Abstract
Carbon quantum dots (CQDs) are photoluminescent nanomaterials (<10 nm) with excellent hydrophilicity, biocompatibility, and low cytotoxicity, making them attractive for biological applications. However, their use in aquaculture nutrition has remained largely unexplored. This study investigated the effects of dietary CQDs on zebrafish (Danio rerio), a model organism with approximately 70% genetic homology with humans. CQDs were synthesized hydrothermally from unripe Citrus limon and characterized by UV–visible (UV-Vis) spectroscopy, UV–vis transillumination, scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray analysis (EDX), Fourier-transform infrared spectroscopy (FT-IR), and photoluminescence (PL) spectroscopy. Zebrafish were fed diets containing varying CQD concentrations, and growth performance, condition factor (K), hematological parameters, enzymatic activity, and tissue morphology were assessed. Feeds supplemented with 2 mL CQDs produced significant improvements in growth and biochemical indicators without adverse effects. Hematological and enzymatic profiles remained within normal ranges, and histological examination revealed no morphological abnormalities, indicating the absence of toxicity. These findings suggest that citrus-derived CQDs can enhance zebrafish growth and maintain physiological health, thereby supporting their potential as safe functional feed additives in aquaculture. This approach may open new opportunities for the application of CQDs in sustainable fish farming and the broader food industry.
1. Introduction
The lack of innovation in aquafeed formulations poses a significant drawback to aquaculture and society as a whole. Conventional feeds often suffer from low digestibility, poor nutrient bioavailability, and limited functional properties, which not only reduces fish growth and survival but also increases feed conversion ratios, production costs, and environmental pollution through nutrient leaching [1]. This ultimately restricts aquaculture productivity, compromises food security, and reduces the sector’s economic benefits. To address these challenges, the incorporation of nanomaterials into aquafeeds has emerged as a promising strategy. Nanotechnology enables the encapsulation and delivery of nutrients, vitamins, minerals, and bioactive compounds in nanosized forms, thereby improving their solubility, stability, and bioavailability [2]. Additionally, nano-encapsulation allows for the controlled release of feed supplements, protecting them from degradation and minimizing nutrient loss into water systems [3]. Beyond growth promotion, nanomaterials can also enhance immunity, reduce disease susceptibility, and reduce dependence on antibiotics in aquaculture systems [4]. Thus, advancements in nanotechnology-based feed supplements represent a sustainable approach to overcoming the limitations of traditional aquafeeds, while supporting the growth, health, and productivity of cultured species. Moreover, NPs can improve the solubility, stability, and bioavailability of poorly absorbed or unstable therapeutic agents, thereby transforming them into effective delivery systems. Quantum dots (QDs) are nanomaterials with sizes ranging from 1 to 20 nm [5,6].
Quantum dots (QDs) are semiconductor nanocrystals that exhibit size-dependent optical and electronic properties owing to quantum confinement, which is typically observed when the particle size approaches or is smaller than the exciton Bohr radius of the material. Depending on the semiconductor, this can extend beyond 20 nm, for example, PbSe QDs [7]. Unlike conventional feed supplements, QDs exhibit excellent water solubility, stability, and biocompatibility, making them suitable for biological applications [8]. Their small size (<10 nm) and large surface area allow for efficient interactions with biological systems, leading to improved nutrient delivery, antioxidant activity, and bioavailability of the active compounds [9]. Additionally, QDs can be functionalized with biomolecules or minerals, enabling them to act as effective carriers for feed supplements, while protecting sensitive nutrients from degradation. In addition to exhibiting comparable optical properties, carbon quantum dots (CQDs) offer advantages, such as low toxicity, ecological sustainability, cost-effectiveness, ease of synthesis, high water solubility, biocompatibility, stable fluorescence with broad excitation spectra, and tunable emission wavelengths. Surface passivation and functionalization of CQDs have been extensively explored to enhance their applications in chemical sensing, biological detection, bioimaging, nanomedicine, photocatalysis, and electrochemical catalysis [10,11,12].
Green, synthesized CQDs are increasingly preferred over chemically synthesized ones because they are eco-friendly, cost-effective, and safer for biological applications, avoiding the use of toxic precursors or harsh conditions [13,14,15,16]. Using natural plant sources for CQD synthesis also provides added biological value, as the phytochemicals present in the raw material often impart functional properties to the CQDs [9]. Among these, lemon Citrus limon is an excellent candidate because of its rich content of citric acid, ascorbic acid, flavonoids, and polyphenols, which not only act as natural precursors for carbonization but also endow the resulting CQDs with intrinsic antioxidant, antimicrobial, and bioactive properties [17]. Lemon-derived CQDs are highly water-soluble and biocompatible, and exhibit strong fluorescence, making them suitable for feed supplementation and bioimaging in aquaculture. When incorporated into aquafeeds, lemon-based CQDs can serve as nanocarriers for nutrients, enhancing their bioavailability and stability while simultaneously contributing to improved growth, immunity, and stress tolerance in fish. Additionally, natural antioxidant activity helps reduce oxidative stress, thereby minimizing disease susceptibility and reliance on synthetic additives [18].
The application of CQDs in aquaculture has been shown to improve disease management, water quality, and feed efficiency, and reduce environmental impacts. Recent studies have highlighted their role in enhancing the efficiency of biological degradation processes in wastewater treatment, which is crucial for maintaining optimal aquaculture environments [19]. Additionally, CQDs have been employed in sensors for detecting contaminants such as ammonia, enabling more effective monitoring of water quality and fish health [20]. Their antimicrobial properties further contribute to disease management by inhibiting pathogen growth in aquatic systems [21]. These findings underscore the potential of CQDs as sustainable and effective tools for aquaculture.
