Nano-Titania Photocatalysis and Metal Doping to Deter Fungal Growth on Outdoor and Indoor Paint Surfaces Using UV and Fluorescent Light
Abstract
1. Introduction
2. Methods
2.1. Paint Preparation
2.2. Determination of Fungal Species Following 9 Months of Outdoor Exposure
2.2.1. Preparation of A. niger Spores
2.2.2. Exposure of Different Paint Formulations Containing Commercial Biocide, TiO2 (Nano-Titania) or Commercial Biocide and TiO2 Seeded with A. niger Spores
2.2.3. Effect of Chemical Doping on A. niger Growth on the Paint Surfaces
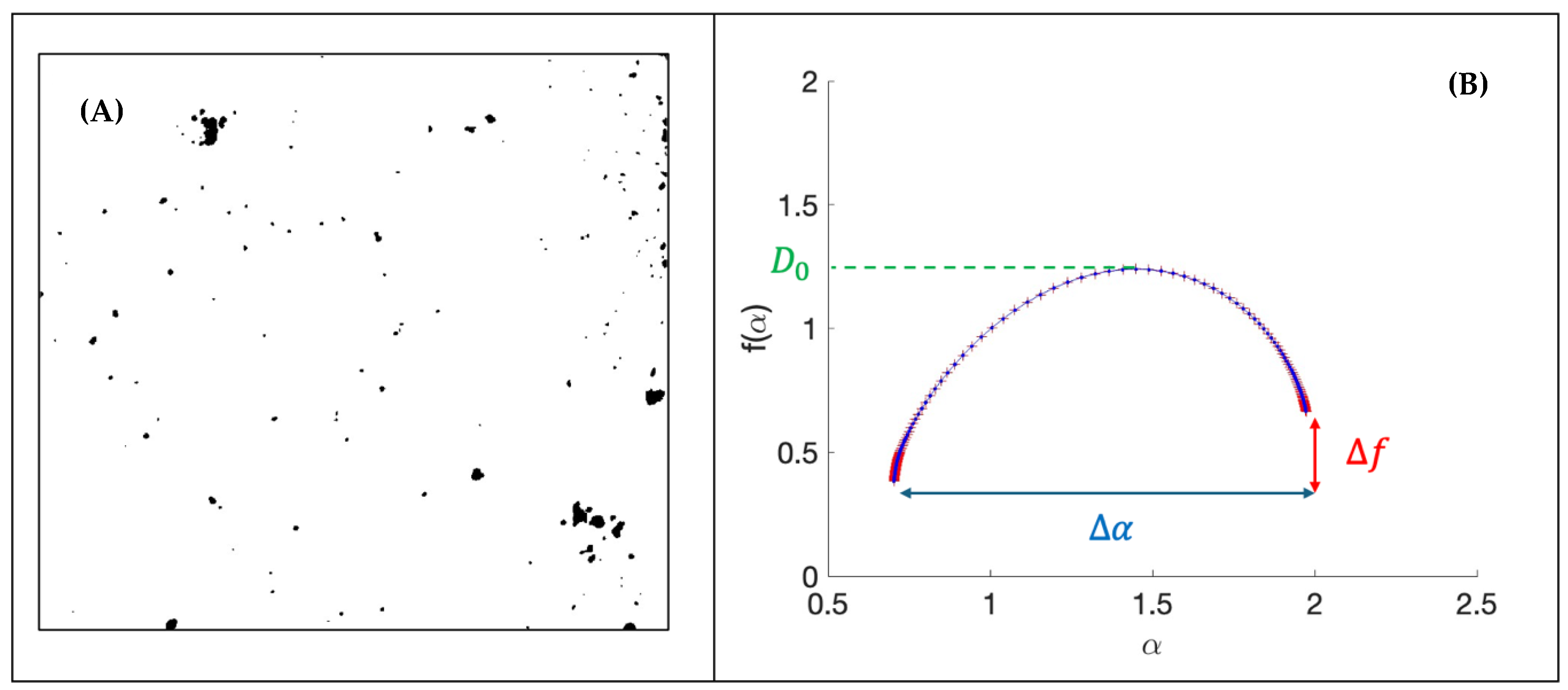
2.2.4. Multifractal Analysis
3. Results
