The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems
Abstract
1. Introduction
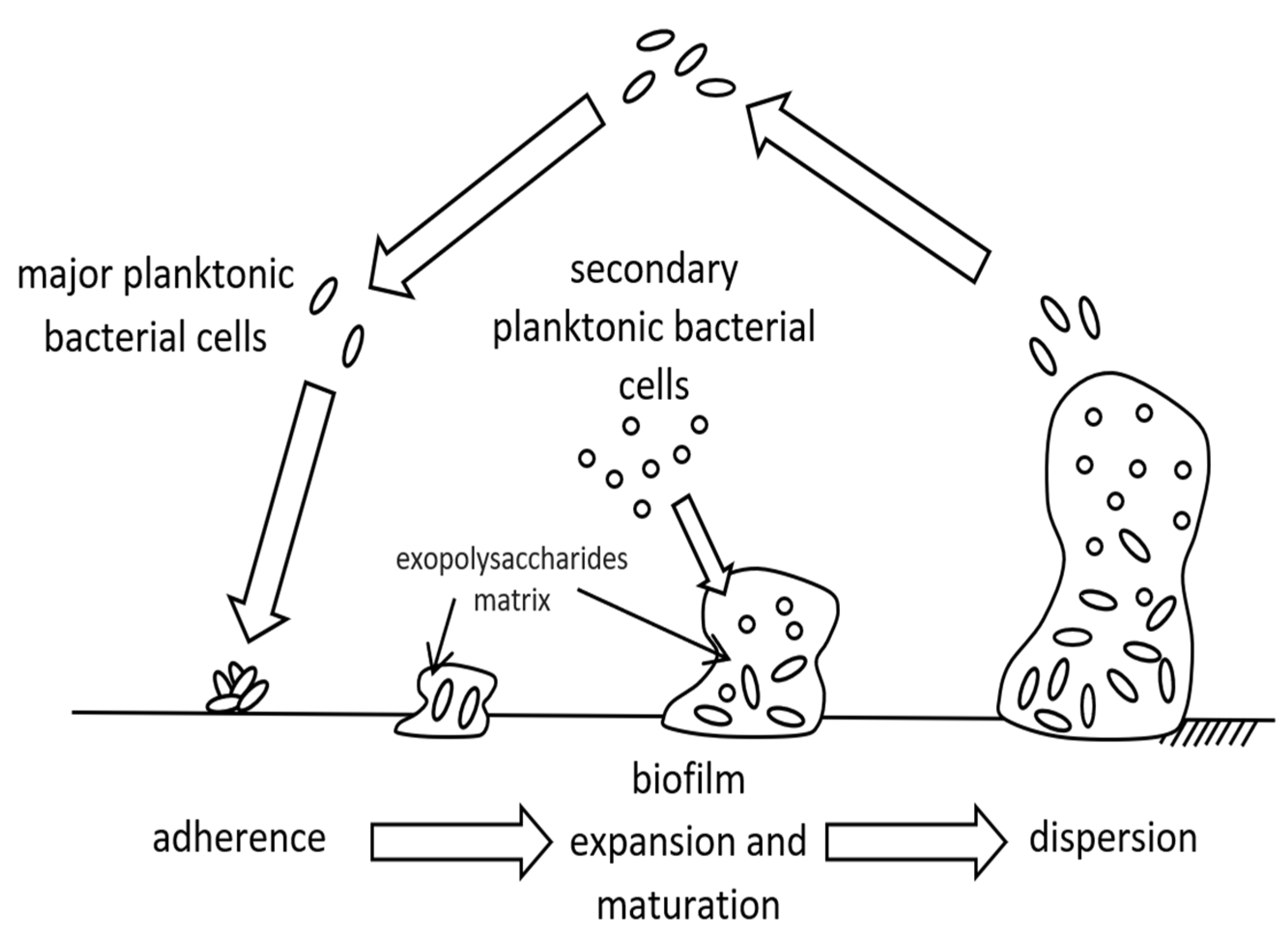
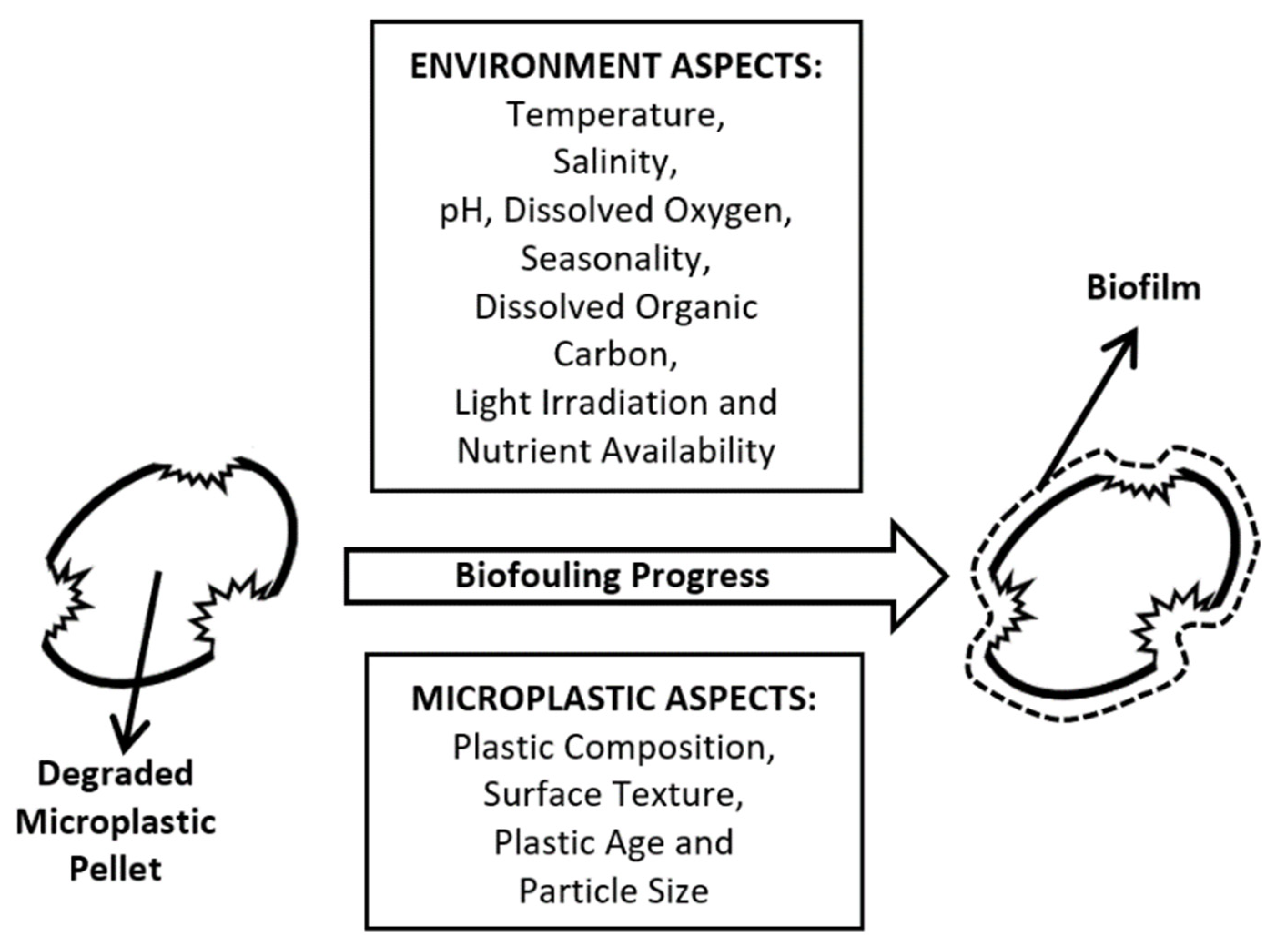
2. Biofouling (Biofilm Formation)
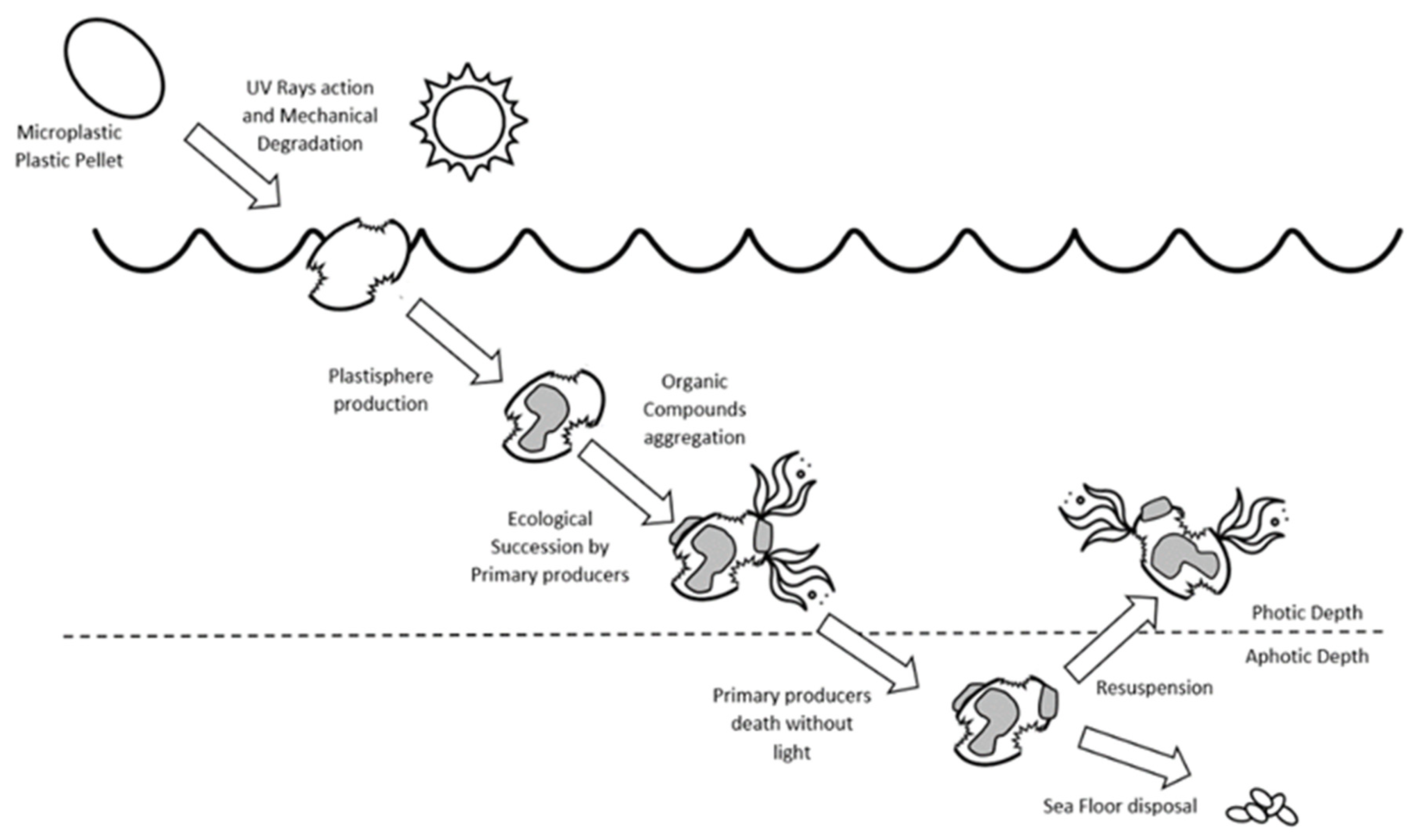
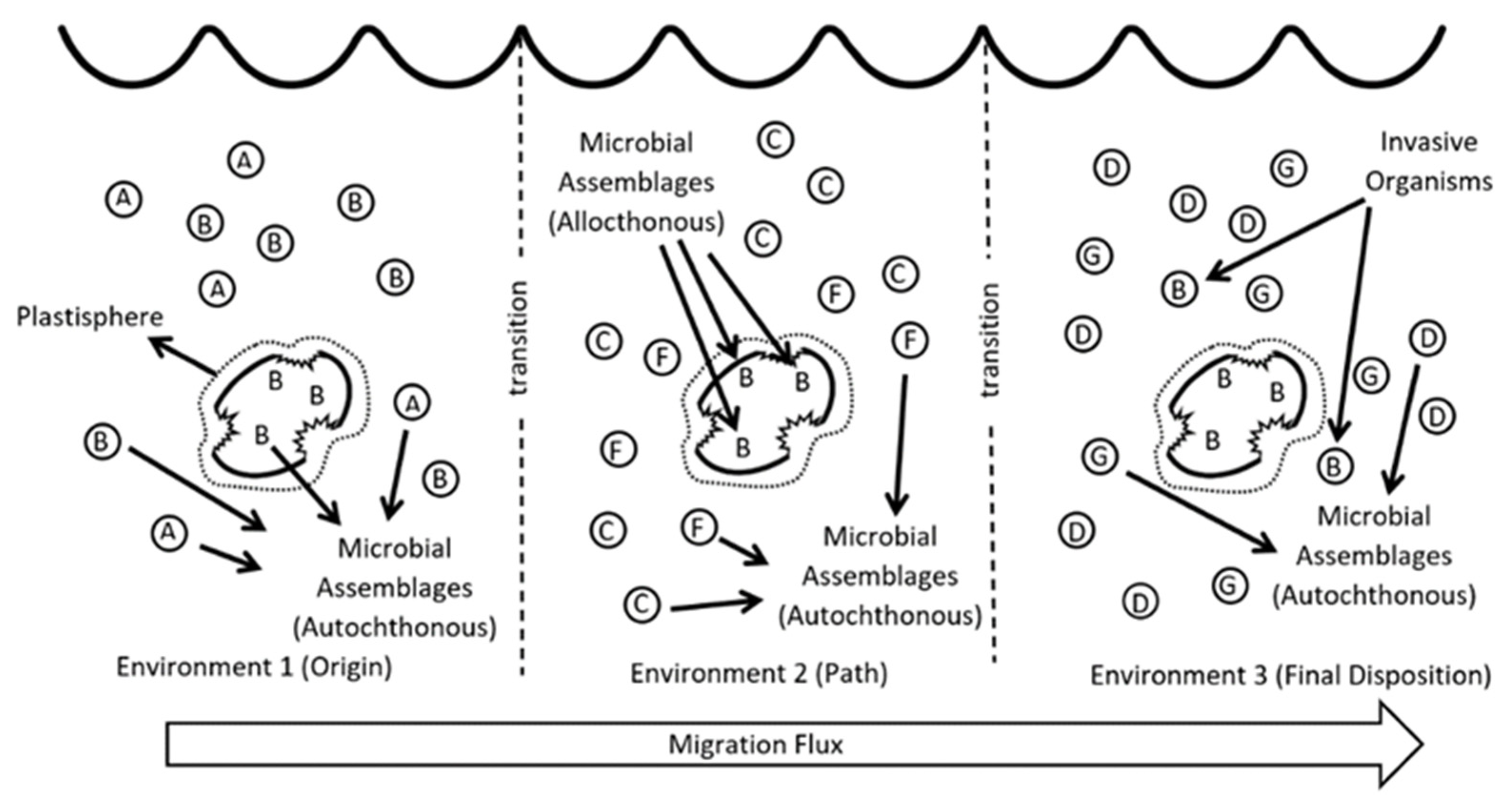
3. The Plastisphere
4. The Plastisphere Micro-Niche and Biodegradation
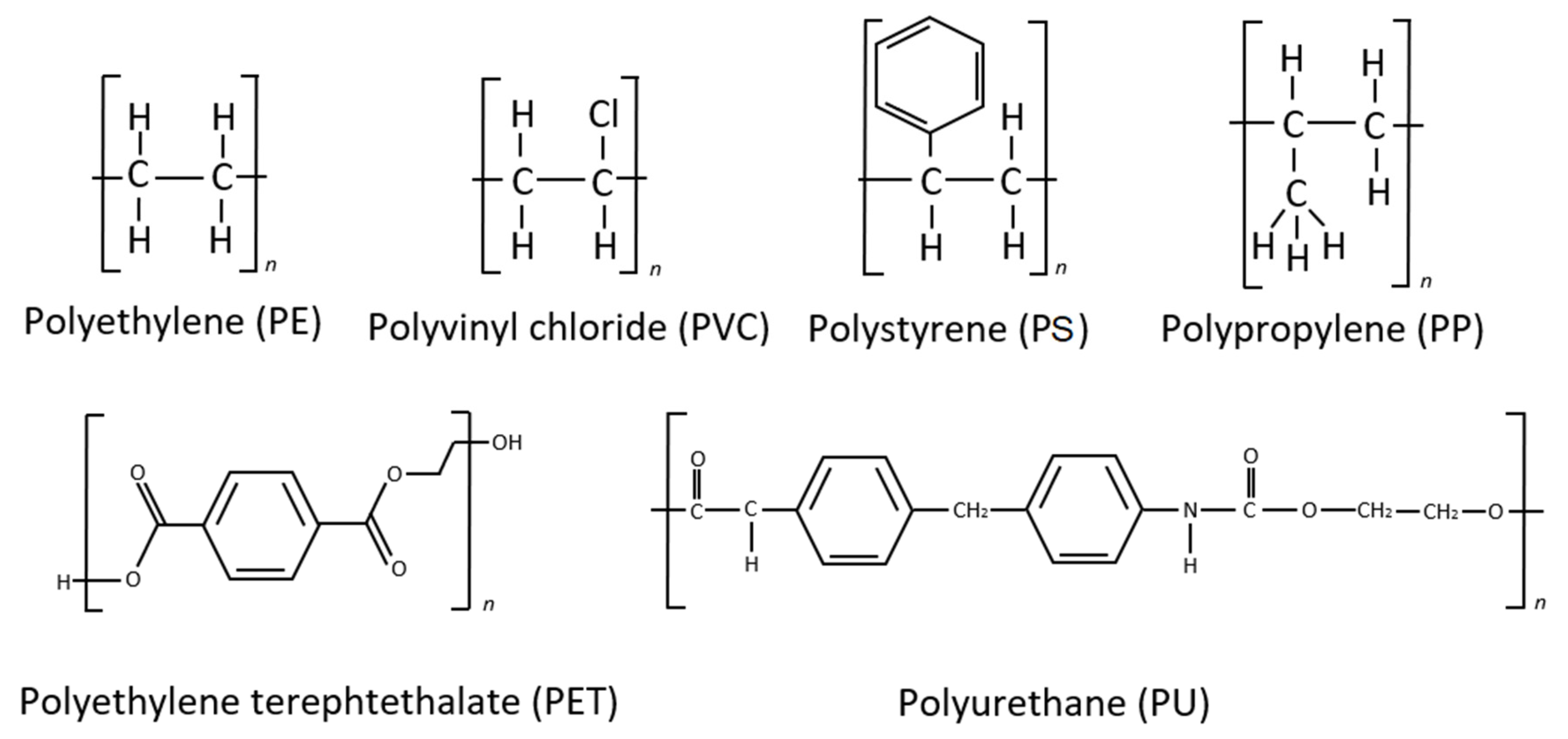
5. Linear Carbon Chain Axis Polymers
6. Polymers with Ester-Linked Backbones and Side Chains
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Löder, M.; Labrenz, M. Marine microplastic-associated biofilms—A review. Environ. Chem. 2015, 12, 551–562. [Google Scholar] [CrossRef]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Let. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Law, K.L. Plastics in the marine environment. Annu. Rev. Mar. Sci. 2017, 9, 205–229. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Mees, J.; Janssen, C.R. Assessment of marine debris on the Belgian Continental Shelf. Mar. Pollut. Bull. 2013, 73, 161–169. [Google Scholar] [CrossRef]
- Woodall, L.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.; Narayanaswamy, B.; Thompson, R. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.-M.; Greer, S.D.; Borrero, J.C. Numerical modelling of floating debris in the world’s oceans. Mar. Pollut. Bull. 2012, 64, 653–661. [Google Scholar] [CrossRef]
- Cózar, A.; Sanz-Martín, M.; Martí, E.; González-Gordillo, J.I.; Ubeda, B.; Gálvez, J.Á.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, 0121762. [Google Scholar] [CrossRef]
- Cózar, A.; Martí, E.; Duarte, C.M.; García-de-Lomas, J.; van Sebille, E.; Ballatore, T.J.; Eguíluz, V.M.; González-Gordillo, J.I.; Pedrotti, M.L.; Echevarría, F.; et al. The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci. Adv. 2017, 3, 1600582. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nat. Commun. 2017, 8, 15611. [Google Scholar]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Baptista Neto, J.A.; Fonseca, E.M. Paint fragments as polluting microplastics: A brief review. Mar. Pollut. Bull. 2021, 162, 9. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, 111913. [Google Scholar] [CrossRef]
- Palatinus, A.; Viršek, M.K.; Robič, U.; Grego, M.; Bajt, O.; Šiljić, J.; Suaria, G.; Liubartseva, S.; Coppini, G.; Peterlin, M. Marine litter in the Croatian part of the middle Adriatic Sea: Simultaneous assessment of floating and seabed macro and micro litter abundance and composition. Mar. Pollut. Bull. 2019, 139, 427–439. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Poll. Bull. 2016, 108, 105–112. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; MCGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of Lagoon of Venice, Italy: First observations. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Ye, S.; Andrady, A.L. Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar. Pollut. Bull. 1991, 22, 608–613. [Google Scholar] [CrossRef]
- Moret-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Artham, T.; Sudhakar, M.; Venkatesan, R.; Madhavan, N.C.; Murty, K.V.G.K.; Doble, M. Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegrad. 2009, 63, 884–890. [Google Scholar] [CrossRef]
- Kerr, A.; Cowling, M.J. The effects of surface topography on the accumulation of biofouling. Philos. Mag. 2003, 83, 2779–2795. [Google Scholar] [CrossRef]
- Carson, H.S.; Norheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Poll. Bull. 2013, 75, 126–132. [Google Scholar] [CrossRef]
