1. Introduction
Autism spectrum disorder (ASD) is a developmental disability that impacts behavior and social communication [
1]. According to the National Institute of Mental Health, ASD can be diagnosed at any age; its onset usually happens in early childhood, and symptoms usually show up in the first two years of life [
2]. In the U.S., around 2.2% of adults and 2.3% of 8-year-old children suffer from ASD [
3]. To date, there are no established biomarkers available for diagnostic screening of ASD as it is primarily diagnosed through clinical examination, behavioral evaluation, and observations by healthcare providers such as neurologists, pediatricians, or psychiatrists [
4]. ASD can present itself in a variety of ways, resulting in a broad range of strengths and challenges for each person. It is characterized by confined and repetitive activities as well as difficulties with social interaction and communication [
5]. Some people may find it difficult to read facial emotions, keep eye contact, or participate in conversation with others [
6]. They could also exhibit repetitive activities or show strong interest in particular subjects.
Despite years of extensive research, no single cause has been attributed to autism spectrum disorder (ASD) [
7]. Researchers have determined that the disorder can be caused by a combination of biological factors, genetic factors, and environmental factors [
8].
Current therapies and interventions exist to combat challenges to ASD that individuals may face. These strategies include behavioral therapy, which is a research-based therapy that provides a 1:1 ratio of services that implement applied behavior analysis, or ABA. Early intensive behavioral intervention, or EIBI, is a specific methodology that ABA incorporates into therapies geared for young children. Included in this approach is a variety of methods such as natural environment teaching (NET) and discrete trial training (DTT) to engage children in a combination of learning activities [
9].
Implementing ABA into the lives of young children diagnosed with ASD has shown to result in decreases in maladaptive behaviors (e.g., property destruction, aggression) often exhibited by children on the spectrum [
10]. Additionally, ABA focuses on replacement skills for those maladaptive behaviors displayed by those with ASD [
11]. This may be accomplished by increasing skills of communication through manding (requesting), increasing verbal utterances, tacting (object identification), and providing eye contact when engaging in conversation. Social skills are also essential when incorporating replacement skills in lieu of problem behaviors. This can include teaching appropriate replacement skills like sharing or providing others personal space. This reduces the chances of isolation that an individual may experience.
In addition to EIBI practices, children diagnosed with ASD often receive occupational therapy, or OT, which is a practice geared toward assisting individuals to take part in activities and occupations essential to everyday life. Self-care, leisure activities, and productivity are some aspects that work toward accomplishing the goal of OT: to support those in need of gaining the tools necessary for independent living [
12].
Speech language therapy (SLT) also known as speech language pathology (SLP), often completes the trifecta of early intervention strategies utilized in ABA. According to the American Speech–Language–Hearing Association, SLT encompasses a wide range of challenges in communication often paired with ASD such as concerns with speech patterns, literacy, social communication, the understanding of linguistics and expression, voice quality, fluency, and cognitive communication skills, as well as feeding or swallowing complications [
13].
In
Figure 1, the different therapeutic treatments of ASD are highlighted to provide a basis of understanding on how treatment for the disorder can be allotted. Some individuals may benefit from different combinations of treatment methodologies, and some therapy types are specific to age and function level.
One should take note that most treatments of ASD are therapeutic; few advancements have been made in terms of affective medication for treating ASD [
15]. Currently, there is no sole medication with the power to produce substantial results toward eliminating ASD; however, there are drugs that can be prescribed to target the varying symptoms often coupled with the disorder. As research into pharmaceutical interventions resume, the focus will often address specific symptoms or co-occurring conditions rather than target the elimination of ASD itself.
Nanotechnology involves the manipulation and control of matter on an exceptionally small scale. Typically, nanoparticles ranging from 1 to 100 nanometers (nm) in size as well as submicron-sized polymer particles ranging from 100 to 1000 nanometers are utilized within this field [
16]. For perspective, a nanometer is approximately 1/100,000th of the diameter of a human hair [
17]. At this scale, materials experience significant alterations in properties due to quantum effects and a heightened ratio of surface area to volume. These changes result in novel physical, chemical, and biological capabilities. Through the design, modification, and utilization of materials at the nanoscale, scientists and engineers can innovate new products, technologies, and applications across various industries. Numerous fields, including electronics, material science, energy, environmental research, and medicine, have found extensive uses for nanotechnology. The application of nanotechnology to medicine for preventive, therapeutic, and diagnostic reasons is known as nanomedicine [
18]. It entails building and modifying nanoscale structures and devices to accomplish particular goals and results. This topic is still growing and has a lot of room for innovation across many scientific and technological fields such as orthopedics, oncology, and radiology [
19].