3.1. Growth and Identification of Fungal Species Following 9 Months of Outdoor Exposure
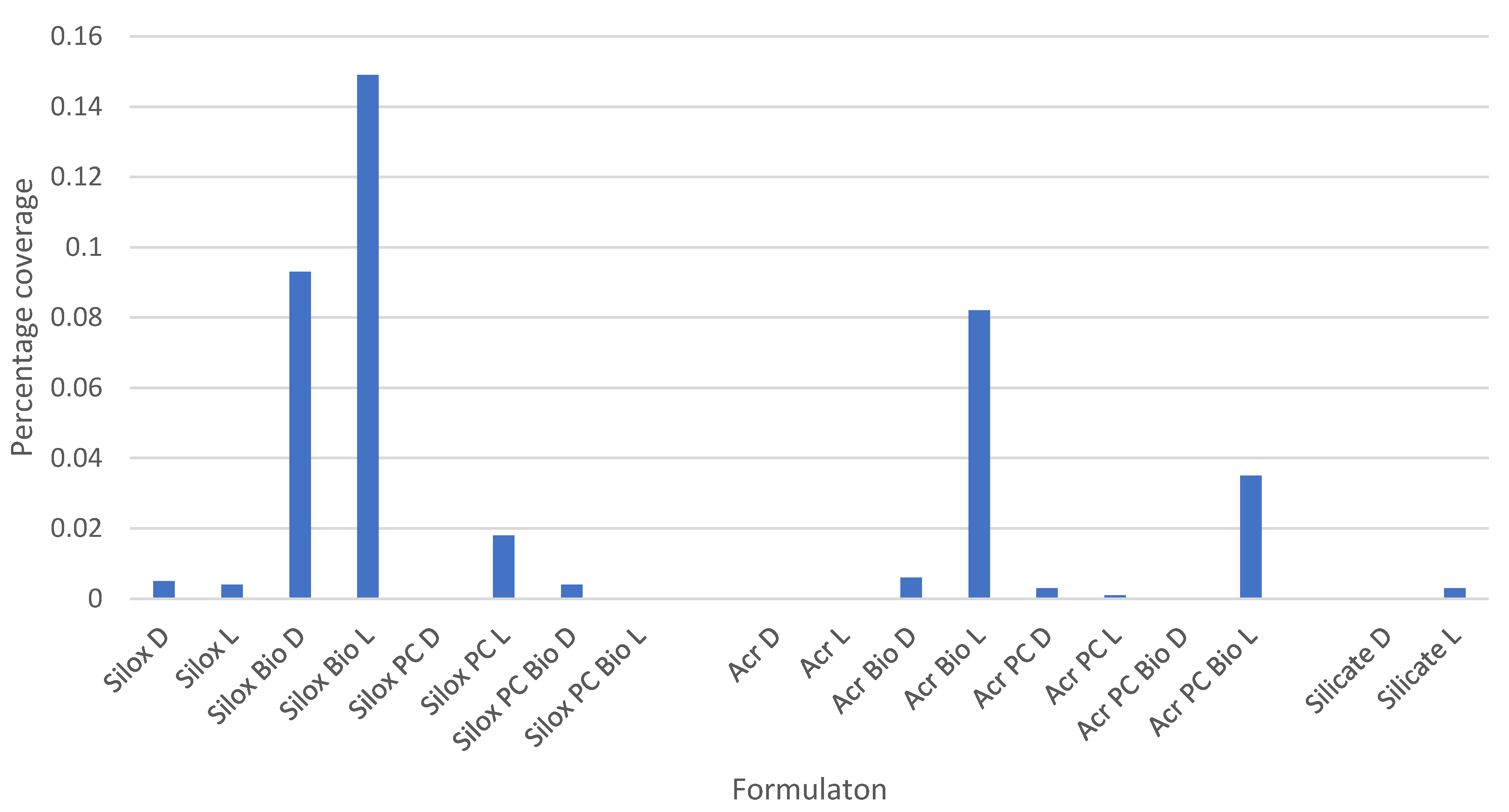
3.2. Exposure of Different Paint Formulations Seeded with A. niger Spores and Exposed to UV
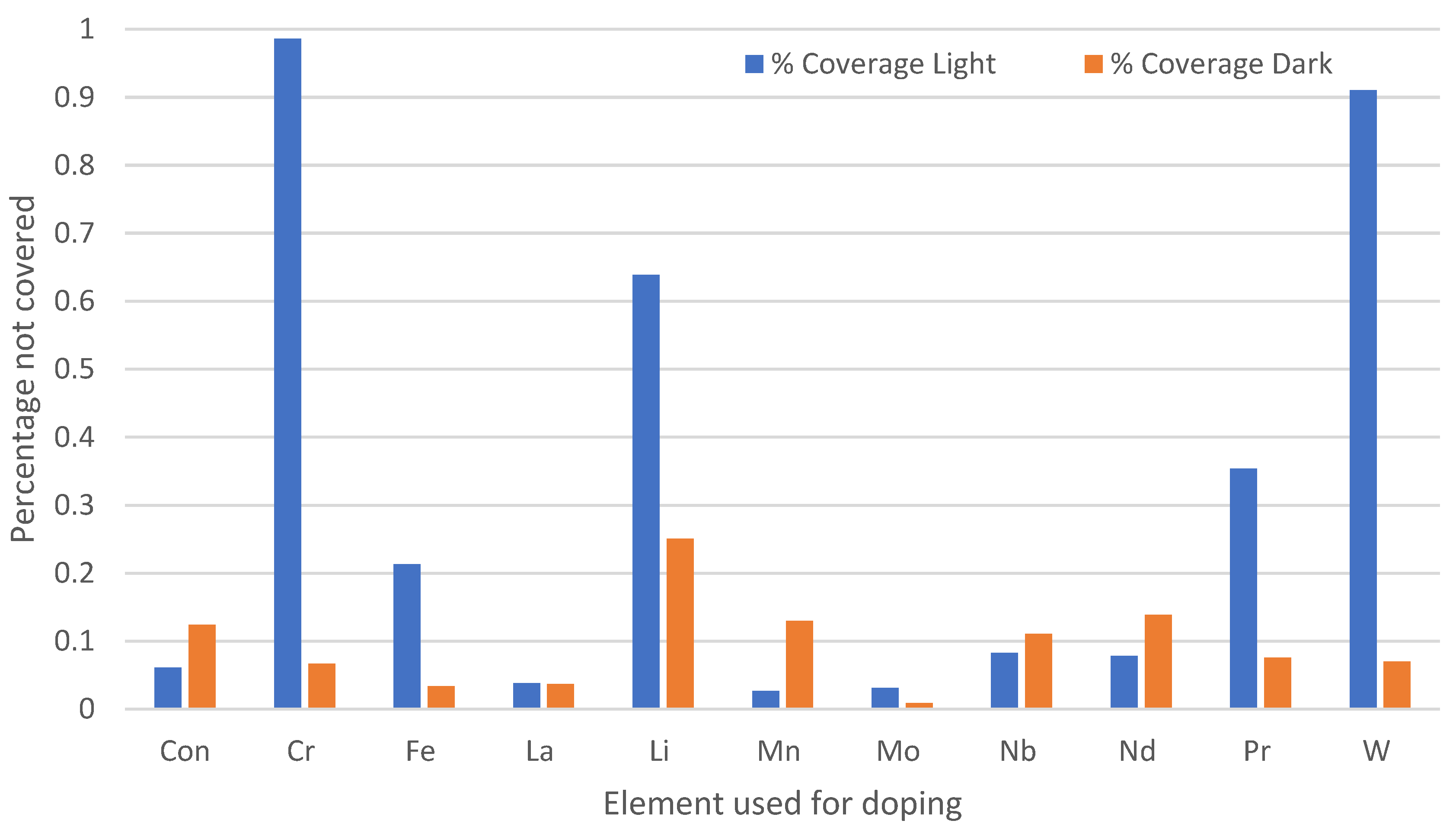
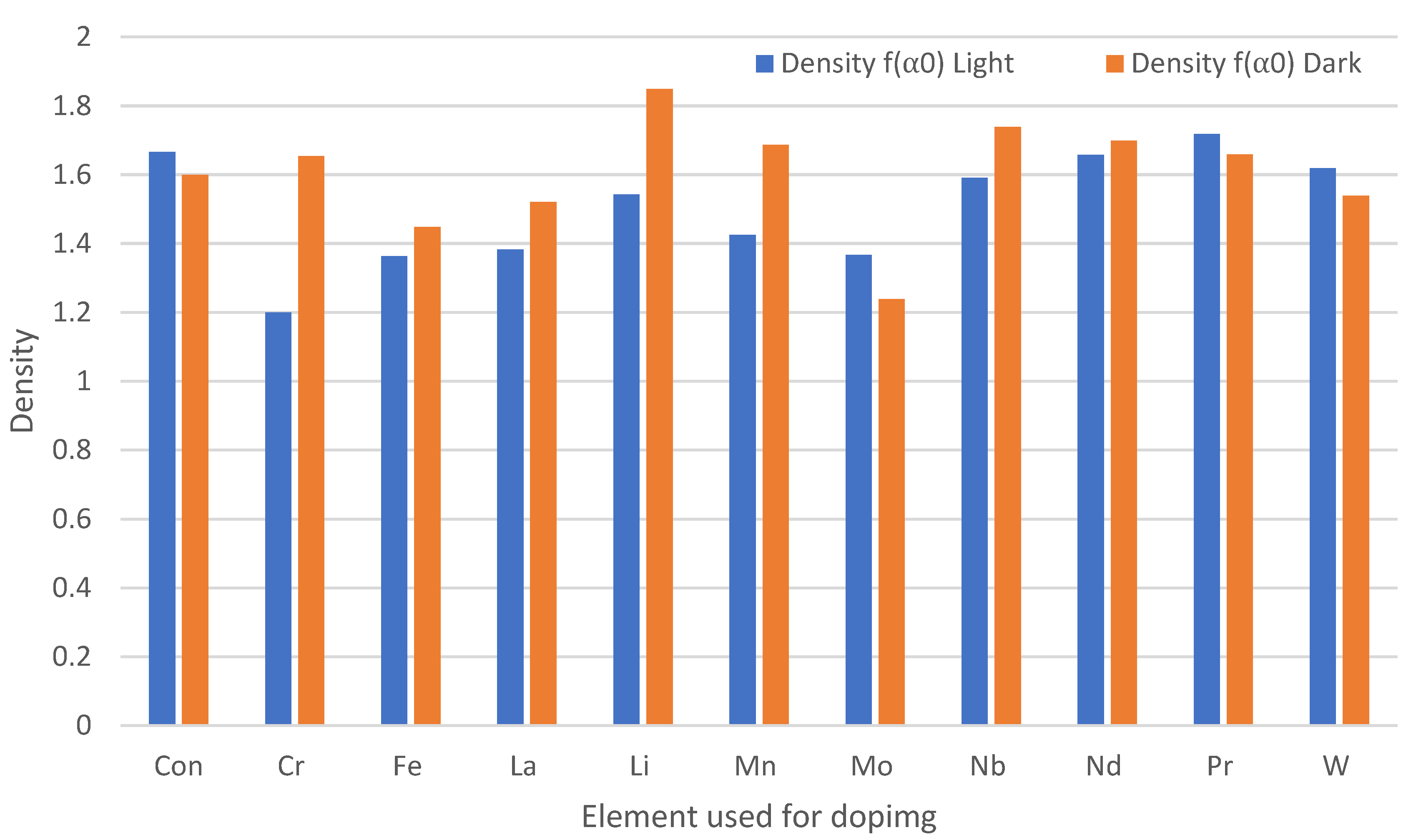
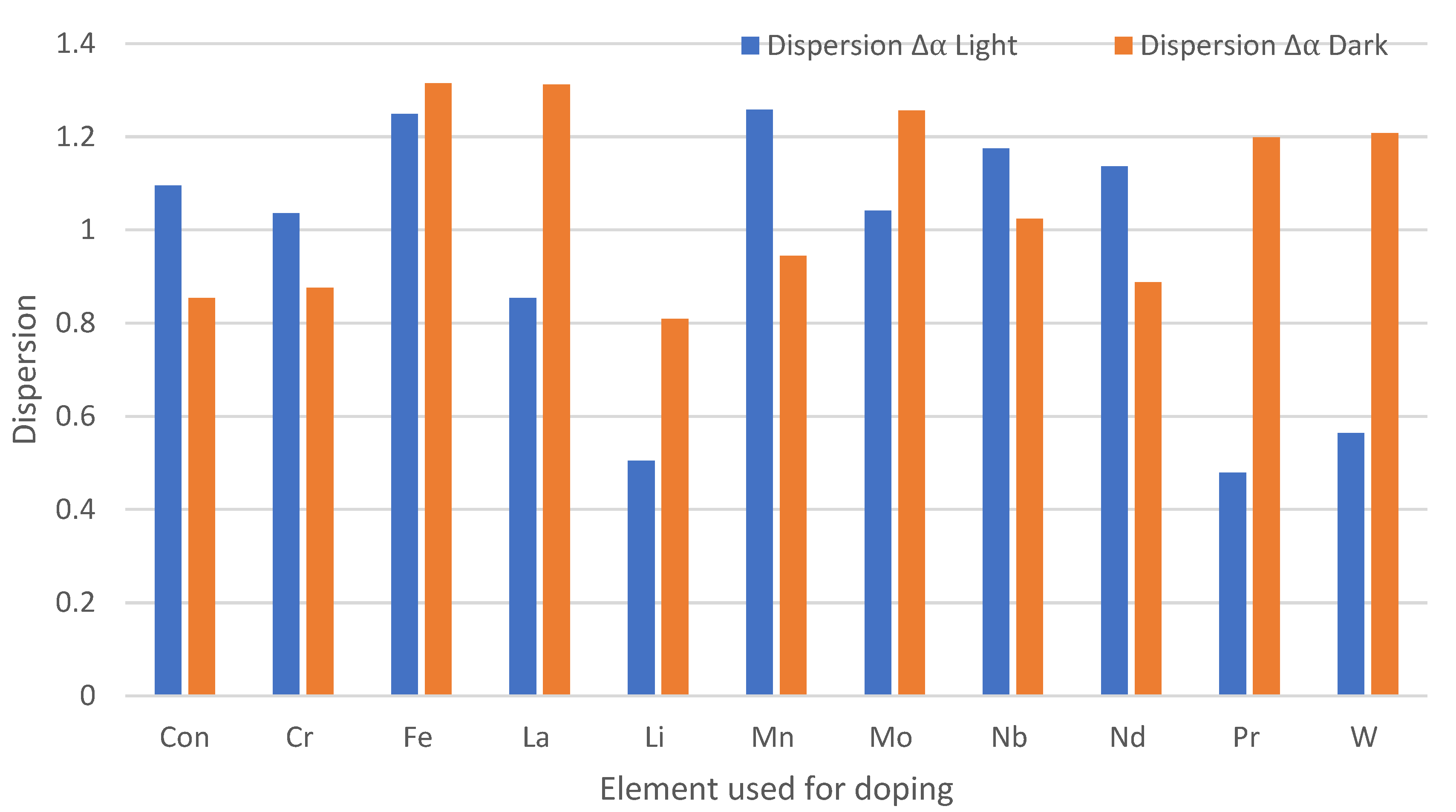
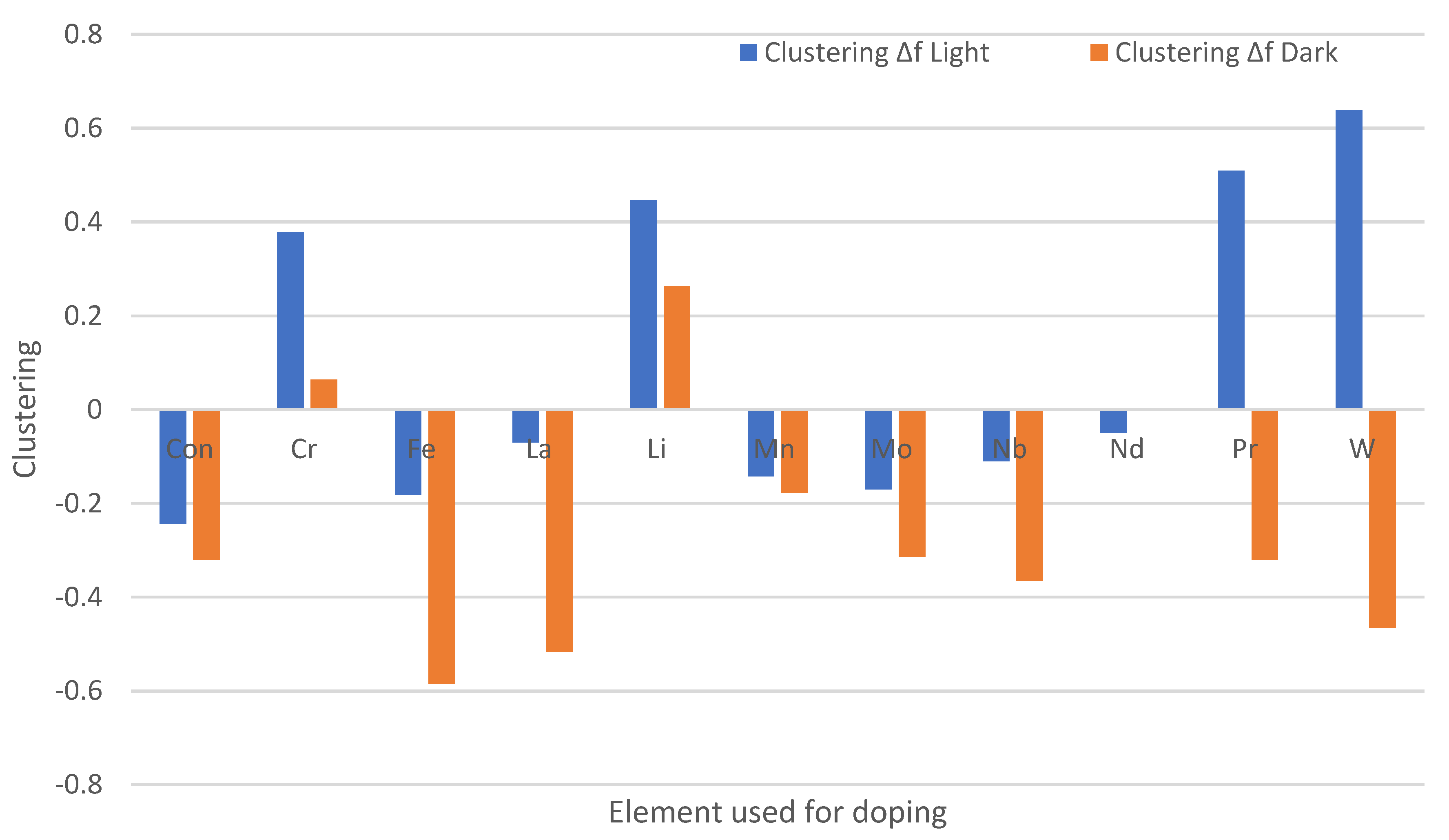
3.3. Effect of Chemical Doping on A. niger Growth on the Paint Surfaces Under Fluorescent Lights
4. Discussion
4.1. Effect of Paints on Outdoor Colonisation by Fungi
4.2. Different Paint Formulations in Light and Dark Conditions on Fungal Growth Using UV
4.3. Antifungal Efficacy of Doped Paints with Fluorescent Lights