- Eich, A.; Mildenberger, T.; Laforsch, C.; Weber, M. Biofilm and diatom succession on polyethylene (PE) and biodegradable plastic bags in two marine habitats: Early signs of degradation in the pelagic and benthic zone? PLoS ONE 2015, 10, 0137201. [Google Scholar] [CrossRef]
- Harrison, J.P.; Schratzberger, M.; Sapp, M.; Osborn, A.M. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014, 14, 232. [Google Scholar] [CrossRef]
- Bryant, J.A.; Clemente, T.M.; Viviani, D.A.; Fong, A.A.; Thomas, K.A.; Kemp, P.; Karl, D.M.; White, A.E.; DeLong, E.F. Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre Systems. ASM J. 2016, 1, e00024-16. [Google Scholar]
- Kettner, M.T.; Rojas-Jimenez, K.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.P. Microplastics alter composition of fungal communities in aquatic ecosystems. Environ. Microbiol. 2017, 19, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Dussud, C.; Meistertzheim, A.L.; Conan, P.; Pujo-Pay, M.; George, M.; Fabre, P.; Coudane, J.; Higgs, P.; Elineau, A.; Pedrotti, M.L.; et al. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ. Poll. 2018, 236, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ogonowski, M.; Motiei, A.; Ininbergs, K.; Hell, E.; Gerdes, Z.; Udekwu, K.I.; Bacsik, Z.; Gorokhova, E. Evidence for selective bacterial community structuring on microplastics. Environ. Microbiol. 2018, 20, 2796–2808. [Google Scholar] [CrossRef]
- Philippot, L.; Andersson, S.G.; Battin, T.J.; Prosser, J.I.; Schimel, J.P.; Whitman, W.B.; Hallin, S. The ecological coherence of high bacterial taxonomic ranks. Nat. Rev. Microbiol. 2010, 8, 523–529. [Google Scholar] [CrossRef]
- Rousk, J.; Bengtson, P. Microbial regulation of global biogeochemical cycles. Front. Microbiol. 2014, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, K.; Xiong, X. Microplastic pollution in inland waters focusing on Asia. In Freshwater Microplastics; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Mistri, M.; Scoponi, M.; Granata, T.; Moruzzi, L.; Massara, F.; Munari, C. Types, occurrence and distribution of microplastics in sediments from the northern Tyrrhenian Sea. Mar. Poll. Bull. 2020, 153, 111016. [Google Scholar] [CrossRef] [PubMed]
- Alomar, C.; Estarellas, F.; Deudero, S. Microplastics in the Mediterranean Sea: Deposition in coastal shallow sediments, spatial variation and preferential grain size. Mar. Environ. Res. 2016, 115, 1–10. [Google Scholar] [CrossRef]
- Critchell, K.; Lambrechts, J. Modelling accumulation of marine plastics in the coastal zone; what are the dominant physical processes? Estuar. Coast. Shelf Sci. 2016, 171, 111–122. [Google Scholar] [CrossRef]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Inter. 2020, 136, 105411. [Google Scholar] [CrossRef]
- Amaral-Zettler, L.; Zettler, E.; Slikas, B.; Boyd, G.; Melvin, D.; Morrall, C.; Proskurowski, G.; Mincer, T. The biogeography of the Plastisphere: Implications for policy. Front. Ecol. Environ. 2015, 13, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Jacquin, J.; Conan, P.; Pujo-Pay, M.; Barbe, V.; George, M.; Fabre, P.; Bruzaud, S.; Ter Halle, A.; Meistertzheim, A.L.; et al. Relative influence of plastic debris size and shape, chemical composition and phytoplankton-bacteria interactions in driving seawater plastisphere abundance, diversity and activity. Front. Microbiol. 2021, 11, 610231. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. T. Enviro. 2020, 698, 134254. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- McGivney, E.; Cederholm, L.; Barth, A.; Hakkarainen, M.; Hamacher-Barth, E.; Ogonowski, M.; Gorokhova, E. Rapid physicochemical changes in microplastic induced by biofilm formation. Front. Bioeng. Biotechnol. 2020, 8, 205. [Google Scholar]