Nanoparticles have been used in recent studies to help understand the pharmacological mechanisms underlying the treatment of ASD. One of the drugs that have shown success in targeting the treatment of ASD is the FDA-approved drug bumetanide. Bumetanide has shown a positive outlook on the treatment of ASD symptoms, specifically on the social deficits that an individual may encounter. Despite the impact that bumetanide has demonstrated within animal models and individuals with ASD, there is persistent controversy, as the regions within the brain as well as specific cell type mechanisms through which bumetanide operates in individuals with ASD remain unclear. The accessibility of nanoparticles offers a feasible method for unveiling pharmacological mechanisms applicable in addressing ASD [
15]. In a recent study titled “Nanoformulated Bumetanide Ameliorates Social Deficiency in BTBR Mice Model of Autism Spectrum Disorder”, researchers administered bumetanide along with a controlled drug to BTBR mice as they were subjected to various behavioral tests. The administration of 100 μM of poly (ethylene glycol)-poly(l-lactide) (PEG-PLA) nanoparticles, namely NP(bumetanide), in the medial prefrontal cortex (mPFC) in a region-specific manner ameliorated the social abnormalities seen in BTBR animals. Compared to control mice, untreated BTBR mice exhibited less interaction and spent less time in the social stimulus box during the sociability stage. These societal deficiencies were not completely resolved by bumetanide. To counteract the preference for familiar stimuli observed in untreated BTBR mice, treated animals spent more time with a new stimulus during the social novelty stage. Additionally, bumetanide reduced recurrent grooming habits. In BTBR mice, the medication had no discernible effect on anxiety or locomotor activity. Overall, in BTBR mice, bumetanide demonstrated promise in reducing autistic-like behaviors without significantly affecting anxiety or locomotion [
15].
Poly (ethylene glycol)-poly(l-lactide) (PEG-PLA) is just an example of one type of nanoparticle used for the delivery of drugs within the central nervous system.
Figure 2 highlights the varying types of nanoparticles along with some of their attributes.
Figure 2 delineates various types of nanoparticles (NPs) employed for drug delivery to the central nervous system, including: (a) a schematic representation of the dendrimer structure, highlighting the core, interior, and periphery; (b) a depiction of the molecular structure of carbon nanotubes; (c) various NPs utilized for transporting drugs to the central nervous system; (d) a schematic representation of a typical nanomicelle; (e) an illustration of a standard drug-loaded nanoliposome; and (f) a depiction of a typical carbon-60 fullerene-based structure used for encapsulating neuroprotective compounds and nanocapsules embedding drugs in the polymer matrix [
20].
2. Prenatal Prevention
In another study conducted by Bolandparvaz et al., the findings revealed that approximately 25% of cases of autism spectrum disorder (ASD) have a connection to maternal autoantibodies that cross the placenta and interact with particular proteins in the developing fetal brain, which may cause neurodevelopmental problems in ASD. Because of the permeability of the blood–brain barrier during fetal development, maternal antibodies, especially those of the Immunoglobulin G (IgG) type, can enter the developing fetal brain. These antibodies are transported across the placenta through the neonatal Fc receptor [
21]. Seven proteins in the embryonic brain that these maternal autoantibodies target have been found recently, which raises questions regarding their possible effects on fetal development. Although the precise etiology of these autoantibodies is unknown, genetic predisposition, molecular mimicry, and the loss of self-tolerance are all potential explanations. Research conducted on animal models has demonstrated that these IgG antibodies, when transferred from the mothers of children diagnosed with ASD, cause long-term behavioral alterations in offspring that closely resemble the characteristics of autistic individuals [
22]. Eliminating these mother autoantibodies may help prevent infants from developing autism.