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, N.S.; Edge, M.; Verran, J.; Stratton, J.; Maltby, J.; Bygott, C. Photocatalytic titania based surfaces: Environmental benefits. Polym. Degrad. Stab. 2008, 93, 1632–1646. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Buxbaum, G.; Pfaff, G. (Eds.) Industrial Inorganic Pigments, 3rd ed.; WILEY-VCH Verlag GmbH&Co: Hoboken, NJ, USA, 2005. [Google Scholar]
- Wakamura, M. Antibacterial, Antifouling Paint for Construction Materials and Construction Materials Coated Therewith. U.S. Patent US7157503B2, 2 January 2007. [Google Scholar]
- Goodwin, G.; Stratton, J.; McIntyre, R. Coating Composition Having Surface Depolluting Properties. U.S. Patent US20070167551A1, 3 July 2007. [Google Scholar]
- Gambogi, J. Titanium and Titanium Dioxide. In Mineral Commodity Summaries; U.S. Department of the Interior, U.S. Geological Survey, Science for a Changing World: Reston, VA, USA, 2009; pp. 176–177. [Google Scholar]
- Stratton, J. Depolluting Coating Composition. U.S. Patent US20080097018A1, 24 April 2008. [Google Scholar]
- Caballero, L.; Whitehead, K.A.; Allen, N.S.; Verran, J. Inactivation of E. coli on immobilized TiO2 using fluorescent light. J. Photochem. Photobiol. A: Chem. 2009, 202, 92–98. [Google Scholar] [CrossRef]
- Caballero, L.; Whitehead, K.A.; Allen, N.S.; Verran, J. Photoinactivation of Escherichia coli on acrylic paint formulations using fluorescent light. Dye. Pigment. 2010, 86, 56–62. [Google Scholar] [CrossRef]
- Caballero, L.; Whitehead, K.A.; Allen, N.A.; Verran, J. Photocatalytic inactivation of Escherichia coli using doped titanium dioxide under fluorescent irradiation. J. Photochem. Photobiol A Chem. 2014, 276, 50–57. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C: Photochem. Rev. 2005, 6, 186–205. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherichia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Cat. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Pichat, P.; Guillard, C.; Amalric, L.; Renard, A.; Plaidy, O. Assessment of the importance of the role of H2O2 and O2•- in the photocatalytic degradation of 1, 2-dimethoxybenzene. Solar Energy Mat. Solar Cells. 1995, 38, 391–399. [Google Scholar] [CrossRef]
- Rincon, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Cat. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- ASTM D5590-17; Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay. ASM International D5590 Standard Test Method for Determining the Resistance of Paint Films and Related Coatings to Fungal Defacement by Accelerated Four-Week Agar Plate Assay. ASTM: West Conshohocken, PA, USA, 2021.