Among aquaculture species, zebrafish (Danio rerio) are widely used as model organisms because of their high genetic similarity to humans, with approximately 70% of human genes represented. This makes them ideal for in vivo imaging studies, including fluorescent protein imaging, visualization of blood flow in heart defects, and bone calcification [12,22]. Recent studies have demonstrated efficient uptake and distribution of CQDs in zebrafish tissues, particularly in neural tissues [23]. The intricate neural architecture of zebrafish allows for precise examination of behavioral changes and neurodevelopmental alterations induced by fluorescent CQDs. Neurotoxicity assessments reveal both short- and long-term effects, ranging from immediate behavioral changes to subtle alterations in neuronal morphology. These findings highlight the need for standardized methodologies to evaluate neurological outcomes and emphasize the ethical considerations in nanomaterial research. Furthermore, CQDs synthesized via hydrothermal methods have been shown to modulate dopamine receptors in the zebrafish brain, thereby enhancing dopamine production and increasing locomotor activity [24]. Overall, CQDs exhibited low cytotoxicity and high biocompatibility, highlighting their potential for biomedical and aquaculture applications. However, further investigations are required to assess the effects of CQD-supplemented feed on zebrafish growth, hematological parameters, and tissue histology to fully understand their safety and functional benefits.
CQDs possess a high surface area and abundant functional groups that facilitate interactions with digestive enzymes and the intestinal epithelium. These interactions may enhance the efficiency of nutrient absorptions—particularly of amino acids and minerals which are essential for growth and metabolic function. The general assumption is that feed additives can alter the fish microbiota which, in turn, interacts with the host immune system [25,26]. Moreover, CQDs appear to modulate key biochemical processes such as enzyme activity and protein synthesis. Zebrafish fed with CQD-supplemented diets exhibited elevated activities of growth-related enzymes, including alkaline phosphatase and protease, which play critical roles in digestion and tissue development. The improved feed conversion ratio observed in CQD-fed groups indicates a more efficient conversion of feed into body mass, suggesting that CQDs may optimize metabolic processes and energy utilization [27].
At the molecular level, CQDs may influence the expression of growth-related genes and signaling pathways such as the insulin-like growth factor and mTOR cascades, which are fundamental to cell proliferation, differentiation, and protein synthesis. Owing to their nanoscale size and cell-penetrating capacity, CQDs can potentially modulate intracellular signaling and gene expression, thereby contributing to enhanced growth performance and overall physiological efficiency [28,29].
The aim of this study was to develop and evaluate aquafeeds incorporated with lemon-derived CQDs and assess their impact on growth, feed utilization efficiency, and overall health of zebrafish (Danio rerio). Specifically, this study aimed to determine whether lemon-based CQDs could enhance nutrient bioavailability, promote growth, reduce oxidative stress, and provide a sustainable alternative to conventional feed additives. This study is novel because it explores the use of lemon-derived CQDs as functional nanomaterials for aquafeed supplementation, which has not been widely reported in aquaculture nutrition research. Unlike conventional feed additives or synthetic nanomaterials, lemon-based CQDs combine the dual advantages of green synthesis from a natural, renewable source and the inherent bioactivity derived from lemon phytochemicals. The incorporation of CQDs into zebrafish aquafeed has shown promising effects on growth performance; however, the source and synthesis route of CQDs critically influence both their biological efficacy and environmental compatibility. Chemically synthesized CQDs, although structurally consistent and crystalline, exhibit relatively low bioavailability and only moderate growth enhancement, likely due to residual reagents and limited surface passivation. In contrast, lemons act as macronutrient supplements, whereas lemon-derived CQDs function as nano-bioenhancers. The lemon-derived CQDs provided targeted interactions with the zebrafish gut and systemic physiology, potentially activating growth-related pathways more efficiently than the bulk lemon extract. The ability to act as both a nutrient nanocarrier and a bioactive agent with antioxidant and antimicrobial properties provides a unique approach to improve feed efficiency, fish health, and sustainability. This study bridges nanotechnology and aquaculture nutrition by demonstrating the potential of plant-derived CQDs as innovative aqueous supplements.
2. Materials and Methods
2.1. Preparation of Lemon Extract
Fresh, unripen green lemons (Citrus limon) were collected directly from a farm in Sengattampatti Village, Dindigul, Tamil Nadu, India. The lemons were rinsed twice with distilled water to remove dust and impurities. Seeds and pulp were separated using a sieve, and the resulting pulp-free lemon juice was diluted with distilled water in a 1:2 ratio. The mixture was stirred at 200 rpm for 15 min to ensure thorough homogenization. The prepared lemon juice was collected in a clean glass container for the subsequent CQD synthesis (Figure 1).
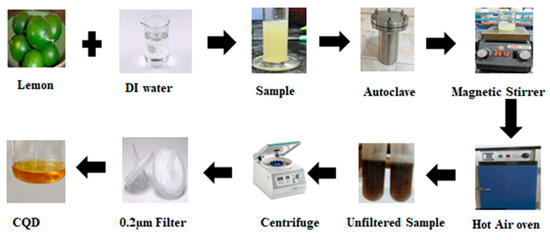
Figure 1.
Schematic illustration of the synthesis workflow for citrus-derived CQDs.
2.2. Synthesis of CQDs
The CQDs were synthesized using a green hydrothermal method. Pulp-free lemon juice (ivory-white solution) and deionized water were mixed in a 1:2 ratio and stirred for 10 min to ensure uniform distribution. The solution was then transferred to a Teflon-lined stainless-steel autoclave and subjected to hydrothermal treatment at 180 °C for 8 h. After the completion of the reaction, the autoclave was allowed to cool naturally to room temperature. Carbonization of the solution was indicated by a color change from ivory white to dark brown, confirming the formation of CQDs. The resulting solution was centrifuged at 10,000 rpm for 30 min, and the supernatant was collected using a syringe and further purified using a 0.2 µm filter. The purified CQD solution was stored at 4 °C until subsequent use (Figure 1).
