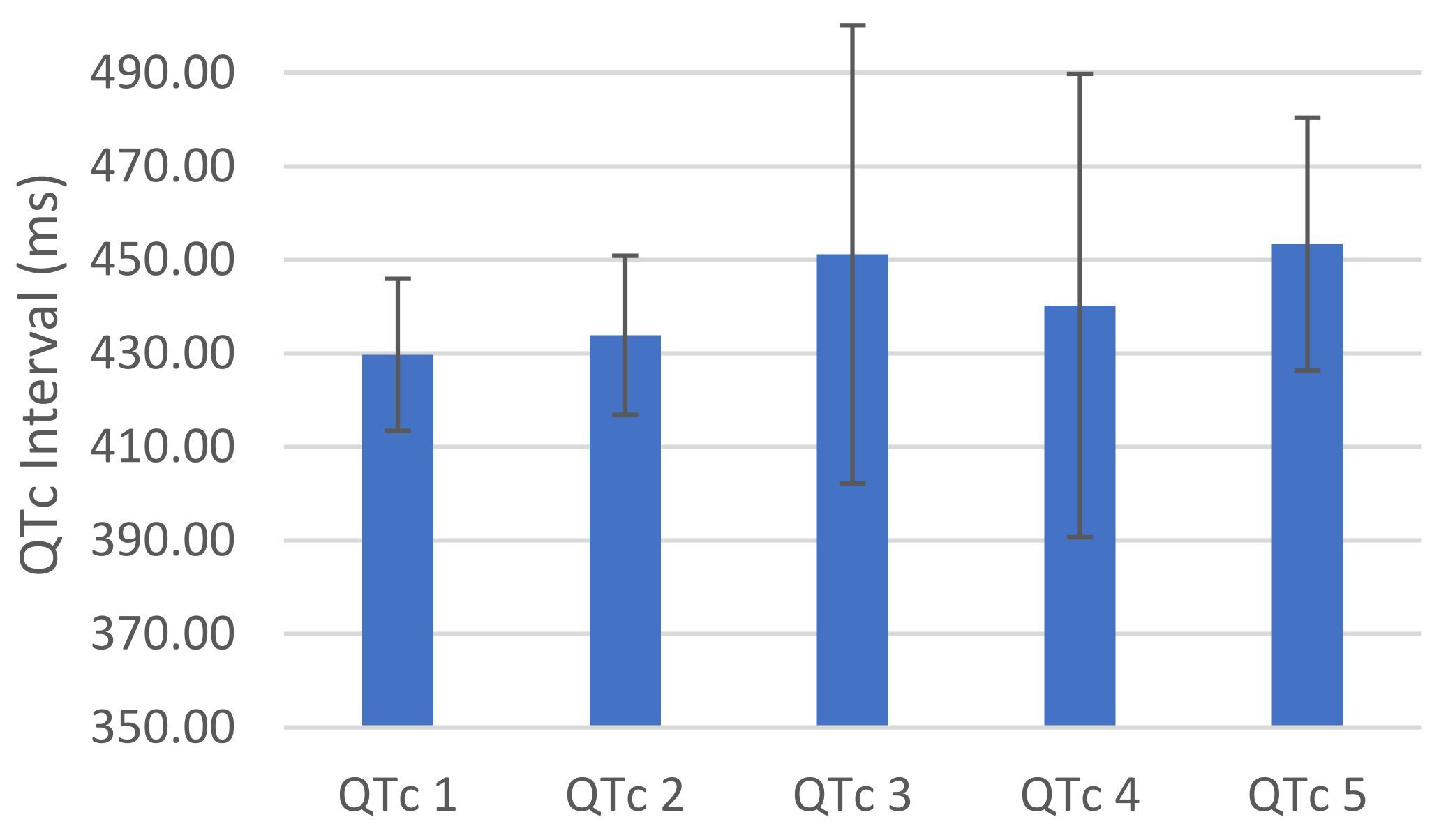
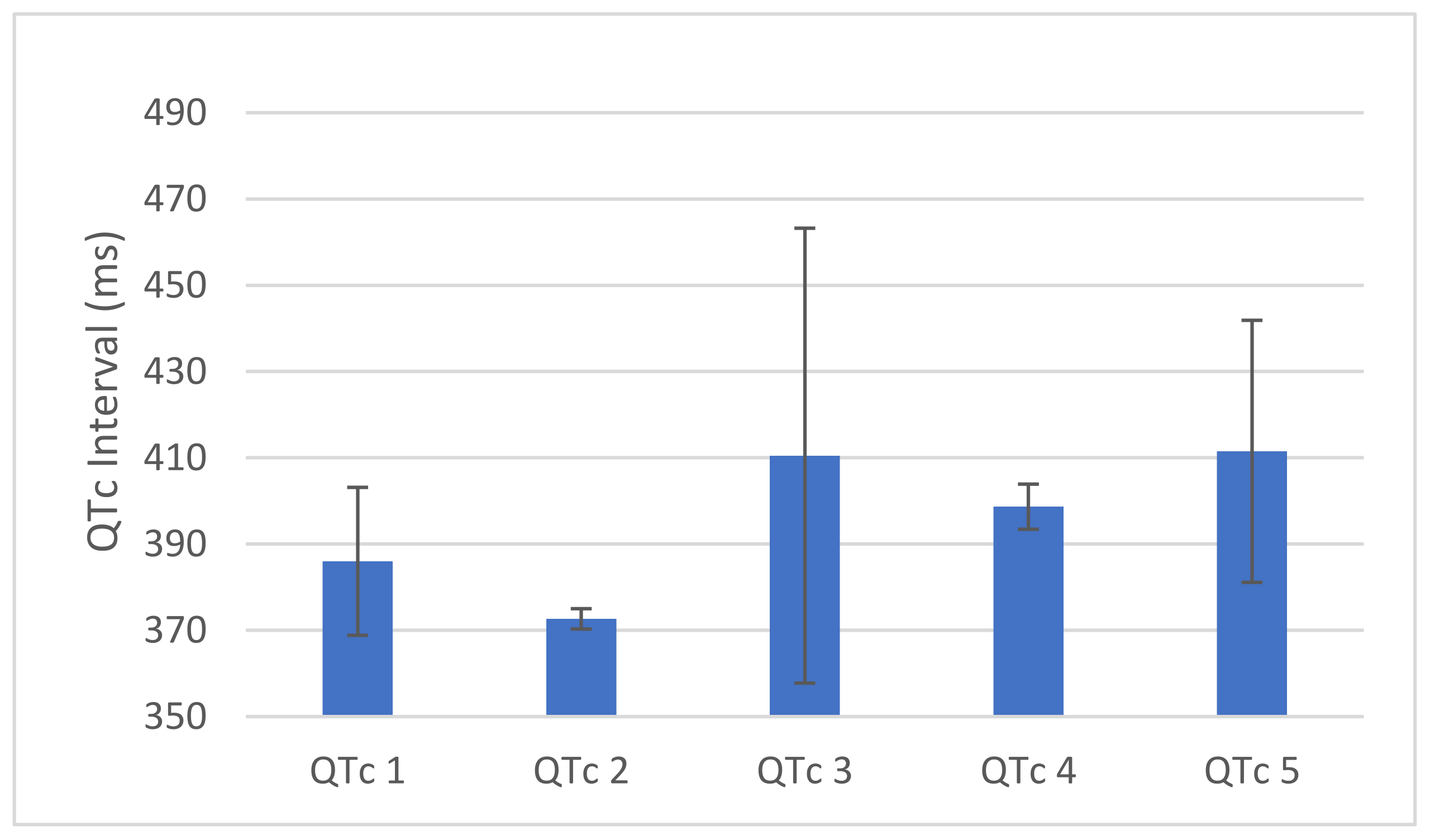
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Cases in the US. 2020. Available online: https://covid.cdc.gov/covid-data-tracker/#datatracker-home (accessed on 22 June 2021).
- Centers for Disease Control and Prevention (CDC). Information for Clinicians on Therapeutic Options for COVID-19 Patients. 2020. Available online: https://www-cdc-gov.proxy2.cl.msu.edu/coronavirus/2019-ncov/hcp/therapeutic-options.html#r8 (accessed on 1 May 2020).
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—preliminary report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19 (accessed on 1 May 2020).
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef]
- Hoffmann, M.; Mösbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Krüger, N.; Gassen, N.C.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nat. Cell Biol. 2020, 585, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Wang, Y.-H.; Chen, Y.-L.; Tsai, W.-C.; Lee, C.-H.; Chuang, K.-P.; Chen, Y.-M.; Yuan, C.-H.; Ho, S.-Y.; Yang, M.-H.; et al. Chloroquine and Hydroxychloroquine: Efficacy in the Treatment of the COVID-19. Pathogens 2021, 10, 217. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. medRxiv 2020. Preprint. Available online: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v2 (accessed on 22 June 2021).
