Formulation of Pharmaceutical Tablets Containing β-Cyclodextrin-4-Methyl-Umbelliferone (Hymecromone) Inclusion Complexes and Study of the Dissolution Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of 4-Methyl-Umbelliferone (4-MU)
2.2. Preparation of β-CD—4-Methyl-Umbelliferone Inclusion Complexes (β-CD-4-MU ICs)
2.3. Characterization of the β-CD—4-Methyl-Umbelliferone ICs
2.3.1. Inclusion Efficiency of the β-CD—4-Methyl-Umbelliferone ICs
2.3.2. Dynamic Light Scattering (DLS)
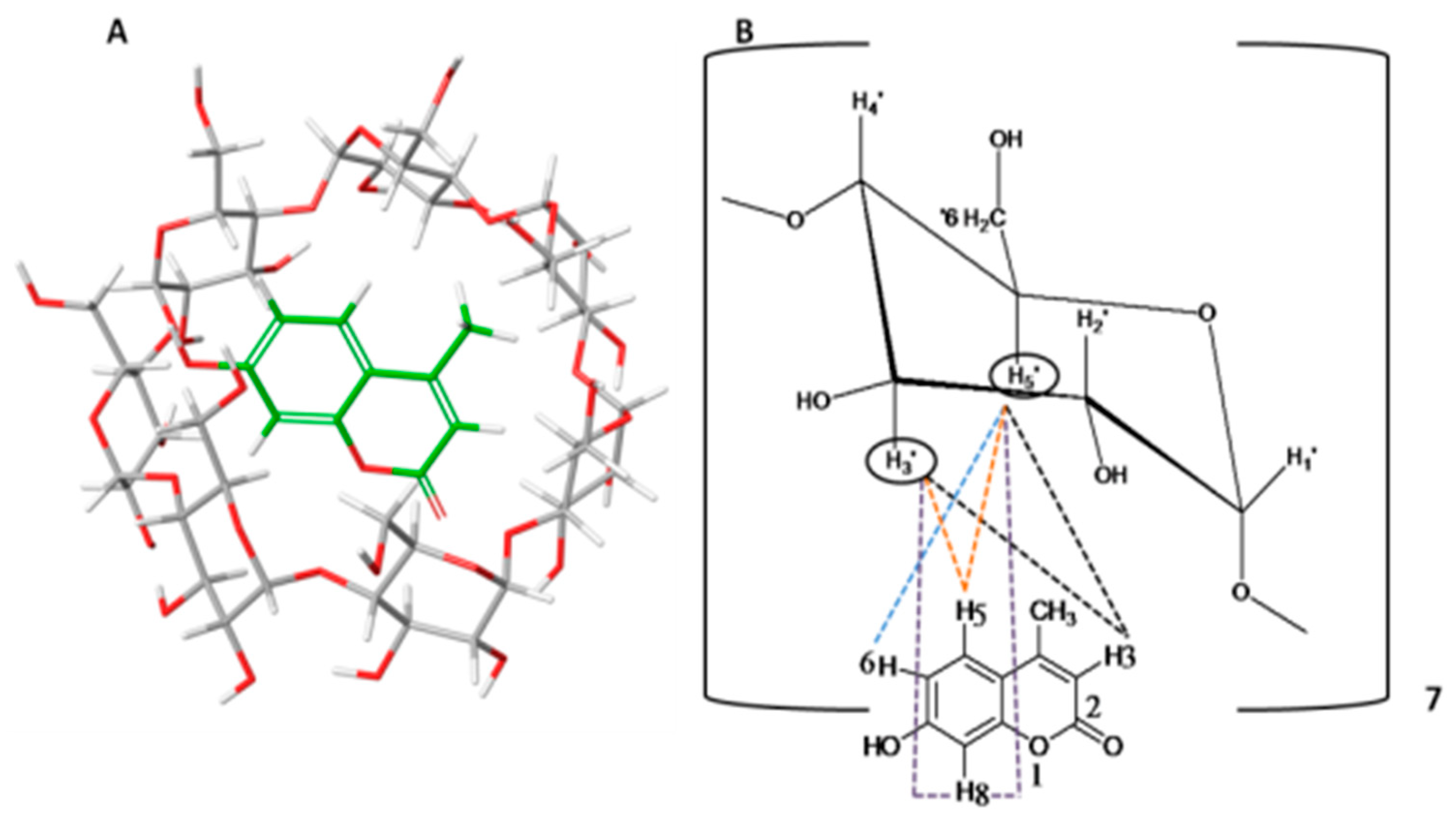
2.3.3. Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy)
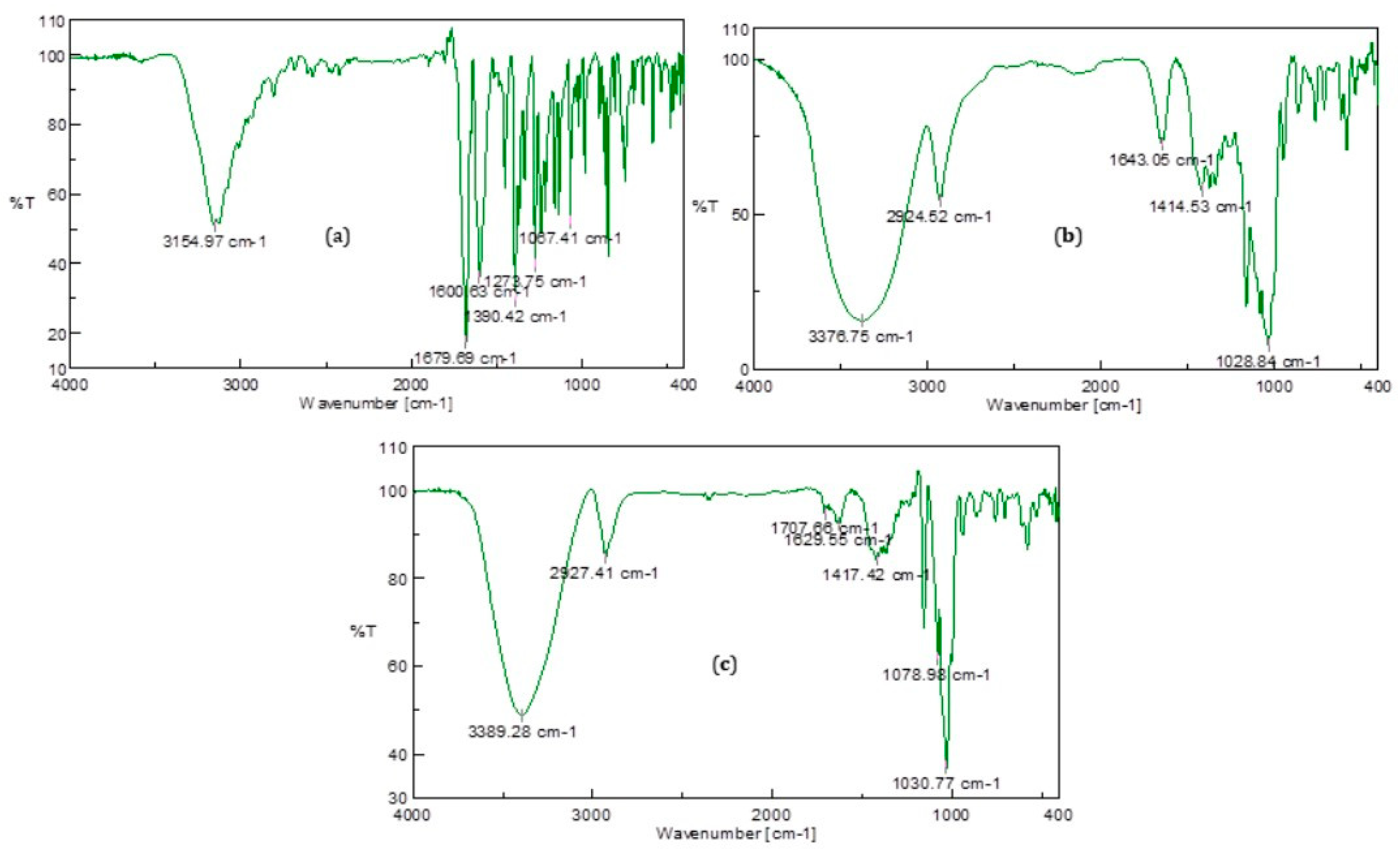
2.3.4. FT-IR Spectroscopy
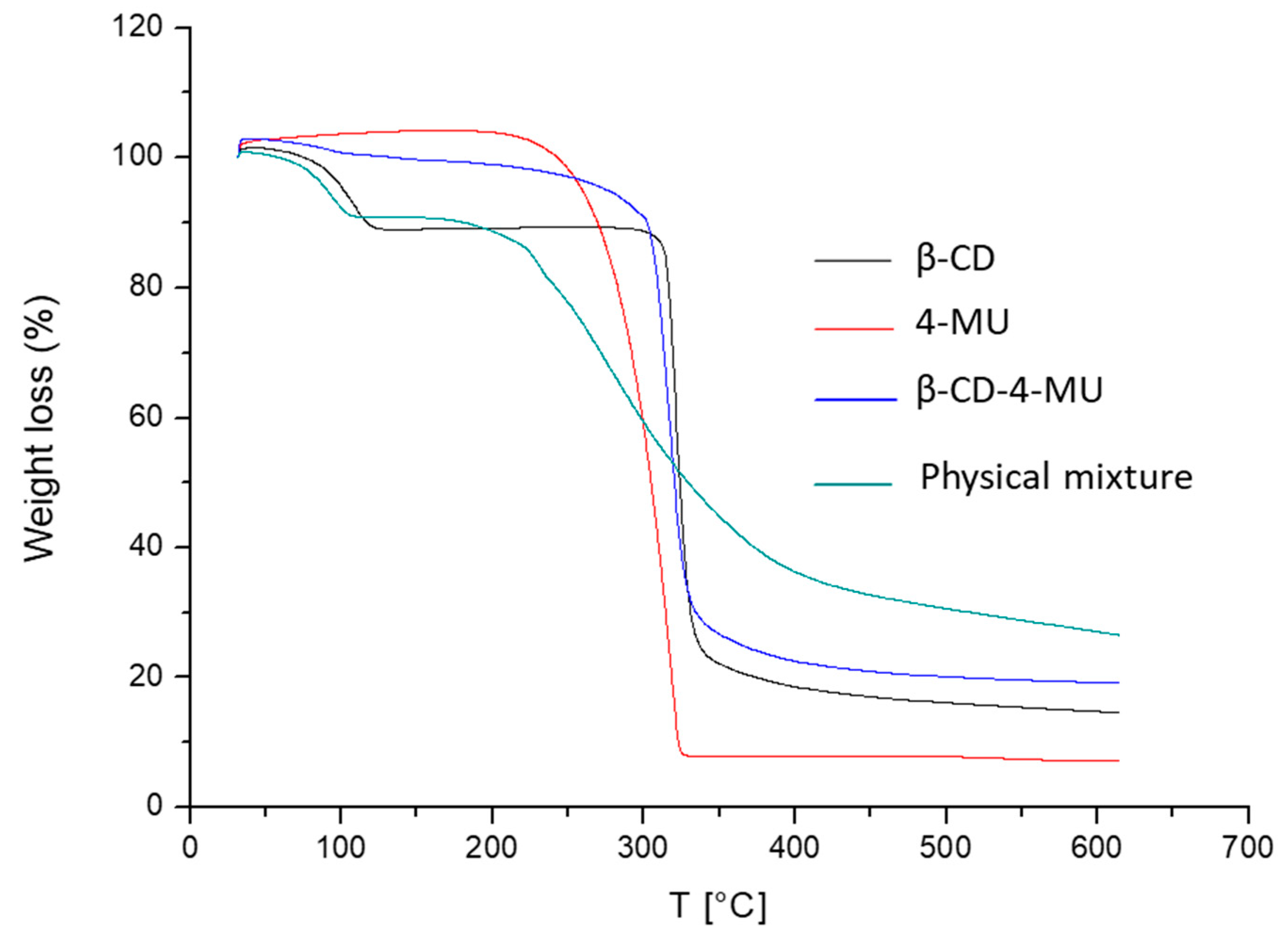
2.3.5. Thermogravimetric Analysis (TGA)
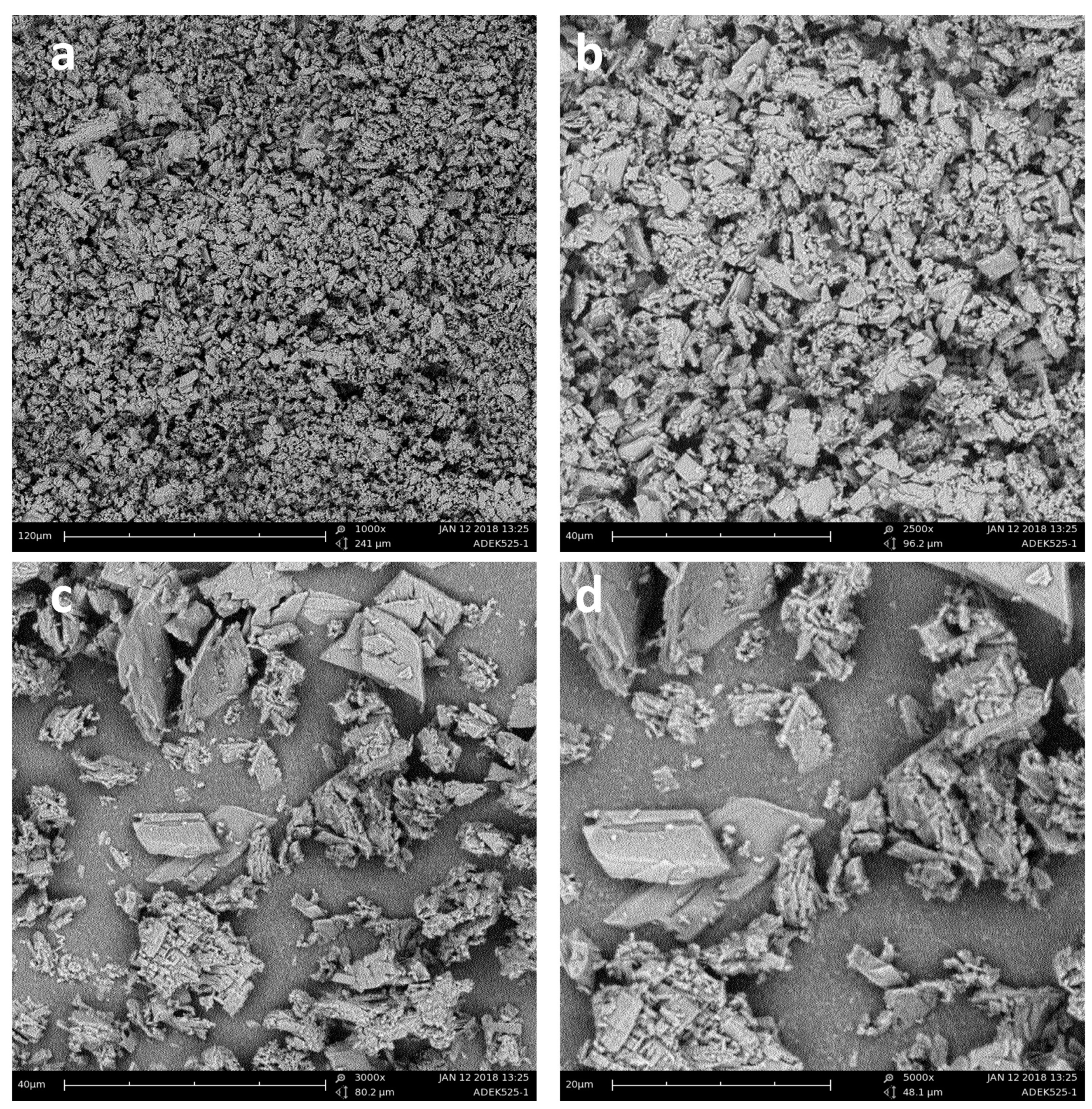
2.3.6. Scanning Electron Microscopy (SEM)
2.4. Biological Activity Evaluation of the β-CD—4-Methyl-Umbelliferone ICs
2.4.1. Determination of Reducing Activity Using the Stable Radical 1,1-Diphenyl-Picrylhydrazyl (DPPH)
2.4.2. Inhibition of Linoleic Acid Peroxidation
2.4.3. Soybean Lipoxygenase Inhibition Study
2.5. In Vitro Release Studies of the β-CD—4-Methyl-Umbelliferone ICs
2.6. Preliminary Tests
2.7. Preparation of Pharmaceutical Tablets
2.8. Dissolution Testing
3. Results and Discussion
3.1. Characterization of the β-CD-4-MU ICs
3.1.1. Inclusion Efficiency, Process Yield, Size, Size Distribution, and Zeta-Potential
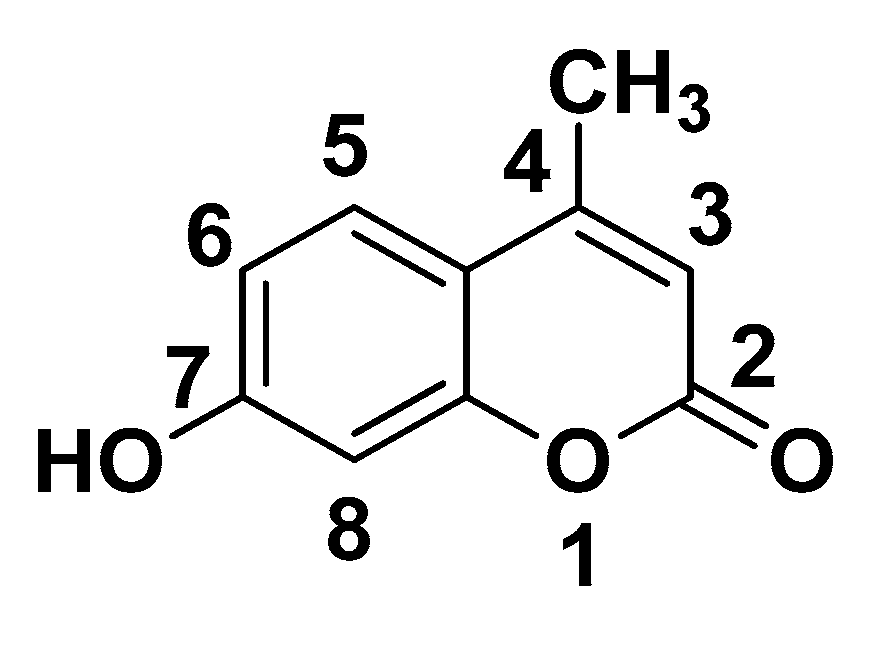
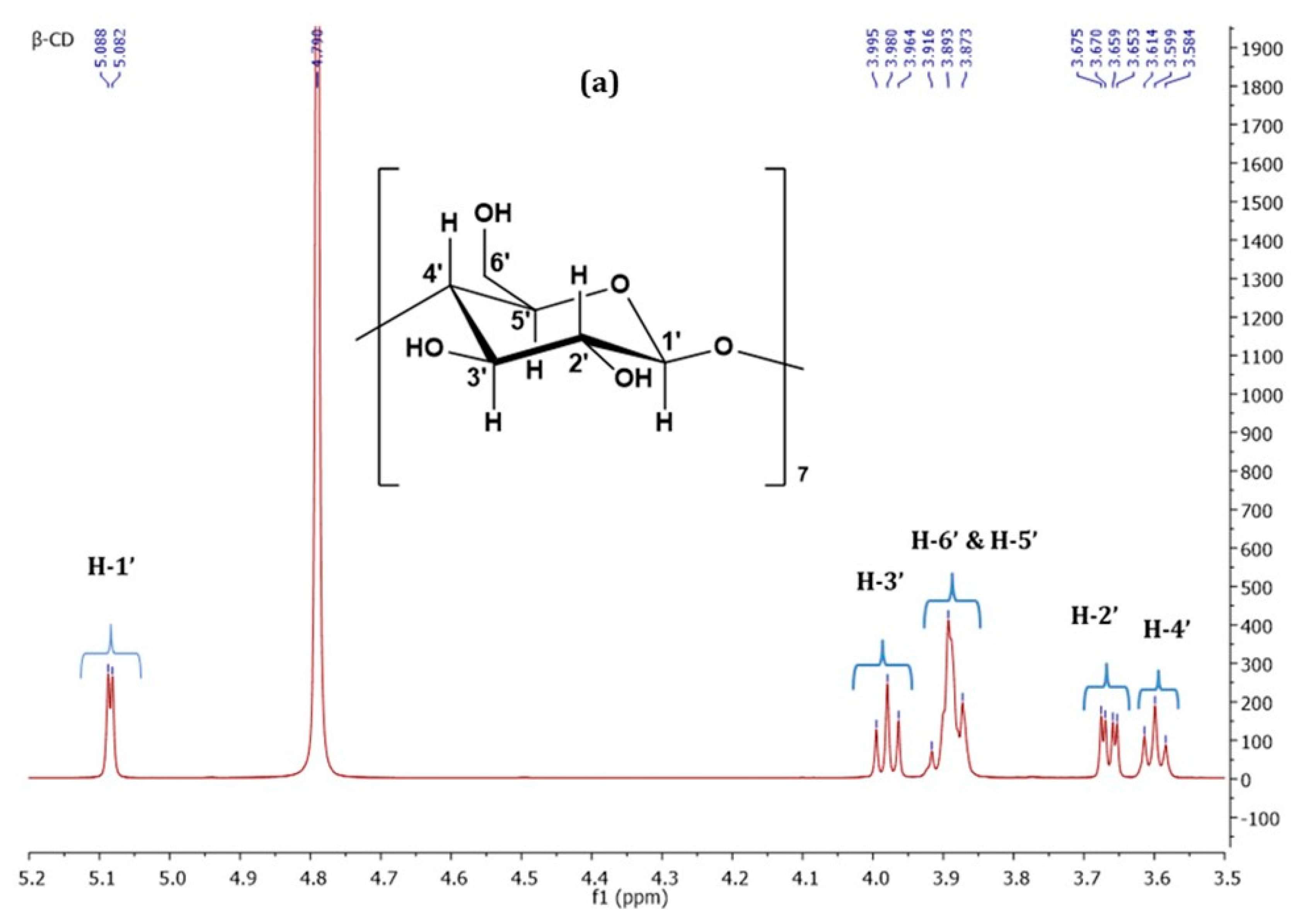
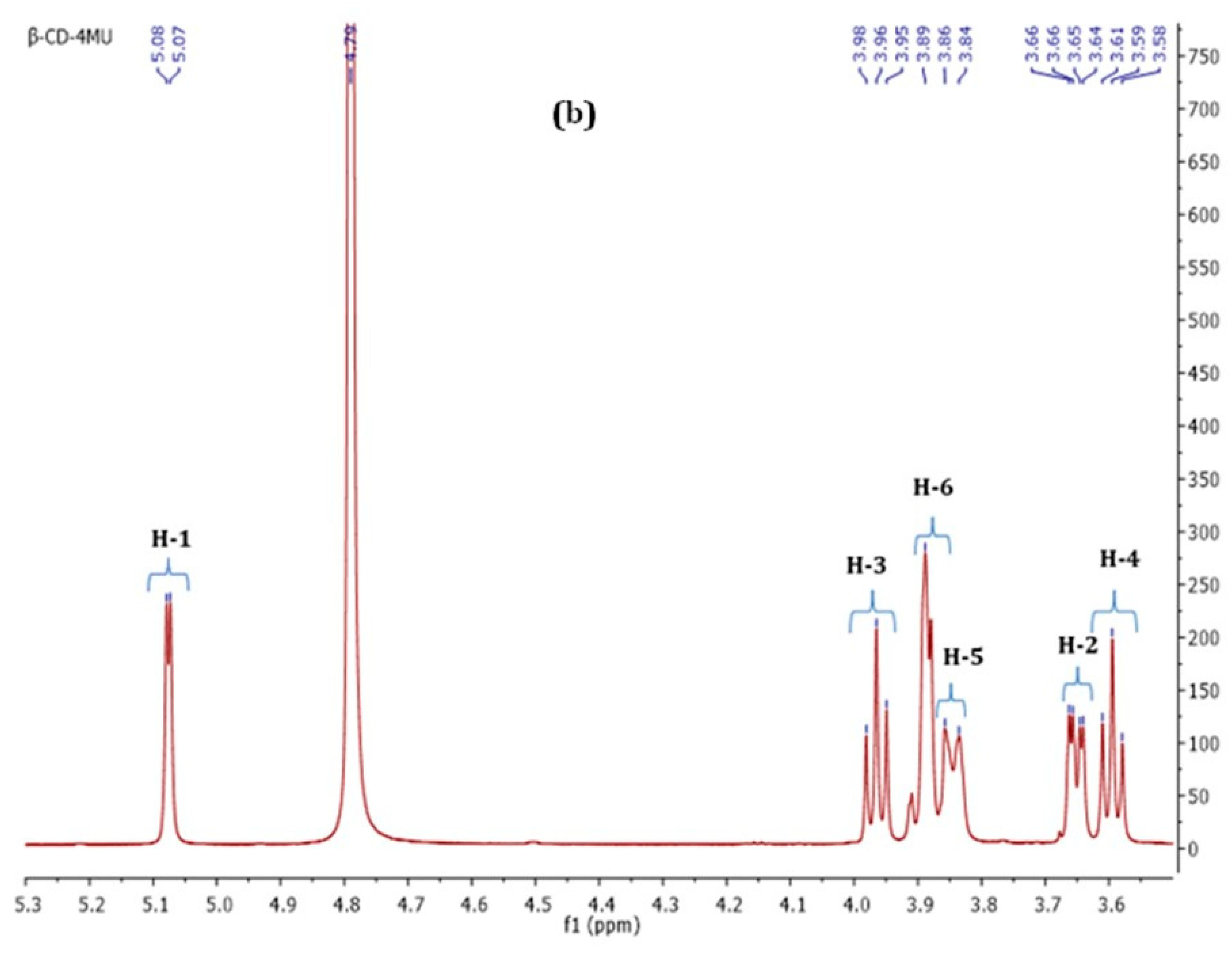
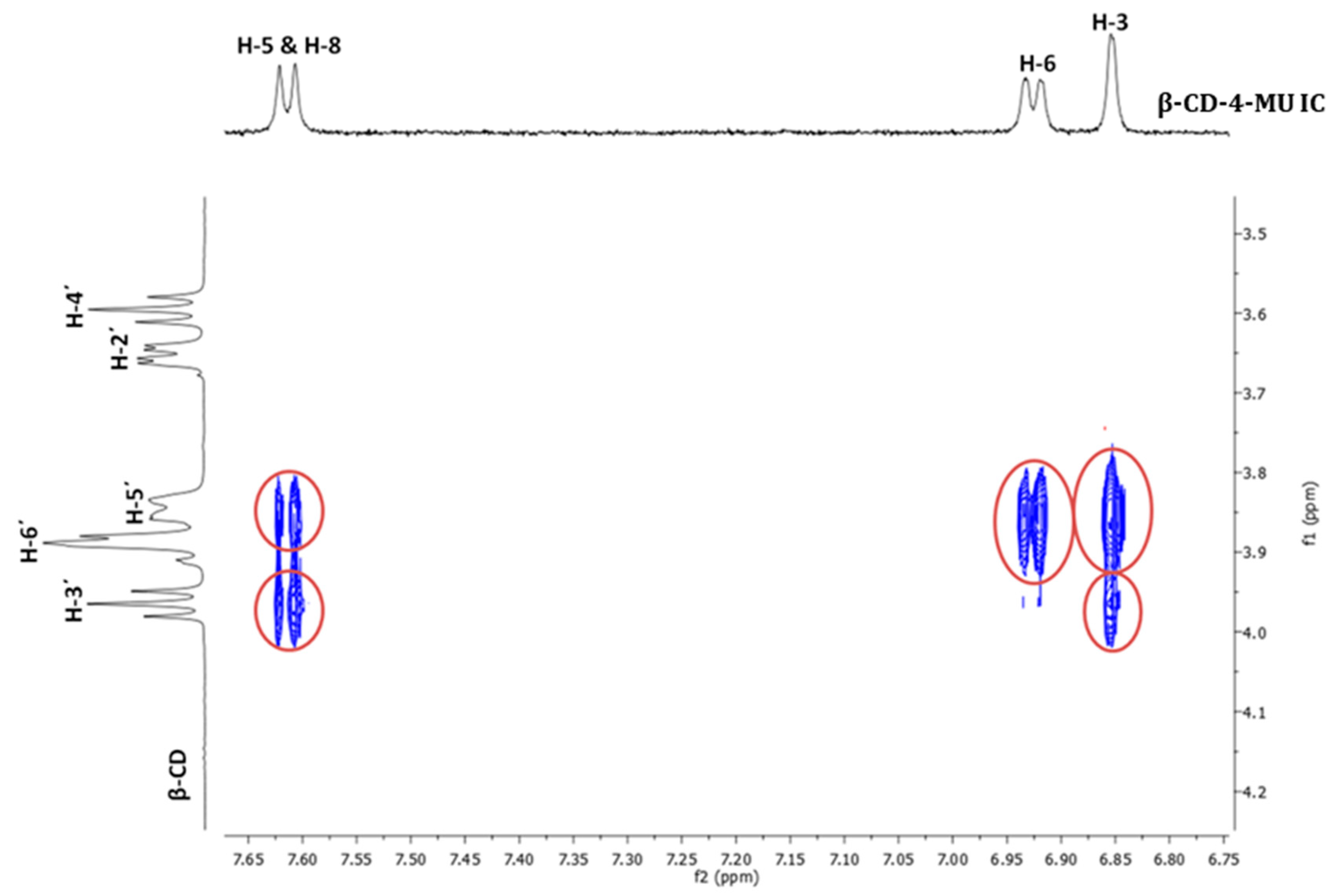
3.1.2. Nuclear Magnetic Resonance Spectroscopy (NMR Spectroscopy)
3.1.3. FT-IR Spectroscopy
3.1.4. Thermogravimetric Analysis (TGA)
3.1.5. Scanning Electron Microscopy (SEM)
3.2. Biological Activity Evaluation
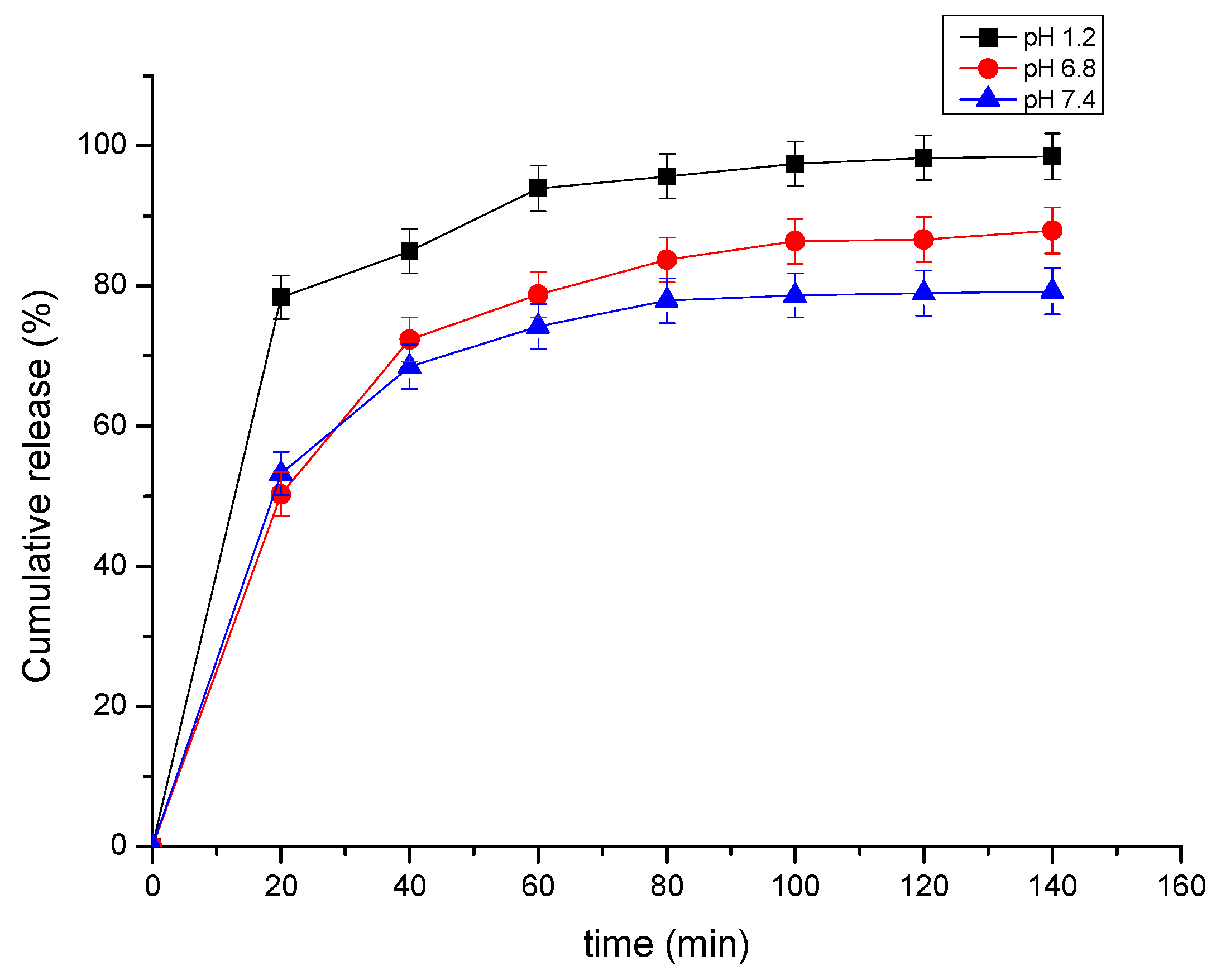
3.3. In Vitro Release Studies of the 4-MU from β-CD—4-Methyl-Umbelliferone ICs