2.3. Characterization of CQDs
The successful synthesis of the green CQDs was confirmed using multiple characterization techniques. The optical properties, including the absorption spectrum and fluorescence, were analyzed using a UV–visible (UV-Vis) spectrophotometer (GENESYSTM 180 UV-Vis Spectrophotometer; Thermo Scientific, Waltham, MA, USA) and a UV-Vis transilluminator (MEDOX-BIO® UV Transilluminator-Regular; Medox Biotech, India Pvt. Ltd., Chennai, India). Photoluminescence (PL) spectra were recorded using a spectrofluorometer (FP-8500; Jasco International Co., Ltd.; Tokyo, Japan). Morphological features and elemental compositions were examined using scanning electron microscopy (SEM; VEGA3, Tescan, Kohoutovice, Czech Republic), transmission electron microscopy (TEM; Bruker Nano GmbH, Berlin, Germany), and energy-dispersive X-ray (EDX; VEGA3, Tescan, Kohoutovice, Czech Republic). The functional groups present on the CQD surface were identified by Fourier-transform infrared (FT-IR) spectroscopy (FT/IR 4700 Spectrometer; Jasco International Co., Ltd.; Tokyo, Japan).
2.4. Experimental Study and Feed Preparation
Zebrafish fingerlings (1.340 ± 0.540 g) were acquired from the Aqua Garden Fish Farm in Kadachanenthal, Madurai, Tamil Nadu, India, and transported to the laboratory in polythene bags containing oxygen-enriched water. Upon arrival, the fish were acclimated in glass aquaria [60 cm length (L) × 45 cm width (W) × 45 cm height (H)] for 15 d at a controlled temperature of 28 ± 2 °C. During acclimation, a formulated dry pellet diet composed of fish meal, groundnut oil cake, wheat flour, and rice bran was provided. The raw materials for feed preparation were carefully selected for their ability to supply essential nutrients—proteins, carbohydrates, and lipids—at low cost while ensuring optimal digestibility. The protein content of each ingredient was determined using the Micro-Kjeldahl method, and the feed formulation was developed following Pearson’s square method, a scientifically validated and widely accepted approach for balanced ration formulation in aquaculture [30]. Protein and carbohydrate sources included fish meal, groundnut oil cake, tapioca powder, and wheat flour. All ingredients were dried, pulverized, and sieved through a 425 µm mesh prior to preparation. The weighed ingredients were thoroughly mixed, and 130–150 mL of sterile distilled water was added to achieve a homogeneous mixture.
The feed mixture (Table 1) was autoclaved at 100 °C for 30 min and cooled to room temperature before incorporating fish oil and sunflower oil as lipid sources, along with Supplevite mix, sodium chloride, sodium benzoate, and varying quantities of carbon quantum dots (CQDs) (0.5, 1.0, 1.5, 2.0, and 2.5 mL). The final mixture was extruded into pellets using a pelletizer and stored in airtight containers at −20 °C to prevent microbial contamination and nutrient degradation.

Table 1.
Composition of different ingredients in the experimental feed (g/100 g) of zebrafish.
This control diet served as the baseline formulation, enabling accurate comparison with the experimental diets containing graded levels of CQDs and ensuring a standardized and scientifically robust evaluation of CQD supplementation on zebrafish growth and physiological performance.
For the growth studies, uniformly sized zebrafish (Danio rerio, 1.34 ± 0.54 g) were selected and introduced into rectangular glass tanks (45 cm L × 22 cm W × 22 cm H) with an 18 L capacity. Ten fish were stocked in each tank, and each treatment was conducted in triplicate. During the rearing period, the fish were fed a prepared diet ad libitum twice daily for 1 h each, from 8:30 to 9:30 am and 4 to 5 pm. After 1 h of feeding, unfed fish were collected without disturbing the tank and dried to a constant weight. Fecal matter was collected daily before water changes, again with minimal disturbance to the fish, and was dried at 950 °C. Approximately 70% of the tank water was replaced by tap water. This procedure was continued for 28 days. On the 29th day, the length and weight of the fish were measured while they were still in a live state. The blood, liver, gills, and muscle of fish from all experimental tanks were collected for further analysis. The feeding trial in our study was designed and conducted in strict accordance with the OECD Test Guideline 215: Fish, Juvenile Growth Test (https://doi.org/10.1787/9789264070205-en), which specifically recommends a 28-day exposure period for evaluating the effects of chemical substances on the growth and health of juvenile fish, including zebrafish. This internationally recognized protocol is widely accepted as sufficient for assessing sub-chronic toxicity and growth-related endpoints, ensuring compliance with established international standards for aquatic toxicity and nutritional assessment.
3. Results and Discussion
3.1. Optical Characterization
The optical properties of the CQDs were analyzed using UV-Vis spectroscopy and transillumination. The UV-Vis absorption spectrum (Figure 2A) exhibited two prominent peaks at 295 and 355 nm. The 295 nm peak corresponds to π–π* transitions of C=C bonds within aromatic sp2 domains of the carbon core, while the 355 nm peak is attributed to the n–π* transitions of C=O groups and other oxygen-containing surface functionalities. These features confirm the presence of a conjugated carbon structure and effective surface passivation, which are characteristic of well-formed CQDs with photoluminescence properties. Hydrothermally synthesized CQDs generally exhibit absorption edges between 250 and 350 nm [31], which is consistent with previous reports [32,33,34].