- Tourinho, P.S.; Koci, V.; Loureiro, S.; Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Vianello, A.; Vollertsen, J. Retention of microplastics in sediments of urban and highway stormwater retention ponds. Environ. Pollut. 2019, 255, 113335. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, J.; Zheng, Y.; Yang, Y.; Zhang, Y.; He, F.; Chen, H.; Quan, G.; Yan, J.; Li, T.; et al. Environmental occurrences, fate, and impacts of microplastics. Ecotoxicol. Environ. Saf. 2019, 30, 184. [Google Scholar] [CrossRef]
- Da Fonseca, E.M.; Gaylarde, C.C.; Baptista Neto, J.A.; Camacho Chab, J.C.; Ortega-Morales, O. Microbial interactions with particulate and floating pollutants in the oceans: A review. Micro 2022, 2, 257–276. [Google Scholar] [CrossRef]
- Almeida, M.P.; Gaylarde, C.C.; Baptista Neto, J.A.; Neves, C.V.; Fonseca, E.M. Particulate and Floating Pollutants in the Oceans. Encycl. J. 2022, 1, 1. [Google Scholar]
- Amaral-Zettler, L.A.; Zettler, E.R.; Mincer, T.J. Ecology of the plastisphere. Nat. Rev. Microbiol. 2020, 18, 139–151. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption behavior and mechanisms of organic contaminants to nano and microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- Bhagwat, N.R.; Owens, S.N.; Ito, M.; Boinapalli, J.V.; Poa, P.; Ditzel, A.; Kopparapu, S.; Mahalawat, M.; Davies, O.R.; Collins, S.R.; et al. SUMO is a pervasive regulator of meiosis. Elife 2021, 10, 57720. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; van der Plaats, R.Q.; van der Wielen, P.W.; Bauerlein, P.S.; de Roda Husman, A.M. Riverine microplastic and microbial community compositions: A field study in the Netherlands. Water Res. 2021, 192, 116852. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, A. A roadmap for a Plastisphere. Mar. Poll. Bull. 2021, 167, 112322. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 1, 415. [Google Scholar] [CrossRef]
- Wang, R.; Neoh, K.G.; Shi, Z.; Kang, E.T.; Tambyah, P.A.; Chiong, E. Inhibition of escherichia coli and proteus mirabilis adhesion and biofilm formation on medical grade silicone surface. Biotechnol. Bioeng. 2011, 109, 336–345. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.; Nadagouda, M.; Aminabhavi, T. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Engin. J. 2020, 408, 127317. [Google Scholar] [CrossRef]
- Reisser, J.; Shaw, J.; Hallegraeff, G.; Proietti, M.; Barnes, D.K.; Thums, M.; Wilcox, C.; Hardesty, B.D.; Pattiaratchi, C. Millimeter-sized marine plastics: A new pelagic habitat for microorganisms and invertebrates. PLoS ONE 2014, 9, 100289. [Google Scholar] [CrossRef] [PubMed]
- Debroas, D.; Mone, A.; Ter Halle, A. Plastics in the North Atlantic garbage patch: A boat-microbe for hitchhikers and plastic degraders. Sci. Total Environ. 2017, 599, 1222–1232. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Intern. 2019, 123, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Hao, X.; Wang, J.; Zhang, Y. Distribution of low-density microplastics in the mollisol farmlands of northeast China. Sci. Total Environ. 2020, 708, 135091. [Google Scholar] [CrossRef] [PubMed]