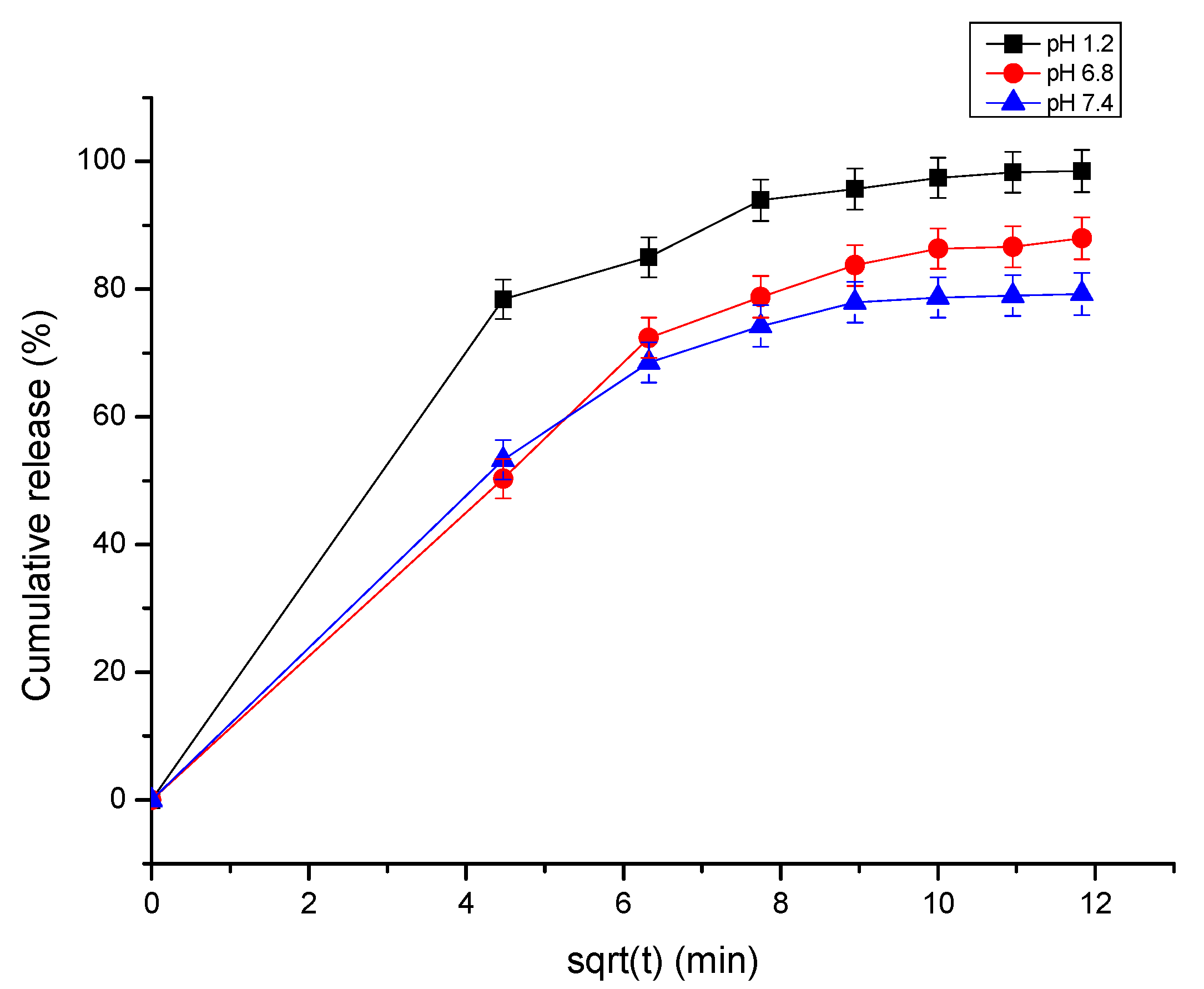
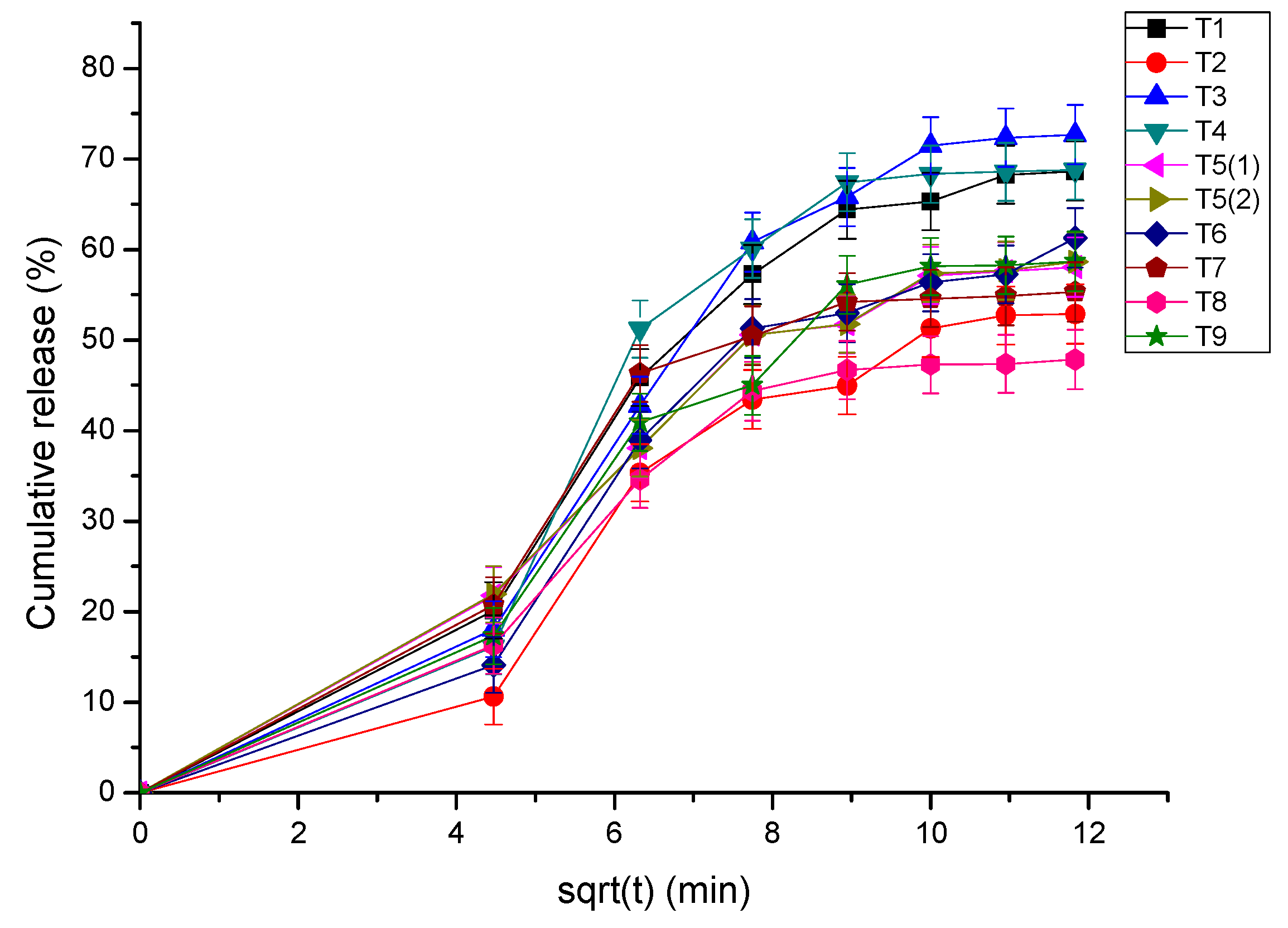
3.4. Dissolution Testing
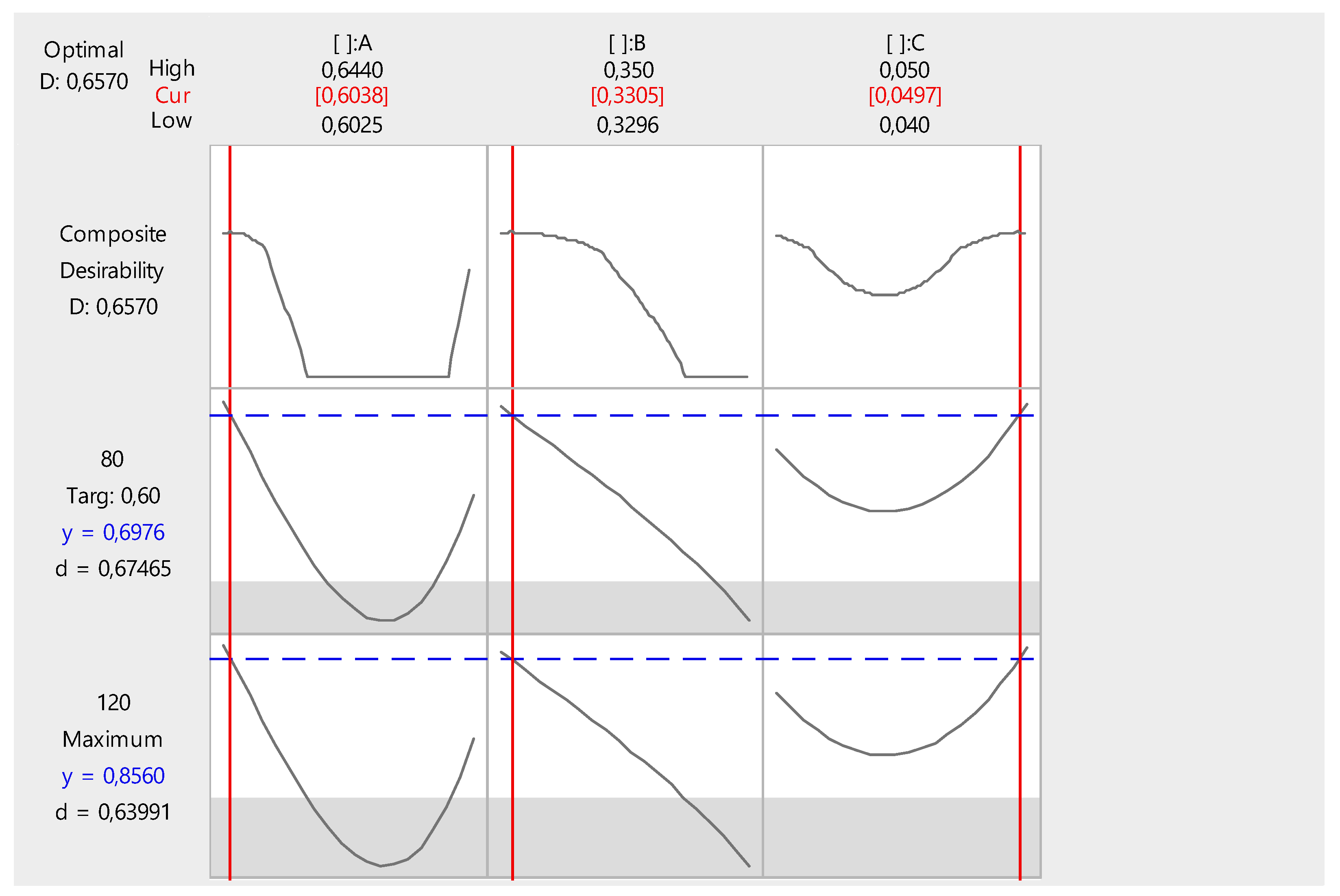
3.5. Formulation Optimization by Means of Extreme Vertices Mixture Design
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantri, R.V.; Sanghvi, R.; Zhu, H.J. Chapter 1-Solubility of Pharmaceutical Solids. In Developing Solid Oral Dosage Forms, 2nd ed.; Qiu, Y., Chen, Y., Zhang, G.G., Yu, L., Mantri, R.V., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 3–22. [Google Scholar] [CrossRef]
- Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Kavetsou, E.; Gkionis, L.; Galani, G.; Gkolfinopoulou, C.; Argyri, L.; Pontiki, E.; Chroni, A.; Hadjipavlou-Litina, D.; Detsi, A. Synthesis of prenyloxy coumarin analogues and evaluation of their antioxidant, lipoxygenase (LOX) inhibitory and cytotoxic activity. Med. Chem. Res. 2017, 26, 856–866. [Google Scholar] [CrossRef]
- Tamura, R.; Yokoyama, Y.; Yoshida, H.; Imaizumi, T.; Mizunuma, H. 4-Methylumbelliferone inhibits ovarian cancer growth by suppressing thymidine phosphorylase expression. J. Ovarian Res. 2014, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, H.; Zhu, W.; Wang, L.; Liu, H.; Xu, X.; Yin, W.; Sima, Y.; Xu, S. Antioxidative properties of 4-methylumbelliferone are related to antibacterial activity in the silkworm (Bombyx mori) digestive tract. J. Comp. Physiol. B 2014, 184, 699–708. [Google Scholar] [CrossRef]
- Šarkanj, B.; Molnar, M.; Čačić, M.; Gille, L. 4-Methyl-7-hydroxycoumarin antifungal and antioxidant activity enhancement by substitution with thiosemicarbazide and thiazolidinone moieties. Food Chem. 2013, 139, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Maynard, R. The Merck Index: 12th edition 1996. Occup. Environ. Med. 1997, 54, 288. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.J.B.; et al. Encapsulation of olive leaves extracts in biodegradable PLA nanoparticles for use in cosmetic formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef]