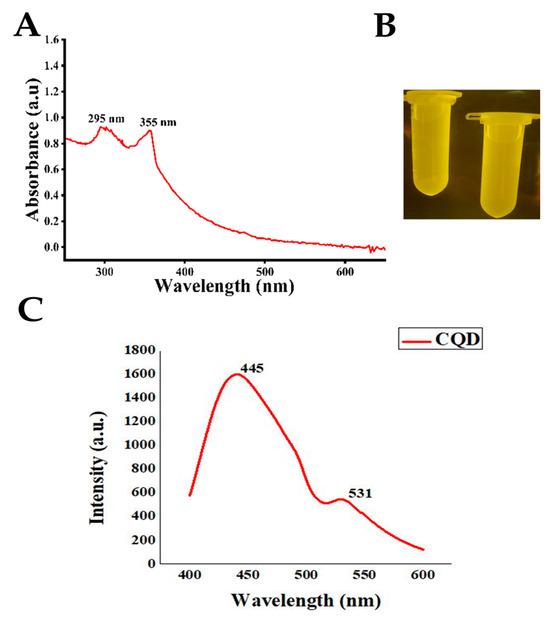
Figure 2.
Optical characterization of CQD: (A) UV–visible absorption spectra, (B) UV–visible transilluminated image, and (C) PL image of CQD.
Under UV-Vis transilluminatior, the lemon juice–derived CQDs exhibited intense green fluorescence, confirming the successful formation of photoluminescent NPs with quantum confinement effects (Figure 2B). This emission is attributed to surface functional groups, including hydroxyl, carboxyl, and amino groups, which enhance light emission by preventing non-radiative recombination. The green fluorescence highlights their potential applications in fluorescent labeling, sensing, and bioimaging. Similar observations have been reported for CQDs synthesized from citric acid and polyethylene glycol (PEG) for DNA studies [35] as well as chemically synthesized CQDs showing green luminescence [36]. Citrus aurantifolia, which is rich in citric and ascorbic acids, is an effective natural carbon source for green CQD synthesis [37]. The hydrothermal carbonization of these acids produces CQDs with well-defined surface functional groups [38]. Using lemon as a carbon precursor offers a sustainable, non-toxic, and environmentally benign approach [39].
The photoluminescence spectroscopy (PL) showed peaks at 445 and 531 nm (Figure 2C). The 400–600 nm range of CQDs is typically a broad, tunable spectrum influenced by the surface chemistry, structure, and environment of the CQDs. Peaks occur between 400 and 600 nm when CQDs are synthesized from biomass or by the green method [40]. In PL microscopy, the emission allows CQDs to act as efficient nanoprobes for bioimaging, where bright and stable luminescence enables the visualization of cells and tissues without the need for additional dyes. The high photostability, low cytotoxicity, and multicolor emission of CQDs make them particularly useful for multiplexed imaging and sensing applications in biological systems [10,41,42].
3.2. SEM and TEM Image Analysis
SEM images of the CQDs at a 2 µm scale (Figure 3A) revealed a uniform surface morphology with well-dispersed spherical particles. Individual CQDs were not fully resolved owing to their nanoscale size; however, their smooth, non-aggregated texture indicated successful synthesis and good colloidal stability. The SEM images also show curved sheets ranging from several dozen to several hundred micrometers [27]. Overall, morphological analysis confirmed that the CQDs predominantly exhibited a spherical shape, consistent with previous reports [43,44], with an average particle size of approximately 4.5 nm [45]. The SEM image analysis of the CQDs at a 2 μm scale, depicted in Figure 3, shows a uniform surface morphology with well-dispersed, spherical particles. Although the individual CQDs were not clearly resolved owing to their nanoscale size, their smooth and non-aggregated textures suggested successful synthesis and good stability.
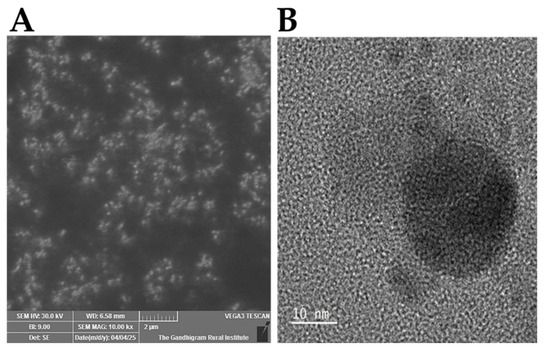
Figure 3.
Image analysis of CQDs: (A) SEM and (B) TEM images of CQDs.
TEM images of the CQDs revealed a highly monodisperse spherical morphology with an average size of 4 nm (Figure 3B). CQDs synthesized from both natural and synthetic carbon sources typically exhibit an amorphous carbon structure; either the entire CQD is amorphous or it possesses a crystalline core surrounded by an amorphous shell [46,47]. The narrow size distribution observed for the CQDs synthesized via thermal decomposition in this study is comparable to that obtained using hydrothermal methods [48]. Notably, achieving such a narrow size distribution using natural carbon sources is unprecedented [46,49].
3.3. EDX Analysis
EDX analysis (Figure 4, Table 2) showed that the synthesized CQDs are primarily composed of carbon (C, 78.46 wt.%, 82.37 at.%), oxygen (O, 15.69 wt.%, 12.36 at.%), and nitrogen (N, 5.86 wt.%, 5.27 at.%). The high carbon content indicates a well-structured carbon core, which is essential for the quantum confinement effects. The presence of oxygen and nitrogen reflects surface functional groups, such as hydroxyl, carboxyl, and amine groups, which enhance the water solubility, photoluminescence, and chemical stability. Nitrogen doping can further modify the electronic structure of CQDs, potentially improving their fluorescence efficiency and quantum yield. The comparable atomic percentages of oxygen and surface carbon suggest extensive surface functionalization, because many carbon atoms are located at the surface and bonded to oxygen-containing groups. Nitrogen is present both within the carbon lattice and at surface sites, contributing to the functional and optical properties of CQDs [50].
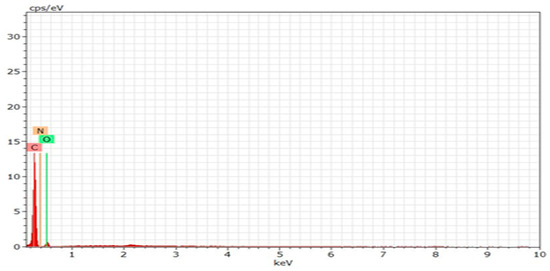
Figure 4.