- Linsebigler, A.L.; Lu, G.Q.; Yates, J.T. Photocatalysis on TiO2 surfaces—principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Solomon, D.H.; Hawthorne, D.G. Chemistry of Pigments and Fillers; Wiley: Hoboken, NJ, USA, 1983; pp. 51–80. [Google Scholar]
- Koleske, J.V. (Ed.) Paint and Coating Testing Manual, 14th ed.ASTM International: West Conshohocken, PA, USA, 1995. [Google Scholar]
- Lynch, S. Python for Scientific Computing and Artificial Intelligence; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Lynch, S. Dynamical Systems with Applications Using MATLAB, 3rd ed.; Springer International Publishing: New York, NY, USA , 2024; in press. [Google Scholar]
- Cappitelli, F.; Vicini, S.; Piaggio, P.; Abbruscato, P.; Princi, E.; Casadevall, A.; Nosanchuk, J.D.; Zanardini, E. Investigation of Fungal Deterioration of Synthetic Paint Binders Using Vibrational Spectroscopic Techniques. Macromol. Biosci. 2005, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wirth, A.; Pacheco, F.; Toma, N.; Valiati, V.; Tutikian, V.; Gomes, L. Analysis of fungal growth on different coatings applied to lightweight systems. Rev. Ing. Construcción 2019, 34, 5–14. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Gaylarde, C.C.; Gaylarde, P.M.; John, V.; Gambale, W. Fungal Colonization and succession on newly painted buildings and the effect of biocide. FEMS Microbiol. Ecol. 2002, 39, 165–173. [Google Scholar] [CrossRef]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Cortesão, M.; de Haas, A.; Unterbusch, R.; Fujimori, A.; Schütze, T.; Meyer, V.; Moeller, R. Aspergillus niger Spores Are Highly Resistant to Space Radiation. Front Microbiol. 2020, 3, 560. [Google Scholar] [CrossRef]
- Singaravelan, N.; Grishkan, I.; Beharav, A.; Wakamatsu, K.; Ito, S.; Nevo, E. Adaptive melanin response of the soil fungus Aspergillus niger to UV radiation stress at “evolution canyon”, mount carmel, Israel. PLoS ONE 2008, 3, e2993. [Google Scholar] [CrossRef]
- Taylor-Edmonds, L.; Lichi, T.; Rotstein-Mayer, A.; Mamane, H. The impact of dose, irradiance and growth conditions on Aspergillus niger (renamed A. brasiliensis) spores low-pressure (LP) UV inactivation. J. Environ. Sci. Health Part A 2015, 50, 341–347. [Google Scholar] [CrossRef]
- Bickley, R.I.; Gonzalez-Carreno, T.; Lees, J.S.; Palmisano, L.; Tilley, R.J.D. Structural investigation of titanium dioxide photocatalysts. J. Solid State Chem. 1991, 92, 178–190. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Liu, J.; Zhou, M.; Xu, H.; Xie, M.; Li, H.; Xie, J. Direct Z-scheme red carbon nitride/rod-like lanthanum vanadate composites with enhanced photodegradation of antibiotic contaminants. Appl. Cat. B Environ. 2020, 277, 119245. [Google Scholar] [CrossRef]
- Khan, A.A.; Partho, A.T.; Arnab, M.H.; Khyam, M.A.; Kumar, N.; Tahir, M. Recent advances in Lanthanum-based photocatalysts with engineering aspects for photocatalytic hydrogen production: A critical review. Mat. Sci. Semicond. Process. 2024, 184, 108809. [Google Scholar] [CrossRef]
- Kemp, T.J.; McIntyre, R.A. Photodegradation of DNA induced by modified forms of titanium dioxide. Prog. React. Kinet. Mech. 2007, 32, 219–229. [Google Scholar] [CrossRef]
- Mir, S.M.; Shaikh, K.R.; Pawar, A.R.; Undre, P.B. Microwave-assisted synthesis and functionalization of nanocrystalline molybdenum trioxide for enhanced antimicrobial and photocatalytic activity. J. Indian Chem. Soc. 2024, 101, 101288. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Pramila, S.; Nagaraju, G.; Lakshmi Ranganatha, V. Enhanced photocatalytic, electrochemical and antimicrobial activities of alpha-Mn2V2O7 nanopebbles. J. Mat. Sci. Mat. Elec. 2021, 33, 617–634. Available online: https://api.semanticscholar.org/CorpusID:244532565 (accessed on 6 September 2024). [CrossRef]
- Alghool, S.; Abd El-Halim, H.F.; Abd El-sadek, M.A.; Yahia, I.S.; Wahab, L.A. Synthesis, thermal characterization, and antimicrobial activity of lanthanum, cerium, and thorium complexes of amino acid Schiff base ligand. J. Thermal Anal. Calorim. 2013, 112, 671. [Google Scholar] [CrossRef]
- Tétault, N.; Gbaguidi-Haore, H.; Bertrand, X.; Quentin, R.; van der Mee-Marquet, N. Biocidal activity of metalloacid-coated surfaces against multidrug-resistant microorganisms. Antimicrob. Resist. Infect. Control. 2012, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- El-Habeeb, A.A.; Refat, M.S. Synthesis and Spectroscopic Investigations of Four New Y(III), Ge(IV), W(VI) and Si(IV) Penicillin Antibiotic Drug Complexes. Spectrosc. Spectr. Anal. 2019, 39, 2982–2988. [Google Scholar]