- Roy, N.; Ghosh, B.; Roy, D.; Bhaumik, B.; Roy, M.N. Exploring the Inclusion Complex of a Drug (Umbelliferone) with α-Cyclodextrin Optimized by Molecular Docking and Increasing Bioavailability with Minimizing the Doses in Human Body. ACS Omega 2020, 5, 30243–30251. [Google Scholar] [CrossRef] [PubMed]
- Halahlah, A.; Kavetsou, E.; Pitterou, I.; Grigorakis, S.; Loupassaki, S.; Tziveleka, L.-A.; Kikionis, S.; Ioannou, E.; Detsi, A. Synthesis and characterization of inclusion complexes of rosemary essential oil with various β-cyclodextrins and evaluation of their antibacterial activity against Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 65, 102660. [Google Scholar] [CrossRef]
- Kavetsou, E.; Pitterou, I.; Katopodi, A.; Petridou, G.; Adjali, A.; Grigorakis, S.; Detsi, A. Preparation, Characterization, and Acetylcholinesterase Inhibitory Ability of the Inclusion Complex of β-Cyclodextrin–Cedar (Juniperus phoenicea) Essential Oil. Micro 2021, 1, 250–266. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of oregano (Origanum onites L.) essential oil in β-cyclodextrin (β-CD): Synthesis and characterization of the inclusion complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J.M.S. Cyclodextrins as excipients in tablet formulations. Drug Discov. Today 2018, 23, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; De Lima, A.N. Cyclodextrin-drug inclusion complexes: In vivo and in vitro approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef]
- Novac, M.; Musuc, A.M.; Ozon, E.A.; Sarbu, I.; Mitu, M.A.; Rusu, A.; Gheorghe, D.; Petrescu, S.; Atkinson, I.; Lupuliasa, D. Manufacturing and Assessing the New Orally Disintegrating Tablets, Containing Nimodipine-hydroxypropyl-β-cyclodextrin and Nimodipine-methyl-β-cyclodextrin Inclusion Complexes. Molecules 2022, 27, 2012. [Google Scholar] [CrossRef] [PubMed]