EDX patterns of CQDs.

Table 2.
Quantitative data for EDX analysis of CQDs.
3.4. FT-IR Analysis
The FT-IR spectrum of the synthesized CQDs (Figure 5 and Table 3) displays characteristic absorption bands at 3341.08, 1636.34, and 647.01 cm−1. The broad peak at 3341 cm−1 corresponds to O–H and N–H stretching vibrations, indicating the presence of hydroxyl and amine groups on the CQD surface, which enhances solubility and photoluminescence. The band at 1636 cm−1 is attributed to C=C or C=O stretching vibrations, reflecting conjugated carbon structures or carbonyl groups. The absorption at 647 cm−1 arises from C–H bending or out-of-plane deformation vibrations, which are typical of aromatic or substituted hydrocarbon rings.
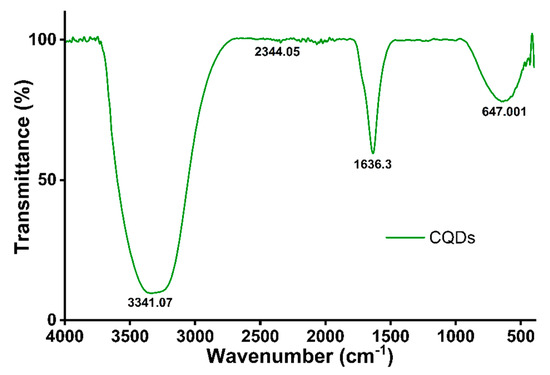
Figure 5.
FT-IR spectrum of CQDs.

Table 3.
FT-IR peak representation of functional groups in CQDs.
Additional features include a peak at 2919 cm−1, corresponding to –CH2 groups, and a broad band around 3352 cm−1, associated with –OH groups in phenolic compounds, suggesting intermolecular hydrogen bonding [51]. These findings confirm the successful surface functionalization of the CQDs, which is consistent with previous reports on plant-derived CQDs [52,53]. The C=O stretching of carboxyl groups at 1636 cm−1 is a typical feature of oxidized carbon nanomaterials [37], while the C–H bending at 647 cm−1 reflects aromatic or unsaturated hydrocarbon structures [54].
3.5. Effect of CQD-Supplemented Feed on Zebrafish Growth and Condition Factor
3.5.1. Condition Factor
The condition factor (K) was assessed to evaluate the overall health status of zebrafish. All diets supplemented with varying concentrations of CQDs improved final K values, indicating enhanced physiological conditions. Feed 5, containing 2 mL of CQDs, resulted in the highest K, suggesting optimal health among the tested groups (Table 4).

Table 4.
Feed utilization and growth parameters of zebrafish in relation to the different quantities of CQDs.
3.5.2. Growth Performance
Zebrafish reared on Feed 1 exhibited a growth of 0.73 mg, whereas those fed Feed 5 achieved a higher growth of 0.29 mg. The percentage growth rate was also highest in Feed 5, demonstrating the positive impact of CQD supplementation on growth (Table 4).
3.5.3. Feed Utilization
Feed utilization, including gross growth efficiency and net growth efficiency, varied significantly among diets. Feed 5 showed superior feed consumption and utilization compared to the other feeds, highlighting the role of CQDs in improving nutrient assimilation and overall growth performance (Table 4).
3.5.4. Comparison with Previous Studies
These findings are consistent with those of earlier reports on nanoparticle supplementation in fish. Rajan and Rohini [55] observed an increase in feed consumption in rigid-fed diets containing 15 mg/g zinc oxide nanoparticles (ZnO NPs). Similarly, Koi carp demonstrated a higher feed conversion efficiency with 100 mg ZnO NPs. Malavika et al. [27] reported improved growth in fish fed 1.5 mL of CQDs synthesized with an L-histidine precursor, whereas Sangeetha and Rajan [56] found enhanced nutrient assimilation in koi carp fed 30 mg iron oxide NPs. Rajan and Meenakumari [57] also reported increased gross and net growth efficiency in common carp fed 40 mg of magnesium oxide NPs.
Overall, these results suggest that optimal levels of CQD supplementation can significantly enhance zebrafish growth, feed utilization, and health status (Table 5).

Table 5.
ANOVA (analysis of variance) of growth parameters (feed consumption, growth, gross growth efficiency, and net growth efficiency) of zebrafish; df indicates degrees of freedom.
3.6. Biochemical, Hematological, Enzymatic, and Histological Analyses
3.6.1. Biochemical Parameters
Biochemical analyses of carbohydrates, proteins, and lipids in the muscles, gills, and liver of zebrafish are presented in Table 6. Among the tested feeds, Feed 5 supplemented with 2 mL of CQDs resulted in the highest levels of carbohydrates, proteins, and lipids across all tissues, indicating improved nutrient assimilation. Similar trends were observed in previous studies using chemically synthesized CQDs, where 1.5 mL supplementation enhanced carbohydrate, protein, and lipid content in fish tissues [27].

Table 6.
ANOVA analyses of biochemical parameters of zebrafish.
3.6.2. Hematological Parameters
Hematological analysis (Table 7) showed that Feed 5 produced the highest counts of red blood cells (RBCs), hemoglobin, hematocrit, white blood cells (WBCs), and platelets, suggesting an improved physiological status and oxygen transport capacity.

Table 7.
ANOVA analyses of hematological parameters of zebrafish.
3.6.3. Enzyme Activity
Enzymatic assays (Table 8) revealed that aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) activities were lowest in Feed 5, indicating minimal cytotoxicity and better liver function compared with other feeds.

Table 8.
ANOVA analyses of enzymatic parameters of zebrafish.