In order to scavenge disease-propagating maternal autoantibodies linked to MAR autism from maternal blood, researchers created Systems for Nanoparticle-based Autoantibody Reception and Entrapment (SNAREs). They created 15 nm dextran iron oxide nanoparticles that were altered using LDH B peptide, citric acid, and methoxy PEG (10 kDa) amine. In lab experiments, the macrophage absorption of SNAREs was considerably lower than that of control nanoparticles. The study’s main accomplishment was proving that SNAREs could successfully eliminate 90% of LDH B autoantibodies from patient-derived serum [
21]. Targeting these IgG antibodies before they can be transferred through the BBB would be quite beneficial. According to this study, SNAREs may be a ground-breaking preventive treatment for MAR autism.
Other studies focus on the prenatal prevention of ASD as well. For example, in the study “Hesperetin and It Nanocrystals Ameliorate Social Behavior Deficits and Oxido-Inflammatory Stress In Rat Model of Autism”, it focuses on prenatal valproic acid (VPA) exposure. VPA is related to increased oxidative and inflammatory stress and behavioral abnormalities induction, which are frequently linked to autism spectrum disorders [
23]. VPA, which is frequently used for specific bipolar disorders, has been linked to learning disabilities, autistic-like symptoms, and aberrant conduct. According to research, in an animal model of autism caused by prenatal VPA exposure, administering Hesperetin (Hst) in the form of nanocrystals averaging 450 nm in size, can lessen oxido-inflammatory stress and ameliorate autistic-like symptoms. Hesperetin treatment, especially in the form of nanocrystals, significantly decreased inflammatory markers in plasma, improved behavioral problems, and reduced oxidative stress in the brain [
24,
25].
3. Overcoming Challenges to the Blood–Brain Barrier
The blood–brain barrier (BBB), a mechanism that stops foreign substances from passing from the blood to the extracellular fluid of the brain, is mostly responsible for controlling the administration of medications to the central nervous system (CNS). Certain medications’ physicochemical characteristics prevent them from passing across the blood–brain barrier and from reaching subtherapeutic concentrations in the tissues they are intended to treat [
26]. Typically, many therapeutic agents are unable to reach the CNS in modern medicine due to the difficulty of transporting medications over the blood–brain barrier (BBB) [
27]. One kind of nanoparticle that has shown promise in medication delivery is carbon dots (CDs). The distinct “core-shell” nanostructures of carbon dots are defined by carbon cores that are usually less than 20 nm. These cores show mild graphitization or extremely dehydrated polymer frames that are crosslinked. These cores are surrounded by shells made up of many polymer chains and functional groups. Because of this specific structure, CDs have more functionalization, compatibility, stability, and ease of modification, which opens up a greater range of possible applications in many disciplines [
28]. In terms of toxicity, CDs display minimal toxicity or non-toxicity as well as exemplary compatibility with biological systems even with elevated concentration levels [
28]. CDs are able to pass right through the BBB. The BBB has been successfully crossed by several CD forms and their conjugates with particular ligands, suggesting that CDs may be used as drug delivery vehicles to treat disorders of the CNS. CDs are adaptable transporters for medicinal chemicals because they have functional groups, such amine and carboxyl groups, on their surface that allow them to bind with a variety of medications [
29]. The categorization, characteristics, and uses of CDs are being researched in this field, with an emphasis on how they might be used to treat neurodegenerative illnesses. Additionally, future studies may look into the mechanisms underlying the movement of molecules across this extremely selective barrier as well as the structure of the blood–brain barrier.
According to recently conducted research, specifically designed acid-responsive dual-targeted nanoparticles containing aspirin, have proven to be highly effective in reducing immunological activation and related symptoms in a mouse model that mimic ASD. Labeled as Asp@TMNPs, within this drug delivery system, polycaprolactone (PCL) and longer poly(ethylene glycol) (PEG) chains were joined with D-T7 using the acid-sensitive linker DAK. At the opposite end of a shorter PCL-PEG chain, MG1 was introduced. These polymers then self-assembled to form nanoparticles that contained aspirin. Asp@TMNPs with diameters of about 50 nm, demonstrated targeted delivery to the BBB’s transferrin receptor (TfR), avoiding lysosomal degradation and permitting transit into the brain parenchyma through the acid-cleavable D-T7. After entering the brain, the nanoparticles, guided by MG1, aimed to target microglial cells and released aspirin gradually to treat ASD. These nanoparticles showed better results than aspirin by itself, indicating that they may be able to alleviate some of the main symptoms associated with ASD [
30]. One important finding is that the aspirin internalization is greatly increased by the nanoparticles, which effectively addresses the difficulties in treating illnesses of the central nervous system caused by the BBB. The work presents a unique design for nanoparticles that facilitates their transport across the blood–brain barrier through a ligand-mediated mechanism. The nanoparticles demonstrated improved BBB penetration, accurate microglial targeting, and successful treatment outcomes in the ASD model. These nanoparticles were coated with specific peptides and a special acid-cleavable linker. The potential of using customized nanoparticles to enhance medication delivery for CNS disorders specifically, such as for the treatment of ASD, is demonstrated by this study.