- Maestrelli, F.; Cirri, M.; De Luca, E.; Biagi, D.; Mura, P. Role of Cyclodextrins and Drug Solid State Properties on Flufenamic Acid Dissolution Performance from Tablets. Pharmaceutics 2022, 14, 284. [Google Scholar] [CrossRef] [PubMed]
- Rincón-López, J.; Almanza-Arjona, Y.C.; Riascos, A.P.; Rojas-Aguirre, Y. When Cyclodextrins Met Data Science: Unveiling Their Pharmaceutical Applications through Network Science and Text-Mining. Pharmaceutics 2021, 13, 1297. [Google Scholar] [CrossRef]
- Kiskan, B.; Yagci, Y. Thermally curable benzoxazine monomer with a photodimerizable coumarin group. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1670–1676. [Google Scholar] [CrossRef]
- Roussaki, M.; Zelianaios, K.; Kavetsou, E.; Hamilakis, S.; Hadjipavlou-Litina, D.; Kontogiorgis, C.; Liargkova, T.; Detsi, A. Structural modifications of coumarin derivatives: Determination of antioxidant and lipoxygenase (LOX) inhibitory activity. Bioorg. Med. Chem. 2014, 22, 6586–6594. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Al Mamun, A.; Hossain, S.; Asaduzzaman; Sarwar, S.; Rashid, M.; Herrera-Calderon, O. In vitro quality evaluation of leading brands of ciprofloxacin tablets available in Bangladesh. BMC Res. Notes 2017, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Martinello, T.; Kaneko, T.M.; Velasco, M.V.R.; Taqueda, M.E.S.; Consiglieri, V.O. Optimization of poorly compactable drug tablets manufactured by direct compression using the mixture experimental design. Int. J. Pharm. 2006, 322, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment-A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Ali, S.M. Molecular recognition of flunarizine dihydrochloride and β-cyclodextrin inclusion complex by NMR and computational approaches. Chem. Cent. J. 2018, 12, 33. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.-Y.; Liu, Y.-Y.; Fu, Y.; Zhao, L.-X.; Li, C.-Y.; Ye, F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers 2019, 11, 1396. [Google Scholar] [CrossRef]