3.6.4. Histological Observations
Histological examination of zebrafish tissues (muscle, gill, and liver) showed healthy organ architecture in feeds with lower CQD concentrations, including Feed 5 (Figure 6). In contrast, Feed 6 induced tissue damage, including vacuole formation and organ denaturation. These results are consistent with those of previous studies on other fish species. For example, common carp fed various diets showed no intestinal or nutritional pathologies [58], whereas replacement of 30% fishmeal with plant-derived proteins (soy, wheat, and corn) caused significant intestinal alterations, including reduced villus length and enterocyte height [59]. Similarly, Poleksić et al. [60] reported that feeds containing only plant proteins without fishmeal significantly reduced enterocyte height in carp. The zebrafish genome exhibits a high degree of similarity to the human genome, with approximately 70% of the human genes having at least one zebrafish ortholog. This genetic homology renders the zebrafish a highly relevant vertebrate model for biomedical and environmental studies. Consequently, zebrafish have been increasingly employed in toxicological and growth assessments because of the conservation of key biological pathways shared by higher vertebrates [61].
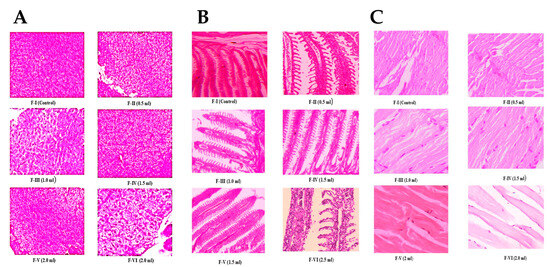
Figure 6.
Histology image zebrafish: (A) liver, (B) gill, and (C) muscle exposed to CQDs.
Overall, these findings indicate that optimal CQD supplementation (2 mL) enhances biochemical composition, hematological health, enzymatic stability, and tissue integrity in zebrafish, whereas excessive concentrations may lead to tissue damage.
4. Conclusions
CQDs were successfully synthesized using a facile, cost-effective, one-step hydrothermal method with unripe lemons as the precursors. The incorporation of 2 mL of CQDs into the zebrafish feed led to significant improvements in growth performance, feed utilization, and biochemical composition, indicating enhanced nutrient absorption and metabolic efficiency. Hematological and enzymatic analyses further confirmed the biocompatibility and low cytotoxicity of the CQDs, whereas histological observations showed a healthy tissue morphology at effective concentrations. These findings suggest that CQDs not only serve as efficient growth enhancers but also hold promise as safe nutritional supplements in aquaculture, potentially improving fish health and productivity. Given the homology between zebrafish and higher vertebrates, these results open new avenues for investigating CQDs in biomedical and clinical contexts, including drug delivery, imaging, and metabolic regulation. Future studies should explore the long-term effects, optimal dosing strategies, and mechanistic pathways underlying the observed benefits to fully realize the potential of CQDs in both aquaculture and translational research.
Author Contributions
Conceptualization, V.M. and M.R.R.; Methodology, V.M.; Validation, K.C.A. and R.K.; formal analysis, V.M.; investigation, V.M., R.K. and K.C.A.; resources, M.R.R.; data curation, V.M.; writing—original draft preparation, V.M.; writing—review and editing, M.R.R. and K.-s.K.; visualization, K.-s.K.; supervision, M.R.R. and K.-s.K. Funding, K.-s.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. RS-2025-22452969).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article.
Acknowledgments
The authors gratefully acknowledge the Department of Biology, The Gandhigram Rural Institute-Deemed to be University, Dindigul, Tamil Nadu, India, for providing laboratory facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kumar, V.; Bhatnagar, A.; Jana, R.K. Challenges and opportunities in aquafeed development. Aquac. Int. 2018, 26, 327–345. [Google Scholar]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Bharathakumar, S.; Gurushankar, K.; Perumal, A.B. Nanomedicine for drug resistant pathogens and COVID-19 using mushroom nanocomposite inspired with bacteriocin–A review. Inorg. Chem. Commun. 2023, 152, 110682. [Google Scholar] [CrossRef]
- Khan, S.K.; Dutta, J.; Ahmad, I.; Rather, M.A. Nanotechnology in aquaculture: Transforming the future of food security. Food Chem X 2024, 24, 101974. [Google Scholar] [CrossRef]
- El Basuini, M.F.; El-Hais, A.M.; Dawood, M.A.O.; Abou-Zeid, A.E.; El-Damrawy, S.Z. Nano-selenium supplementation in aquafeeds: A new approach to improve growth, antioxidant status and immunity in fish. Aquac. Res. 2017, 48, 3371–3381. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Mohapatra, R.; Giri, D.; Vijayakumar, D.; Alagendran, S.; Fernández Saavedra, G.; Jena, J.P.; Sridharan, S.; Karthikeyan, K. Nanomaterials in Biomedical Applications: A Review. J. Adv. Biol. Biotechnol. 2025, 28, 996–1009. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; et al. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des. Dev. Ther. 2024, 18, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Klimov, V.I. Optical nonlinearities and ultrafast carrier dynamics in semiconductor nanocrystals. J. Phys. Chem. B 2000, 104, 6112–6123. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, D. Carbon quantum dots in bioimaging and biomedicines. Front. Bioeng. Biotechnol. 2024, 11, 1333752. [Google Scholar] [CrossRef]
- Dubey, P.; Soni, R.; Dangi, A.K.; Sharma, A.K. Green Synthesis of Nanoparticles: Current Perspectives, Challenges and Future Directions. J. Nanomater. 2024, 2024, 9914079. [Google Scholar] [CrossRef]
- Shahzadi, K.; Shabbir, S.; Ali, S.; Chaudhary, S.; Yousaf, S.; Iqbal, R. A Review on the Green Synthesis of Silver Nanoparticles Using Plant Extracts: Applications and Future Perspectives. RSC Adv. 2025, 15, 12005–12026. [Google Scholar] [CrossRef] [PubMed]