- Chen, J.; Liu, D.; Liu, L.; Liu, P.; Xu, Q.; Xia, L.; Qian, Z. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19). J. Zhejiang Univ. (Med. Sci.) 2020, 49, 215–219. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label nonrandomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Arshad, S.; Kilgore, P.; Chaudhry, Z.S.; Jacobsen, G.; Wang, D.D.; Huitsing, K.; Brar, I.; Alangaden, G.J.; Ramesh, M.S.; McKinnon, J.E.; et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020, 97, 396–403. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group; Horby, P.; Mafham, M.; Linsell, L.; Bell, J.L.; Staplin, N.; Emberson, J.R.; Wiselka, M.; Ustianowski, A.; Elmahi, E.; et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020, 383, 2041–2052. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, m1849. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Pullen, M.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N. Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef]
- Tisdale, J.E.; Jaynes, H.A.; Kingery, J.R.; Mourad, N.A.; Trujillo, T.N.; Overholser, B.R.; Kovacs, R.J. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 479–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, M.; Gabriels, J.; Chang, D.; Kim, B.S.; Mansoor, A.; Mahmood, E.; Makker, P.; Ismail, H.; Goldner, B.; Willner, J.; et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ. Arrhythmia Electrophysiol. 2020, 13, e008662. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Arocutipa, C.; Brañez-Condorena, A.; Hernandez, A.V. QTc prolongation in COVID-19 patients treated with hydroxychloroquine, chloroquine, azithromycin, or lopinavir/ritonavir: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2021, 30, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Oscanoa, T.J.; Vidal, X.; Kanters, J.K.; Romero-Ortuno, R. Frequency of Long QT in Patients with SARS-CoV-2 Infection Treated with Hydroxychloroquine: A Meta-analysis. Int. J. Antimicrob. Agents 2020, 56, 106212. [Google Scholar] [CrossRef] [PubMed]


| Mean | 95% CI | % | ||
|---|---|---|---|---|
| Age | 67.18 | 63.90 | 70.46 | |
| Gender (n) | ||||
| - male | 41 | 54.7 | ||
| - female | 34 | 45.3 | ||
| Ethnicity (n) | ||||
| - Caucasian | 26 | 34.7 | ||
| - African American | 45 | 60.0 | ||
| - Other/unknown | 4 | 5.3 | ||
| BMI | 32.72 | 30.66 | 34.77 | |
| Length of Stay | 8.86 | 7.52 | 10.20 | |
| HTN (n) | 63 | 84.0 | ||
| DM (n) | 38 | 50.7 | ||
| Ventilated (n) | 10 | 13.3 | ||
| ICU (n) | 12 | 16.0 | ||
| n | % | Points | |
|---|---|---|---|
| Completed Course | 74 | 98.67 | 3 |
| Age over 68 | 36 | 48.00 | 1 |
| Female Gender | 34 | 45.33 | 1 |
| Prolong QTc on Admission | 24 | 32.00 | 2 |
| 2 QT prolonging meds | 72 | 96.00 | 3 |
| Acute MI | 0 | 0 | 2 |
| Loop Diuretic? | 23 | 30.67 | 1 |
| >2 SIRS criteria | 49 | 65.33 | 3 |
| Heart failure? | 3 | 4.00 | 3 |
| Serum K < 3.5 mEq/L | 11 | 14.67 | 2 |
| Tisdale Risk Category | |||
| - low | 9 | 12.00 | |
| - moderate | 41 | 54.67 | |
| - high | 25 | 33.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zughaib, M.T.; Singh, R.; Letourneau, M.; Zughaib, M.E. Impact of Hydroxychloroquine Treatment of COVID-19 on Cardiac Conduction: The Beat Goes On. COVID 2021, 1, 458-464. https://doi.org/10.3390/covid1020039
Zughaib MT, Singh R, Letourneau M, Zughaib ME. Impact of Hydroxychloroquine Treatment of COVID-19 on Cardiac Conduction: The Beat Goes On. COVID. 2021; 1(2):458-464. https://doi.org/10.3390/covid1020039
Chicago/Turabian StyleZughaib, Marc Thomas, Robby Singh, Marcel Letourneau, and Marcel Elias Zughaib. 2021. "Impact of Hydroxychloroquine Treatment of COVID-19 on Cardiac Conduction: The Beat Goes On" COVID 1, no. 2: 458-464. https://doi.org/10.3390/covid1020039
APA StyleZughaib, M. T., Singh, R., Letourneau, M., & Zughaib, M. E. (2021). Impact of Hydroxychloroquine Treatment of COVID-19 on Cardiac Conduction: The Beat Goes On. COVID, 1(2), 458-464. https://doi.org/10.3390/covid1020039