- Da Rocha Ferreira, F.; Valentim, I.B.; Ramones, E.L.C.; Trevisan, M.T.S.; Olea-Azar, C.; Perez-Cruz, F.; de Abreu, F.C.; Goulart, M.O.F. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT-Food Sci. Technol. 2013, 51, 129–134. [Google Scholar] [CrossRef]
- Dos Santos Lima, B.; Shanmugam, S.; de Souza Siqueira Quintans, J.; Quintans-Júnior, L.J.; de Souza Araújo, A.A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]


| Tablet | β-CD-4-MU IC (mg) | β-CD (mg) | 4-MU (mg) | Lactose (mg) | Microcrystalline Cellulose (MCC) (mg) | Calcium Phosphate (CaP) (mg) | Magnesium Stearate (Mg-St) (mg) | Starch (St) (mg) | Total Tablet Mass (mg) | Hardness (N) |
|---|---|---|---|---|---|---|---|---|---|---|
| TT1 | - | 150 | - | 30 | 40 | 26 | 2 | 2 | 250 | 19 |
| TT2 | - | 150 | - | 30 | 40 | 26 | 2 | 2 | 250 | 69 |
| TT3 | - | 150 | - | 30 | 40 | 26 | 2 | 2 | 250 | 73 |
| TT4 | - | 150 | - | 24 | 60 | 12 | 2 | 2 | 250 | 77 |
| TT5 | - | 150 | - | - | 84 | 12 | 2 | 2 | 250 | 110 |
| TT6 | - | 150 | - | - | 84 | 12 | 2 | 2 | 250 | 83 |
| TT7 | - | 150 | - | - | 84 | 12 | 2 | 2 | 250 | 110 |
| TT8 | 150 | - | - | - | 84 | 12 | 2 | 2 | 250 | 156 |
| TT9 | - | 135 | 15 | - | 84 | 12 | 2 | 2 | 250 | 108 |
| TT10 | 150 | - | - | - | 84 | 12 | 2 | 2 | 250 | 149 |
| Ingredient | Factor | Low | High |
|---|---|---|---|
| β-CD | x1 | 0.55 | 0.65 |
| MCC | x2 | 0.30 | 0.35 |
| CaP | x3 | 0.04 | 0.05 |
| Tablet | β-CD-4-MU Inclusion Complex X1 (mg) | Microcrystalline Cellulose (MCC) X2 (mg) | Calcium Phosphate (CaP) X3 (mg) | Starch (St) X4 (mg) | Magnesium Stearate (Mg-St) X5 (mg) |
|---|---|---|---|---|---|