- Lithi, U.Z.; Ahmed, S.; Rahman, A.; Chowdhury, S. Green Synthesis of Metal and Metal Oxide Nanoparticles Using Plant Extracts: Advances, Applications, and Future Directions. Nanoscale Adv. 2025, 7, 1610–1635. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Kumar, V.; Pathak, P.; Bhardwaj, R. Green synthesis of fluorescent carbon quantum dots from lemon juice for in vitro bioimaging. Mater. Today Proc. 2020, 28, 246–251. [Google Scholar] [CrossRef]
- Hu, X.; Ma, W.; Zhang, D.; Tian, Z.; Yang, Y.; Huang, Y.; Hong, Y. Application of Natural Antioxidants as Feed Additives in Aquaculture: A Review. Biology 2025, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Z.; Fu, J.; Zhang, J.; He, Q.; Lu, H.; Zhou, Q.; Wang, H. Recent Advances in the Synthesis, Characterization, and Application of Carbon Dots in the Field of Wastewater Treatment: A Comprehensive Review. Water 2025, 17, 210. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shi, C.; Yang, X. A Fluorescence Method Based on N, S-Doped Carbon Dots for Detection of Ammonia in Aquaculture Water and Freshness of Fish. Sustainability 2021, 13, 8255. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, J.; Gu, L.; Tang, Y.; Zhang, X.; Huang, X.; Shen, X.; Zhai, W.; Fodjo, E.K.; Kong, C. Ratiometric Fluorescence Immunoassay Based on Carbon Quantum Dots for Sensitive Detection of Malachite Green in Fish. Biosensors 2023, 13, 38. [Google Scholar] [CrossRef]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a Tractable Model of Human Cardiovascular Disease. Br. J. Pharmacol. 2021, 178, 3988–4006. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, C.; Arya, S.K.; Puri, S.; Khatri, M. Neurological effects of carbon quantum dots on zebrafish: A review. Neuroscience 2024, 560, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, Z.; Dong, J.; Zhu, J.; Liu, C.; Li, G.; Lu, M.; Han, J.; Cao, S.; Chen, L.; et al. Green Synthesis of Chlorella-Derived Carbon Dots and Their Fluorescence Imaging in Zebrafish. RSC Adv. 2024, 14, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, H.; Cheng, X.; Yin, M.; Yao, X.; Ma, J.; Huang, M.; Chen, G.; Liu, H. Zebrafish: An efficient vertebrate model for understanding role of gut microbiota. Mol. Med. 2022, 28, 161. [Google Scholar] [CrossRef] [PubMed]
- Onomu, A.J.; Okuthe, G.E. The role of functional feed additives in enhancing aquaculture sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Patel, D.; Mehta, R. Nanoparticle-induced gene modulation and growth enhancement in zebrafish. Nanotechnol. Environ. Eng. 2024, 9, 393. [Google Scholar] [CrossRef]
- Santos, M.; Oliveira, F. Growth hormone transgenesis and gene expression in fast-growing zebrafish. Front. Endocrinol. 2024, 15, 1369043. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; García-Asuero, A. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Jadhao, M.M.; Paliwal, L.J.; Bhave, N.S. Resin. III. Synthesis, characterization, and ion-exchange properties of a 2,2′-dihydroxybiphenyl-formaldehyde copolymer resin. J. Appl. Polym. Sci. 2008, 109, 508–514. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Cao, F.; Leng, Y. Thermal treatment of hair for the synthesis of sustainable carbon quantum dots and the applications for sensing Hg2+. Sci. Rep. 2016, 6, 35795. [Google Scholar] [CrossRef] [PubMed]
- Rani, U.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E.; Hairom, N.H.H. Photocatalytic degradation of crystal violet dye using sulphur-doped carbon quantum dots. Mater. Today Proc. 2021, 46, 1934–1939. [Google Scholar] [CrossRef]
- Jing, L.; Ding, Q.; Li, X.; Lou, J.; Liu, Z.; Jiang, Y.; Cheng, Z. Bifunctional collagen fiber/carbon quantum dot fluorescent adsorbent for efficient adsorption and detection of Pb2+. Sci. Total Environ. 2023, 871, 161989. [Google Scholar] [CrossRef]
- Jelinek, R. Characterization and physical properties of carbon-dots. In Carbon Quantum Dots: Synthesis, Properties and Applications; Springer: Cham, Switzerland, 2016; pp. 29–46. [Google Scholar] [CrossRef]
- Milosavljevic, V.; Nguyen, H.V.; Michalek, P. Synthesis of carbon quantum dots for DNA labeling and its electrochemical, fluorescent and electrophoretic characterization. Chem. Pap. 2015, 69, 192–201. [Google Scholar] [CrossRef]
- Hidayat, R.N.; Widiyandari, H.; Parasdila, H.; Prilita, O.; Astuti, Y.; Mufti, N.; Ogi, T. Green synthesis of ZnO photocatalyst composited carbon quantum dots (CQDs) from lime (Citrus aurantifolia). Catal. Commun. 2024, 187, 106888. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.Q.; Tang, Z.R.; Xu, Y.J. Recent progress in carbon quantum dots: Synthesis, properties and applications in photocatalysis. J. Mater. Chem. A 2017, 5, 3717–3734. [Google Scholar] [CrossRef]
- Safranko, S.; Stankovic, A.; Hajra, S.; Kim, H.J.; Strelec, I.; Dutour-Sikiric, M.; Jokic, S. Preparation of multifunctional N-doped carbon quantum dots from citrus clementina peel: Investigating targeted pharmacological activities and the potential application for Fe3+ sensing. Pharmaceuticals 2021, 14, 857. [Google Scholar] [CrossRef]
- Chávez-García, D.; Guzman, M.; Sanchez, V.; Cadena-Nava, R.D. Green synthesis of biomass-derived carbon quantum dots for photocatalytic degradation of methylene blue. Beilstein J. Nanotechnol. 2024, 15, 755–766. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H. Quantum-sized carbon dots for bright and colorful photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots: Consensus, debates and challenges. Nano Today 2015, 10, 397–436. [Google Scholar] [CrossRef]
- Malavika, V.; Anumol, R.; Rajan, M.R. Differential quantities of carbon quantum dots incorporated feed on the growth and biochemical traits of zebrafish (Danio rerio). Int. J. Agric. Technol. 2024, 20, 2393–2404. Available online: https://li04.tci-thaijo.org/index.php/IJAT/article/view/5602 (accessed on 4 November 2025).