- Bowley, J.; Baker-Austin, C.; Porter, A.; Hartnell, R.; Lewis, C. Oceanic hitchhikers–assessing pathogen risks from marine microplastic. Trends Microbiol. 2021, 29, 107–116. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Primers. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- De Souza Valente, C.; Wan, A.H. Vibrio and major commercially important vibriosis diseases in decapod crustaceans. J. Invertebrate Pathol. 2021, 181, 107527. [Google Scholar] [CrossRef]
- Silva, M.M.; Maldonado, G.C.; Castro, R.O.; de Sá Felizardo, J.; Cardoso, R.P.; Dos Anjos, R.M.; de Araújo, F.V. Dispersal of potentially pathogenic bacteria by plastic debris in Guanabara Bay, RJ, Brazil. Mar. Poll. Bull. 2019, 141, 561–568. [Google Scholar] [CrossRef]
- Ward, C.S.; Diana, Z.; Ke, K.M.; Orihuela, B.; Schultz, T.P.; Rittschof, D. Microbiome development of seawater-incubated pre-production plastic pellets reveals distinct and predictive community compositions. Front. Mar. Sci. 2022, 8, 2047. [Google Scholar] [CrossRef]
- Erni-Cassola, G.; Wright, R.J.; Gibson, M.I.; Christie-Oleza, J.A. Early colonization of weathered polyethylene by distinct bacteria in marine coastal seawater. Microb. Ecol. 2020, 79, 517–526. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Zhu, X. The plastic cycle–an unknown branch of the carbon cycle. Front. Mar. Sci. 2021, 7, 1227. [Google Scholar] [CrossRef]
- Goudriaan, M.; Morales, V.H.; van der Meer, M.T.; Mets, A.; Ndhlovu, R.T.; van Heerwaarden, J.; Simon, S.; Heuer, V.B.; Hinrichs, K.U.; Niemann, H. A stable isotope assay with 13C-labeled polyethylene to investigate plastic mineralization mediated by Rhodococcus ruber. Mar. Poll. Bull. 2023, 186, 114369. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K. Polythene and Plastics-degrading microbes from the mangrove soil. Rev. De Biol. Trop. 2003, 51, 629–633. [Google Scholar]
- Skariyachan, S.; Patil, A.A.; Shankar, A.; Manjunath, M.; Bachappanavar, N.; Kiran, S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym. Degrad. Stab. 2018, 149, 52–68. [Google Scholar] [CrossRef]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145. [Google Scholar] [CrossRef]
- Puglisi, E.; Romaniello, F.; Galletti, S.; Boccaleri, E.; Frache, A.; Sandro, P. Selective bacterial colonization processes on polyethylene waste samples in an abandoned landfill site. Sci. Rep. 2019, 9, 14138. [Google Scholar] [CrossRef]
- Bardají, D.K.R.; Furlan, J.P.R.; Stehling, E.G. Isolation of a polyethylene degrading Paenibacillus sp. from a landfill in Brazil. Arch. Microbiol. 2019, 201, 699–704. [Google Scholar] [CrossRef]
- Cárdenas Espinosa, M.J.; Colina Blanco, A.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward Biorecycling: Isolation of a Soil Bacterium That Grows on a Polyurethane Oligomer and Monomer. Front. Microbiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Janatunaim, R.Z.; Fibriani, A. Construction and cloning of plastic-degrading recombinant enzymes (MHETase). Recent. Pat. Biotechnol. 2020, 14, 229–234. [Google Scholar] [CrossRef]
- Roy, R.; Mukherjee, G.; Das Gupta, A.; Tribedi, P.; Sil, A.K. Isolation of a soil bacterium for remediation of polyurethane and low-density polyethylene: A promising tool towards sustainable cleanup of the environment. Biotech 2021, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.C.; Anderson, S.O.; Karlsson, S. Mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Deana, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Colin, P.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef]