| Τ1 | 161.00 | 75.00 | 10.00 | 2.00 | 2.00 |
| Τ2 | 156.000 | 78.13 | 11.87 | 2.00 | 2.00 |
| Τ3 | 151.000 | 84.37 | 10.63 | 2.00 | 2.00 |
| Τ4 | 149.75 | 84.38 | 11.87 | 2.00 | 2.00 |
| Τ5 (i, ii) * | 153.50 | 81.25 | 11.25 | 2.00 | 2.00 |
| Τ6 | 158.50 | 75.00 | 12.50 | 2.00 | 2.00 |
| Τ7 | 146.00 | 87.50 | 12.50 | 2.00 | 2.00 |
| Τ8 | 157.25 | 78.12 | 10.63 | 2.00 | 2.00 |
| Τ9 | 148.50 | 87.50 | 10.00 | 2.00 | 2.00 |
| Molar ratio β-CD:4-MU (mol) | β-CD (gr) | Size (nm) | Polydispersity Index (PdI) | Zeta-Potential (mV) | Process Yield % | Inclusion Efficiency (IE) % |
|---|---|---|---|---|---|---|
| 2:1 | 0.5 | 917.9 ± 127.5 | 0.709 ± 0.087 | −16.8 ± 2.6 | 69 | 57 |
| 1:1 | 0.5 | 145.6 ± 52.0 | 0.370 ± 0.025 | −29.5 ± 1.4 | 78 | 75 |
| 1:1 | 4 | 271.0 ± 20.5 | 0.367 ± 0.065 | −29.1 ± 0.9 | 82 | 77 |
| Proton | Chemical Shifts (δ1) of β-CD Protons (ppm) | Chemical Shifts (δ2) of β-CD Protons in β-CD—ICs (ppm) | Δδ = δ2 − δ1 (ppm) |
|---|---|---|---|
| H-1′ | 5.085 | 5.076 | −0.009 |
| H-2′ | 3.664 | 3.652 | −0.012 |
| H-3′ | 3.980 | 3.965 | −0.015 |
| H-4′ | 3.599 | 3.595 | −0.004 |
| H-5′ | 3.893 | 3.845 | −0.048 |
| H-6′ | 3.893 | 3.884 | −0.009 |
| Sample | O–H Stretching Vibration | C–H Stretching Vibration (Aromatic Ring) | C–H Stretching Vibration | C=O Stretching Vibration | Asymmetric C–H Stretching (-CH2) | C=C Stretching Vibration (Aromatic Ring) | O–H Bending Vibration | C–H in Plane Bending Vibration (Aromatic Ring) | C-O Stretching Vibration of the Secondary Alcohol | C–O Stretching Vibration of Phenols |
|---|---|---|---|---|---|---|---|---|---|---|
| 4-MU | - | 3155 | - | 1680 | - | 1601 | 1390 | 1274 | - | 1067 |
| β-CD | 3377 | - | 2925 | - | 1643 | - | 1415 | - | 1029 | - |
| β-CD-4-MU | 3389 | - | 2927 | 1708 | 1630 | - | 1417 | - | 1030 | 1079 |