- Yadav, S.; Choudhary, N.; Sonpal, V.; Paital, A.R. Engineering excitation-independent turn-on fluorescent probe for mercury: Functionalized dendritic silica doped with red-emissive carbon dots towards simultaneous detection and remediation with biosensing application. Chem. Eng. J. 2023, 471, 144715. [Google Scholar] [CrossRef]
- Li, P.; Yang, X.; Zhang, X.; Pan, J.; Tang, W.; Cao, W.; Xing, X. Surface chemistry-dependent antibacterial and antibiofilm activities of polyamine-functionalized carbon quantum dots. J. Mater. Sci. 2020, 55, 16744–16757. [Google Scholar] [CrossRef]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational synthesis, characterization, and application of environmentally friendly (polymer–carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environ. Sci. Eur. 2020, 32, 12. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Ji, W.-Q.; Chen, S. Direct Synthesis of Multicolor Fluorescent Hollow Carbon Spheres Encapsulating Enriched Carbon Dots. Sci. Rep. 2016, 6, 19382. [Google Scholar] [CrossRef]
- Chen, G.; Wu, S.; Hui, L.; Zhao, Y.; Ye, J.; Tan, Z.; Zeng, W.; Tao, Z.; Yang, L.; Zhu, Y. Assembling carbon quantum dots to a layered carbon for high-density supercapacitor electrodes. Sci. Rep. 2016, 6, 19028. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Mandal, T.K. Synthesis of Highly Fluorescence Nitrogen Doped Carbon Quantum Dots Bioimaging Probe, and its in vivo Clearance and Printing Applications. RSC Adv. 2016, 6, 18134–18140. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, L.; Cai, J.; Fang, Q.; Chi, Y.; Chen, G. Natural carbon-based dots from humic substances. Sci. Rep. 2015, 5, 10037. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.; Mahdavi, K. Green synthesis of high photoluminescence nitrogen-doped carbon quantum dots from grass via a simple hydrothermal method for removing organic and inorganic water pollutions. Appl. Surf. Sci. 2019, 463, 283–291. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Fekry, N.A.; Abdelfattah, A.M. Removal of uranium (VI) from water by the action of microwave-rapid green synthesized carbon quantum dots from starch-water system and supported onto polymeric matrix. J. Hazard. Mater. 2020, 397, 122770. [Google Scholar] [CrossRef]
- Pant, M.; Kumar, S.; Kiran, K.; Bisht, N.S.; Pande, V.; Dandapat, A. A universal green approach for the synthesis of NPS-codoped carbon quantum dots with enhanced broad-spectrum antibacterial and antioxidant activities. RSC Adv. 2023, 13, 9186–9194. [Google Scholar] [CrossRef]
- Zhai, Z.; Dong, X.; Qi, H.; Tao, R.; Zhang, P. Carbon quantum dots with high photothermal conversion efficiency and their application in photothermal modulated reversible deformation of poly(n-isopropylacrylamide) hydrogel. ACS Appl. Bio Mater. 2023, 6, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, A.; Hagos, M.; RamaDevi, D.; Basavaiah, K.; Belachew, N. Fluorescent-nitrogen-doped carbon quantum dots derived from citrus lemon juice: Green synthesis, mercury (II) ion sensing, and live cell imaging. ACS Omega 2020, 5, 3889–3898. [Google Scholar] [CrossRef]
- Rajan, M.R.; Rohini, R. Effects of zinc oxide nanoparticles on growth and hematological parameters of Cirrhinus mrigala. J. Water Environ. 2021, 6, 62–71. [Google Scholar] [CrossRef]
- Sangeetha, K.; Rajan, M.R. Evaluation of Different Quantity of Iron Oxide Nanoparticles on Growth, Haematological and Biochemical Characteristics of Koi Carp. Agric. Sci. Dig. 2021, 41, 338–344. [Google Scholar] [CrossRef]
- Rajan, M.R.; Meenakumari, B. Impact of differential quantities of magnesium oxide nanoparticles on growth, haematological and biochemical characteristics of common carp Cyprinus carpio. Int. J. Creat. Res. Thoughts 2023, 11, d811–d822. [Google Scholar]
- Rašković, B.; Stanković, M.; Marković, Z.; Poleksić, V. Histological methods in the assessment of different feed effects on liver and intestine of fish. J. Agric. Sci. 2011, 56, 87–100. [Google Scholar] [CrossRef]
- Rašković, B.; Stanković, M.; Dulić, Z.; Marković, Z.; Lakić, N.; Poleksić, V. Effects of different source and level of protein in feed mixtures on liver and intestine histology of the common carp (Cyprinus carpio, Linnaeus, 1758). Comp. Biochem. Physiol. A 2009, 153, S112. [Google Scholar] [CrossRef]
- Poleksić, V.; Rašković, B.; Marković, Z.; Dulić, Z.; Stanković, M.; Živić, I.; Lakić, N. Effects of different dietary protein sources on intestine and liver morphology of carp yearlings. In Proceedings of the 3rd Serbian Congress for Microscopy; Serbian Microscopy Society: Belgrade, Serbia, 2007; Volume 56, pp. 237–238. [Google Scholar]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).