- Pirt, S.J. Microbial degradation of synthetic polymers. J. Chem. Technol. Biotechnol. 1980, 30, 176–179. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Karlsson, S. Aspects of biodeterioration of inert and degradable polymers. Int. Biodeterior. Biodegrad 1993, 31, 161–170. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2022: An Analysis of European Plastics Production, Demand and Waste Data 2022; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and enzymatic degradation of synthetic plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbio. 2019, 65, 224–234. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Karlsson, S. The influence of biotic and abiotic environments on the degradation of polyethylene. Prog. Polym. Sci. 1990, 15, 177–192. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Shimpi, N.; Borane, M.; Mishra, S.; Kadam, M. Biodegradation of polystyrene (PS)-poly(lactic acid) (PLA) nanocomposites using Pseudomonas aeruginosa. Macromol. Res. 2012, 20, 181–187. [Google Scholar] [CrossRef]
- Jadaun, J.S.; Bansal, S.; Sonthalia, A.; Rai, A.K.; Singh, S.P. Biodegradation of plastics for sustainable environment. Bioresour. Technol. 2022, 1, 126697. [Google Scholar] [CrossRef]
- Schlemmer, D.; Sales, M.J.; Resck, I.S. Degradation of different polystyrene/thermoplastic starch blends buried in soil. Carbohydr. Polym. 2009, 75, 58–62. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Weber, R.W.; Dumais, J.J.; Hanna, M.A. Biodegradation characteristics of starch–polystyrene loose-fill foams in a composting medium. Bioresour. Technol. 2010, 101, 7258–7264. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, V.; Veličković, S.; Antonović, D.G.; Popović, A.R. Biodegradation of starch-graft-polystyrene and starch-graft-poly (methacrylic acid) copolymers in model river water. J. Serb. Chem. Soc. 2013, 78, 1425–1441. [Google Scholar] [CrossRef]
- Cacciari, I.; Quatrini, P.; Zirletta, G.; Mincione, E.; Vinciguerra, V.; Lupattelli, P.; Giovannozzi, S.G. Isotactic polypropylene biodegradation by a microbial community: Physicochemical characterization of metabolites produced. Appl. Environ. Microbiol. 1993, 59, 3695–3700. [Google Scholar] [CrossRef]
- Iwamoto, A.; Tokiwa, Y. Effect of the phase structure on biodegradability of polypropylene/poly(ε-caprolactone) blends. J. Appl. Polym. Sci. 1994, 52, 1357–1360. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Roan, M.-L.; Kuo, M.-C.; Lu, W.-L. Effect of compatibiliser on the biodegradation and mechanical properties of highcontent starch/low-density polyethylene blends. Polym. Degrad. Stab. 2005, 90, 95–105. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Oldak, D.; Malanowski, P.; Chaberska, H. Effect of short wavelength UV-irradiation on ageing of polypropylene/cellulose compositions. Polym. Degrad. Stab. 2005, 88, 189–198. [Google Scholar] [CrossRef]
- Ramis, X.; Cadenato, A.; Salla, J.M.; Morancho, J.M.; Valles, A.; Contat, L.; Ribes, A. Thermal degradation of polypropylene/starch-based materials with enhanced biodegradability. Polym. Degrad. Stab. 2004, 86, 483–491. [Google Scholar] [CrossRef]
- Arkatkar, A.; Juwarkar, A.A.; Bhaduri, S.; Uppara, P.V.; Doble, M. Growth of Pseudomonas and Bacillus biofilms on pretreated polypropylene surface. Int. Biodeterior. Biodegrad. 2010, 64, 530–536. [Google Scholar] [CrossRef]