| %DPPH Scavenging Ability ± SD # (100 μM) 20/60 min | % Lipid Peroxidation Inhibition (AAPH) ± SD # (100 μM) | Inhibition of Soybean Lipoxygenase Enzyme (LOX) IC50 (μΜ) ± SD # | |
|---|---|---|---|
| 4-MU | No/No | 93 ± 1.5 | No |
| β-CD | No/No | 18 ± 0.6 | 51.5 ± 1.7 |
| β-CD-4-MU | 63 (±2.2)/78 (±3.1) | 100 ± 2.6 | 10.0 ± 0.2 |
| Trolox | - | 88 ± 0.8 | - |
| NDGA | 84 (±1.6)/83 (±1.8) | - | 0.45 ± 0.1 |
| pH | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | Higuchi | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | R2 | n | Equation |
| 1.2 | 0.808 | 0.341 | 0.941 | 0.872 | 0.9151 | y = 2.003x + 78.58 |
| 7.4 | 0.539 | 0.365 | 0.823 | 0.893 | 0.8787 | y = 6.142x + 16.95 |
| 6.8 | 0.663 | 0.427 | 0.899 | 0.917 | 0.9144 | y = 7.369x + 12.73 |
| Tablet | Zero-Order | First-Order | Higuchi | Korsmeyer–Peppas | Higuchi | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | Equation | |
| 1 | 0.775 | 0.536 | 0.925 | 0.968 | 0.8865 | y = 6.377x + 0.6819 |
| 2 | 0.799 | 0.594 | 0.915 | 0.971 | 0.8454 | y = 4.9163x − 0.9497 |
| 3 | 0.870 | 0.589 | 0.957 | 0.982 | 0.9047 | y = 6.8621x − 1.4428 |
| 4 | 0.699 | 0.54 | 0.859 | 0.961 | 0.9003 | y = 6.358x + 1.6102 |
| 5 i | 0.799 | 0.523 | 0.953 | 0.965 | 0.8442 | y = 5.2721x + 2.1428 |
| 5 ii * | 0.917 | 0.648 | 0.932 | 0.990 | 0.845 | y = 5.3008x + 2.0575 |
| 6 | 0.814 | 0.577 | 0.932 | 0.975 | 0.8644 | y = 5.6027x − 0.6935 |
| 7 | 0.694 | 0.493 | 0.890 | 0.951 | 0.8393 | y = 5.004x + 4.4583 |
| 8 | 0.795 | 0.536 | 0.946 | 0.967 | 0.814 | y = 4.3748x + 2.4986 |
| 9 | 0.678 | 0.515 | 0.857 | 0.958 | 0.8556 | y = 5.4429x + 0.7738 |
| Run | x1 | x2 | x3 | f80 | f120 |
|---|---|---|---|---|---|
| 1 | 0.6440 | 0.3000 | 0.0400 | 0.5772 | 0.7054 |
| 2 | 0.6240 | 0.3125 | 0.0475 | 0.4347 | 0.5355 |
| 3 | 0.6040 | 0.3375 | 0.0425 | 0.6023 | 0.7421 |
| 4 | 0.5990 | 0.3375 | 0.0475 | 0.5932 | 0.7251 |
| 5 i | 0.6140 | 0.3250 | 0.0450 | 0.4929 | 0.5989 |
| 5 ii | 0.6140 | 0.3250 | 0.0450 | 0.49462 | 0.60116 |