Overcoming the challenges presented with the BBB has been a lengthy journey for researchers throughout the years. Conventional ways of efficiently delivering nanoparticles to the brain are hindered by the BBB [
31]. Focused ultrasound (FUS) has become a viable method for improving the distribution of nanoparticles into the brain by opening up the BBB [
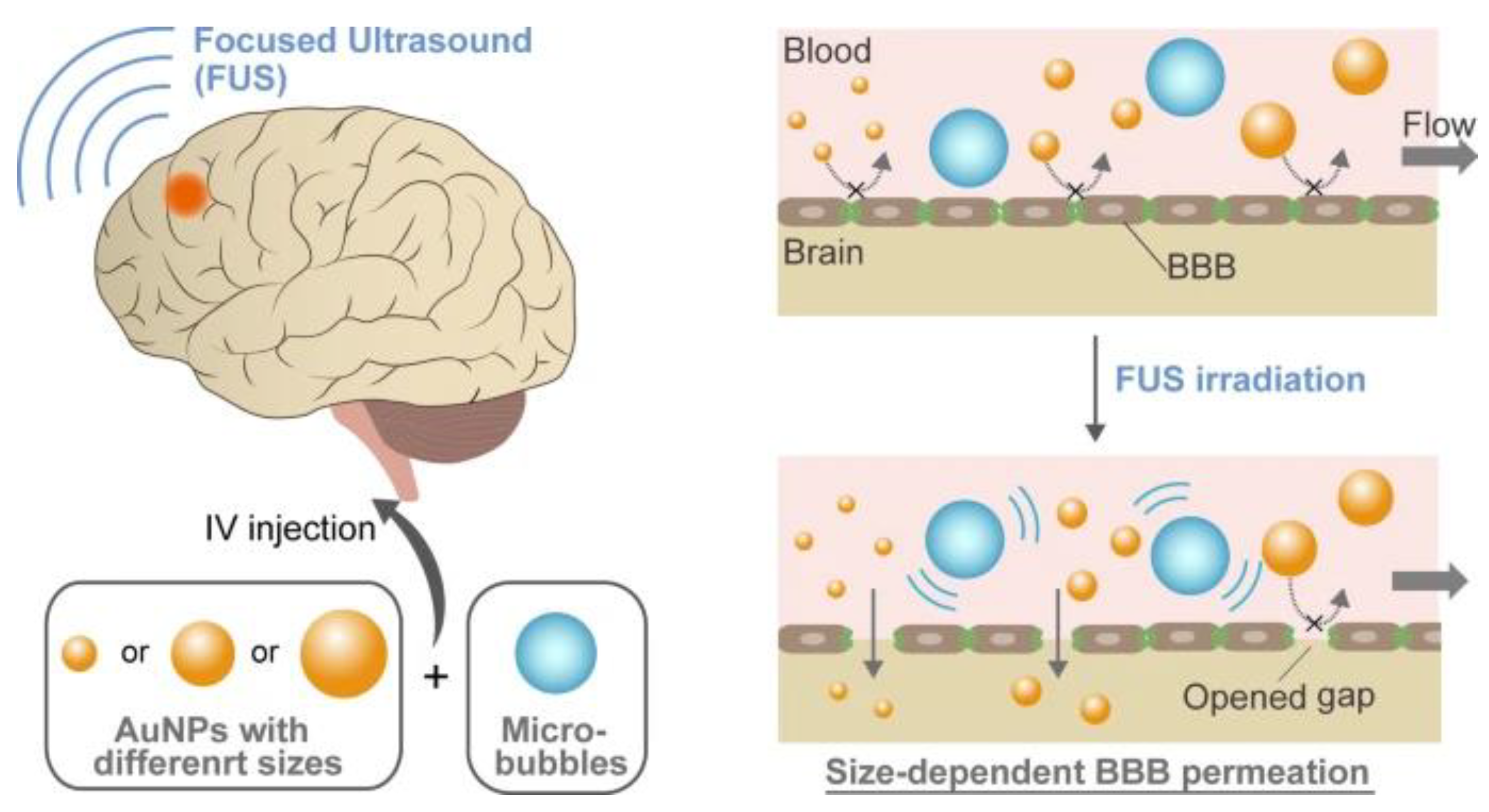
32]. Nevertheless, little is known about the precise methods by which nanoparticles move across these created holes in the blood–brain barrier. In a study conducted by Ohta et al., gold nanoparticles (AuNPs) with varying diameters (3, 15, and 120 nm) were utilized to examine the effect of nanoparticle size on their distribution into the brain with FUS-induced BBB opening. Smaller AuNPs (3 and 15 nm) exhibited a much higher penetration ability across an in vitro BBB model with FUS exposure, according to the findings; however, smaller diameters did not always translate into better delivery in mice exposed to transcranial FUS in vivo. Interestingly, compared to both smaller (3 nm) and bigger (120 nm) particles, medium-sized (15 nm) AuNPs showed the highest delivery efficiency [
32]. According to a computational model, the ideal size was established by balancing the removal of the particles from the bloodstream with their ability to pass through the gaps in the blood–brain barrier. These findings could improve treatments for disorders of the central nervous system by enabling the fabrication of nanoparticles specifically suited for brain delivery.
Figure 3 shows that focused ultrasound (FUS) combined with microbubbles (MBs) causes stable or inertial cavitation, which creates a mechanical force that causes the BBB’s tight connections to momentarily swell [
33].
4. Drawbacks and Critiques
Both nanotechnology and applied behavior analysis carry drawbacks that are often emphasized in terms of their application. The use of nanotechnology in medicine or nanomedicine has an enormous potential to improve drug delivery, diagnosis, and individualized care. It does, however, also come with a number of disadvantages and difficulties that should be carefully considered. The biocompatibility and possible toxicity of nanoparticles employed in medical applications are major causes for concern [
34]. These materials’ potential to illicit an immunological response and their long-term consequences on human health are still unknown, which raises concern regarding their safety [