- Umar, M.; Ajaz, H.; Javed, M.; Mansoor, S.; Iqbal, S.; Rauf, A.; Alhujaily, A.; Awwad, N.S.; Ibrahium, H.S.; Althobiti, R.A.; et al. Design of a highly efficient heterostructure of transition metal tellurides with outstanding photocatalytic and antimicrobial potential. J. Saudi Chem. Soc. 2023, 27, 101760. [Google Scholar] [CrossRef]
- Mohandoss, N.; Renganathan, S.; Subramaniyan, V.; Nagarajan, P.; Elavarasan, V.; Subramaniyan, P.; Vijayakumar, S. Investigation of Biofabricated Iron Oxide Nanoparticles for Antimicrobial and Anticancer Efficiencies. Appl. Sci. 2022, 12, 12986. [Google Scholar] [CrossRef]
- Ismail, A.S.; Tawfik, S.M.; Mady, A.H.; Lee, Y.-I. Preparation, Properties, and Microbial Impact of Tungsten (VI) Oxide and Zinc (II) Oxide Nanoparticles Enriched Polyethylene Sebacate Nanocomposites. Polymers 2021, 13, 718. [Google Scholar] [CrossRef]
- Blatzer, M.; Latgé, J.P. Metal-homeostasis in the pathobiology of the opportunistic human fungal pathogen Aspergillus fumigatus. Curr. Opin. Microbiol. 2017, 40, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Todorova, D.; Nedeva, D.; Abrashev, R.; Tsekova, K. Cd (II) stress response during the growth of Aspergillus niger B 77. J. Appl. Microbiol. 2008, 104, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Lobos, A.; Harwood, V.J.; Scott, K.M.; Cunningham, J.A. Tolerance of three fungal species to lithium and cobalt: Implications for bioleaching of spent rechargeable Li-ion batteries. J. Appl. Microbiol. 2021, 131, 743–755. [Google Scholar] [CrossRef]
- Dursun, A.Y.; Uslu, G.; Cuci, Y.; Aksu, Z. Bioaccumulation of copper(II), lead(II) and chromium(VI) by growing Aspergillus niger. Process Biochem. 2003, 38, 1647–1651. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Lynch, S.; Amin, M.; Deisenroth, T.; Liauw, C.M.; Verran, J. Effects of Cationic and Anionic Surfaces on the Perpendicular and Lateral Forces and Binding of Aspergillus niger Conidia. Nanomaterials 2023, 11, 2932. [Google Scholar] [CrossRef] [PubMed]


| Type of paint | Fungal species recovered | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | Pen1 | Asp1 | Gli | Asp2 | Asp3 | Asp4 | Pen2 | Asp5 | Asp6 | Y1 | Hun | Pae | Y2 | Gli | Cla | Total |
| Siloxane | 14 | |||||||||||||||
| Acrylic | 16 | |||||||||||||||
| Silicone | 10 | |||||||||||||||
| Control | 7 | |||||||||||||||
| (B) | Pen1 | Asp1 | Gli | Asp2 | Asp3 | Asp4 | Pen2 | Asp5 | Asp6 | Y1 | Hun | Pae | Y2 | Gli | Cla | Total |
| Siloxane | 12 | |||||||||||||||
| Acrylic | 12 | |||||||||||||||
| Silicone | 8 | |||||||||||||||
| Control | 4 | |||||||||||||||
| (C) | Pen1 | Asp1 | Gli | Asp2 | Asp3 | Asp4 | Pen2 | Asp5 | Asp6 | Y1 | Hun | Pae | Y2 | Gli | Cla | Total |
| Siloxane | 13 | |||||||||||||||
| Acrylic | 12 | |||||||||||||||
| Silicone | 11 | |||||||||||||||
| Control | 10 | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whitehead, K.A.; Brown, M.; Caballero, L.; Lynch, S.; Edge, M.; Hill, C.; Verran, J.; Allen, N.S. Nano-Titania Photocatalysis and Metal Doping to Deter Fungal Growth on Outdoor and Indoor Paint Surfaces Using UV and Fluorescent Light. Micro 2025, 5, 5. https://doi.org/10.3390/micro5010005
Whitehead KA, Brown M, Caballero L, Lynch S, Edge M, Hill C, Verran J, Allen NS. Nano-Titania Photocatalysis and Metal Doping to Deter Fungal Growth on Outdoor and Indoor Paint Surfaces Using UV and Fluorescent Light. Micro. 2025; 5(1):5. https://doi.org/10.3390/micro5010005
Chicago/Turabian StyleWhitehead, Kathryn A., Mark Brown, Lucia Caballero, Stephen Lynch, Michele Edge, Claire Hill, Joanna Verran, and Norman S. Allen. 2025. "Nano-Titania Photocatalysis and Metal Doping to Deter Fungal Growth on Outdoor and Indoor Paint Surfaces Using UV and Fluorescent Light" Micro 5, no. 1: 5. https://doi.org/10.3390/micro5010005
APA StyleWhitehead, K. A., Brown, M., Caballero, L., Lynch, S., Edge, M., Hill, C., Verran, J., & Allen, N. S. (2025). Nano-Titania Photocatalysis and Metal Doping to Deter Fungal Growth on Outdoor and Indoor Paint Surfaces Using UV and Fluorescent Light. Micro, 5(1), 5. https://doi.org/10.3390/micro5010005