- Zuchowska, D.; Steller, R.; Meissner, W. Structure and properties of degradable polyolefin-starch blends. Polym. Degrad. Stab. 1998, 60, 471–480. [Google Scholar] [CrossRef]
- Morancho, J.M.; Ramis, X.; Fernández, X.; Cadenato, A.; Salla, J.M.; Vallés, A.; Contat, L.; Ribes, A. Calorimetric and thermogravimetric studies of UV-irradiated polypropylene/starch-based materials aged in soil. Polym. Degrad. Stab. 2006, 91, 44–51. [Google Scholar] [CrossRef]
- Weiland, M.; Daro, A.; David, C. Biodegradation of thermally oxidised polyethylene. Polym. Degrad. Stab. 1995, 48, 275–289. [Google Scholar] [CrossRef]
- Farzi, A.; Dehnad, A.; Fotouhi, A.F. Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatal. Agric. Biotechnol. 2019, 17, 25–31. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J.; et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Zhu, B.; Ye, Q.; Seo, Y.; Wei, N. Enzymatic degradation of polyethylene terephthalate plastics by bacterial Curli display PETase. Environ. Sci. Technol. Lett. 2022, 9, 650–657. [Google Scholar] [CrossRef]
- Zurier, H.S.; Goddard, J.M. A high-throughput expression and screening platform for applications-driven PETase engineering. Biotechnol. Bioeng. 2023, 1, 1–15. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Liu, X.; Dong, S.; Tian, Y.E.; Qiao, Y.; Mitra, R.; Han, J.; Li, C.; Han, X.; et al. Computational redesign of a PETase for plastic biodegradation under ambient conditions by the GRAPE strategy. ACS Catal. 2021, 11, 1340–1350. [Google Scholar] [CrossRef]
- Deng, B.; Yue, Y.; Yang, J.; Yang, M.; Xing, Q.; Peng, H.; Wang, F.; Li, M.; Ma, L.; Zhai, C. Improving the activity and thermostability of PETase from Ideonella sakaiensis through modulating its post-translational glycan modification. Commun. Biol. 2023, 6, 39. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Guo, Z.; Yan, T.; Jin, C.; Wu, J. Enhancement of the degradation capacity of IsPETase for PET plastic degradation by protein engineering. Sci. Total Environ. 2022, 834, 154947. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, L.Q.; Jiang, W. Biodegrading plastics with a synthetic non-biodegradable enzyme. Chem 2022, 4, 363–375. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Xue, R.; Xu, B.; Qian, X.; Xin, F.; Blank, L.M.; Zhou, J.; Wei, R.; Dong, W.; et al. Biodegradation and up-cycling of polyurethanes: Progress, challenges, and prospects. Biotechn. Ad. 2021, 48, 107730. [Google Scholar] [CrossRef] [PubMed]
- Klrbas, Z.; Güner, N.K.A. Biodegradation of Polyvinylchloride (PVC) by white rot fungi. Bull. Environ. Contam. Toxicol. 1999, 63, 335–342. [Google Scholar]
- Das, G.; Bordoloi, N.K.; Rai, S.K.; Mukherjee, A.K.; Karak, N. Biodegradable and biocompatible epoxidized vegetable oil modified thermostable poly(vinyl chloride): Thermal and performance characteristics post biodegradation with Pseudomonas aeruginosa and Achromobacter sp. J. Hazard. Mater. 2012, 209–210, 434–442. [Google Scholar] [CrossRef]
- Vivi, V.K.; Martins-Franchetti, S.M.; Attili-Angelis, D. Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiol. 2019, 64, 1–7. [Google Scholar] [CrossRef]
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and molecular characterization of polyvinyl chloride (PVC) plastic degrading fungal isolates. J. Basic Microbiol. 2014, 54, 18–27. [Google Scholar] [CrossRef]