35]. The distinct difficulties presented by nanomedicine fall outside the scope of existing regulatory frameworks, necessitating more precise standards for approval, testing, and continuing observation. Additionally, consistent manufacturing and quality control at the nanoscale are highly challenging due to the complexity and expense at scaling up nanomedical goods for mass production [
36]. When ensuring exact distribution while maintaining its efficacy and safety, it will be imperative to address these challenges.
Applied behavior analysis is a widely utilized therapy for individuals with autism spectrum disorder as well as other developmental disorders and has demonstrated efficacy in skill development and behavior modification. However, it is not without criticisms and drawbacks. Its intensive nature requires significant time and resources from caregivers, therapists, and the individual, which raises a point of concern. Proponents of the neurodiversity perspective have expressed a number of reservations regarding the use of ABA therapies with autistic people. These issues include the long hours associated with early intensive behavioral intervention (EIBI) and particular ABA procedures like the use of aversives [
37]. Debates concerning the appropriateness and effectiveness of such interventions are sparked by criticisms that claim that certain components of ABA may be harmful or possibly damaging to individuals with autism. In addition, a primary critique centers on its emphasis on altering visible actions instead of attending to underlying causes or emotional needs, which may overlook internal experiences. Ethical concerns have also been raised, especially with regard to the employment of aversive techniques; in the past, ABA has faced criticism for possible limitations on the generalization of skills and an excessive focus on compliance, which may potentially undermine an individual’s autonomy. The consistent responsibility of providing outside therapy may also be seen as a burden to parents and other caregivers [
38]. Due to its cost and limited availability, ABA therapy may be out of reach for many [
39]. Despite the effectiveness of ABA, there is ongoing discussion on the quality data supporting it and how it can affect a person’s natural development. Resolving these issues entails improving ABA procedures, emphasizing a person-centered strategy that values individual autonomy, and combining behavior modification methods with a more comprehensive understanding of each person’s needs [
40]. Practitioners and researchers are working hard to address these issues and move ABA in the direction of a more person centered and comprehensive approach.