| 6 | 0.6340 | 0.3000 | 0.0500 | 0.4941 | 0.6067 |
| 7 | 0.5840 | 0.3500 | 0.0500 | 0.4907 | 0.5908 |
| 8 | 0.6290 | 0.3125 | 0.0425 | 0.4178 | 0.5066 |
| 9 | 0.5940 | 0.3500 | 0.0400 | 0.4953 | 0.6055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavetsou, E.; Pitterou, I.; Dimitrioglou, N.G.; Kikionis, S.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.; Hatziavramidis, D.T.; Detsi, A. Formulation of Pharmaceutical Tablets Containing β-Cyclodextrin-4-Methyl-Umbelliferone (Hymecromone) Inclusion Complexes and Study of the Dissolution Kinetics. Micro 2023, 3, 892-911. https://doi.org/10.3390/micro3040061
Kavetsou E, Pitterou I, Dimitrioglou NG, Kikionis S, Kritsi E, Zoumpoulakis P, Pontiki E, Hadjipavlou-Litina D, Hatziavramidis DT, Detsi A. Formulation of Pharmaceutical Tablets Containing β-Cyclodextrin-4-Methyl-Umbelliferone (Hymecromone) Inclusion Complexes and Study of the Dissolution Kinetics. Micro. 2023; 3(4):892-911. https://doi.org/10.3390/micro3040061
Chicago/Turabian StyleKavetsou, Eleni, Ioanna Pitterou, Nikos G. Dimitrioglou, Stefanos Kikionis, Eftichia Kritsi, Panagiotis Zoumpoulakis, Eleni Pontiki, Dimitra Hadjipavlou-Litina, Dimitris T. Hatziavramidis, and Anastasia Detsi. 2023. "Formulation of Pharmaceutical Tablets Containing β-Cyclodextrin-4-Methyl-Umbelliferone (Hymecromone) Inclusion Complexes and Study of the Dissolution Kinetics" Micro 3, no. 4: 892-911. https://doi.org/10.3390/micro3040061
APA StyleKavetsou, E., Pitterou, I., Dimitrioglou, N. G., Kikionis, S., Kritsi, E., Zoumpoulakis, P., Pontiki, E., Hadjipavlou-Litina, D., Hatziavramidis, D. T., & Detsi, A. (2023). Formulation of Pharmaceutical Tablets Containing β-Cyclodextrin-4-Methyl-Umbelliferone (Hymecromone) Inclusion Complexes and Study of the Dissolution Kinetics. Micro, 3(4), 892-911. https://doi.org/10.3390/micro3040061