- Kemona, A.; Piotrowska, M. Polyurethane recycling and disposal: Methods and prospects. Polymers 2020, 12, 1752. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Chen, J.; Gnanasekar, P.; Ma, X.; Qin, D.; Na, H.; Zhu, J.; Yan, N. A facile preparation strategy of polycaprolactone (PCL)-based biodegradable polyurethane elastomer with a highly efficient shape memory effect. New J. Chem. 2020, 44, 658–662. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, D.; Zheng, Y.; Zhao, L.; Xu, T.; Guo, Z.; Hussain, M.I.; Zeng, J.; Lou, L.; Sun, Y.; et al. A novel waterborne polyurethane with biodegradability and high flexibility for 3D printing. Biofabric 2020, 12, 035015. [Google Scholar] [CrossRef]
- Guo, Y.; An, X.; Qian, X. Biodegradable and reprocessable cellulose-based polyurethane films for bonding and heat dissipation in transparent electronic devices. Ind. Crops Prod. 2023, 193, 116247. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, P.; Tanwar, S.; Varshney, G.; Yadav, S. Assessment of bio-based polyurethanes: Perspective on applications and bio-degradation. Macromol 2022, 2, 284–314. [Google Scholar] [CrossRef]
- Skleničková, K.; Abbrent, S.; Halecký, M.; Kočí, V.; Beneš, H. Biodegradability and ecotoxicity of polyurethane foams: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 157–202. [Google Scholar] [CrossRef]
- Miao, L.; Li, W.; Adyel, T.M.; Yao, Y.; Deng, Y.; Wu, J.; Zhou, Y.; Yu, Y.; Hou, J. Spatio-temporal succession of microbial communities in plastisphere and their potentials for plastic degradation in freshwater ecosystems. Wat. Res. 2023, 229, 119406. [Google Scholar] [CrossRef]
- Roosen, M.; Mys, N.; Kusenberg, M.; Billen, P.; Dumoulin, A.; Dewulf, J.; Van Geem, K.; Ragaert, K.; De Meester, S. Detailed analysis of the composition of selected plastic packaging waste products and Its implications for mechanical and thermochemical recycling. Environ. Sci. Tech. 2020, 54, 13282–13293. [Google Scholar] [CrossRef]
- Wiesinger, H.; Wang, Z.; Hellweg, S. Deep dive into plastic monomers, additives, and processing aids. Environ. Sci. Technol. 2021, 55, 9339–9351. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Envir. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- Matjašič, T.; Simčič, T.; Medvešček, N.; Bajt, O.; Dreo, T.; Mori, N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021, 752, 141959. [Google Scholar] [CrossRef]
- Dey, S.; Anand, U.; Kumar, V.; Kumar, S.; Ghorai, M.; Ghosh, A.; Kant, N.; Suresh, S.; Bhattacharya, S.; Bontempi, E.; et al. Microbial strategies for degradation of microplastics generated from COVID-19 healthcare waste. Environ. Res. 2023, 216, 114438. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Strzelecki, M.C.; Kociuba, W.; Franczak, L.; Mironczuk, A.M. Isolation and characterization of Arctic microorganisms decomposing bioplastics. AMB Express 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme—Laccase—In the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeter. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ. Sci. Pollut. Res. 2012, 20, 4146–4153. [Google Scholar] [CrossRef]
- Webb, H.K.; Crawford, R.J.; Sawabe, T.; Ivanova, E.P. Poly(ethylene terephthalate) polymer surfaces as a substrate for bacterial attachment and biofilm formation. Microbes Environ. 2009, 24, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Pajk, J.; Drozd-Bratkowicz, M.; Rymarz, G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int. Biodeterior. Biodegradation. 2011, 65, 757–767. [Google Scholar] [CrossRef]