5. Conclusions
Applied behavior analysis (ABA) has often faced scrutiny in its approach toward treating autism spectrum disorder (ASD) [
41]. Despite the prevalent misrepresentations and misunderstandings surrounding the use of ABA in the context of ASD, research findings consistently demonstrate significant improvements in various skill sets for children with autism. These enhancements often include notable progress in behavior management, cognitive abilities, receptive language, and other vital developmental skills. Despite criticisms and misconceptions, the empirical evidence consistently supports the effectiveness of ABA in fostering crucial developmental advancements for individuals with ASD, showcasing its potential to positively impact their lives and capabilities [
42]. However, with the potential integration of nanomedicine, there is an optimistic view that future implications could extend beyond merely curing the disorder. The combination of ABA with advancements in nanomedicine might lead to a more positive outlook on ASD treatment, potentially offering not just cures but more effective and targeted interventions. Nanomedicine’s precision in drug delivery to the brain and its potential to develop personalized treatments could enhance the efficacy and acceptability of ABA, leading to more favorable outcomes and perceptions of ASD therapy. This synergy between ABA and nanomedicine holds promise in not only addressing the condition, but also in changing the perception and experience of ASD treatment for individuals, families, and communities. Embedding nanotechnology within medicine dedicated to autism spectrum disorder has elicited many benefits even in its preliminary stages. Unlike many other disorders, ASD lacks root causes as well as genetic markers for determining its probability of occurring. Other medical illnesses such as down syndrome (trisomy 21) or polydactyly are predicted in the fetus early on as there are abnormalities in the DNA on genomes Hsa21 and GLI3, respectively [
43,
44]. Nanotechnology offers a promising new area for autism research in terms of possible improvements in the disorder’s knowledge, diagnosis, and therapy. Nanotechnology is being used in autism spectrum disorder (ASD) research to provide new avenues for therapeutic treatments, drug delivery, and diagnostic imaging. The goal of developing nanoparticles is to get through the blood–brain barrier and deliver drugs directly to the brain, which could increase the effectiveness of treatment. Furthermore, developments in nanotechnology support the creation of tailored medication delivery systems, which help to alleviate particular ASD symptoms. Techniques based on nanotechnology have great promise for improving early diagnosis, tracking the course of the illness, and investigating the neurological causes of ASD [
45]. Although there are promising prospects for this field, more research and clinical studies are needed to confirm the safety, effectiveness, and usefulness of implementing nanotechnology to treat the complex challenges surrounding autism. However, the use of nanotechnology for the study of ASD offers a creative and promising avenue for improving our comprehension and treatment of this intricate neurodevelopmental disorder.
6. Future Trends
Future developments in nanotechnology could have a substantial impact on autism spectrum disorder (ASD) in a number of important ways. First and foremost, it presents the possibility of precise and focused drug administration to the brain by bypassing the blood–brain barrier, which could greatly improve the management of symptoms associated with ASD [
46]. Furthermore, the early and precise detection of ASD through the use of particular biomarkers or genetic indicators may be made possible through sophisticated nanotechnology-based diagnostic technologies [
47]. This would allow for timely therapies and continuous monitoring of the disease’s course. In terms of therapeutic approaches, novel nanotechnology-based therapies could potentially address the particular neurobiological components of ASD, potentially reducing core symptoms and improving cognitive, social, and behavioral functioning in affected individuals. Additionally, nanotechnology presents a chance to explore the complex cerebral networks and cellular processes linked to ASD, offering important new understandings of the biology underlying the illness [
48]. Furthermore, nanotechnology may be used in the future to create personalized treatment plans that would better target a patient’s genetic and biological characteristics with tailored interventions. Scientists are actively investigating and utilizing the potential uses of nanotechnology in the diagnosis, treatment, and prevention of numerous diseases as a result of their growing awareness of the benefits of nanomedicine. Because of their special qualities, nanomaterials present exciting possibilities for the development of more effective diagnostic instruments, focused drug delivery systems, and creative preventive measures [
49]. Using nanotechnology to enhance medical outcomes across a wide range of diseases and health conditions has sparked great interest in ongoing research due to its accuracy, adaptability, and promise for personalized therapy [
50]. Finally, researchers may be able to gain a deeper knowledge of ASD by using nanotechnology to investigate neuronal connectivity, genetic variables, and cellular processes associated with the illness. To establish the effectiveness, safety, and practical application of nanotechnology-based therapies in ASD, however, substantial research, rigorous clinical trials, and demanding safety evaluations are necessary.