- Rogers, K.L.; Carreres-Calabuig, J.A.; Gorokhova, E.; Posth, N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. Lett. 2020, 5, 18–36. [Google Scholar] [CrossRef]
- Pathak, V.M.; Navneet. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 15. [Google Scholar] [CrossRef]
- Urbanek, A.K.; Rymowicz, W.; Mirończuk, A.M. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl. Microbiol. Biotechnol. 2018, 102, 7669–7678. [Google Scholar] [CrossRef]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. Plastic DB: A database of microorganisms and proteins linked to plastic biodegradation. Database 2022, 2022, baac008. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J. Haz. Mat. 2019, 380, 120899. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, N.R.; Tessman, M.; Zhen, D.; Johnson, L.; Evans, P.; Clements, S.M.; Pomeroy, R.S.; Burkart, M.D.; Simkovsky, R.; Mayfield, S.P. Biodegradation of renewable polyurethane foams in marine environments occurs through depolymerization by marine microorganisms. Sci. Total Environ. 2022, 850, 158761. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Poll. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.; Doble, M.; Murthy, P.S.; Venkatesan, R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeter. Biodegrad. 2008, 61, 203–213. [Google Scholar] [CrossRef]
- Devi, R.S.; Rajesh Kannan, V.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef]
- Mohanrasu, K.N.; Premnath, G.; Siva, P.; Sudhakar, M.; Boobalan, T.; Arun, A. Exploring multi potential uses of marine bacteria; an integrated approach for PHB production, PAHs and polyethylene biodegradation. J. Photochem. Photobiol. B 2018, 185, 55–65. [Google Scholar] [CrossRef]
- Wright, R.J.; Bosch, R.; Langille, M.G.; Gibson, M.I.; Christie-Oleza, J.A. A multi-OMIC characterisation of biodegradation and microbial community succession within the PET plastisphere. Microbiome 2021, 9, 141. [Google Scholar] [CrossRef]
- Kumar, A.G.; Hinduja, M.; Sujitha, K.; Rajan, N.N.; Dharani, G. Biodegradation of polystyrene by deep-sea Bacillus paralicheniformis G1 and genome analysis. Sci. Total Environ. 2021, 774, 145002. [Google Scholar] [CrossRef]
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2017, 8, 2709. [Google Scholar] [CrossRef]
- Khandare, S.D.; Chaudhary, D.R.; Jha, B. Marine bacterial biodegradation of low-density polyethylene (LDPE) plastic. Biodegradation 2021, 32, 127–143. [Google Scholar] [CrossRef]
- Delacuvellerie, A.; Benali, S.; Cyriaque, V.; Moins, S.; Raquez, J.M.; Gobert, S.; Wattiez, R. Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 2021, 419, 126526. [Google Scholar] [CrossRef]
- Suzuki, M.; Tachibana, Y.; Oba, K.; Takizawa, R.; Kasuya, K.I. Microbial degradation of poly (ε-caprolactone) in a coastal environment. Polym. Degrad. Stab. 2018, 149, 1–8. [Google Scholar] [CrossRef]
- Won, S.J.; Yim, J.H.; Kim, H.K. Functional production, characterization, and immobilization of a cold-adapted cutinase from Antarctic Rhodococcus sp. Protein Expr. Purif. 2022, 195, 106077. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, S.; Zhang, B.; Li, G.; Fu, X.; Yan, P.; Shao, Z. Biodegradation of polystyrene (PS) by marine bacteria in mangrove ecosystem. J. Hazard. Mater. 2023, 442, 130056. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.L.; Rincón, A.F.C.; Jackson, S.A.; Dobson, A.D. In silico screening and heterologous expression of a polyethylene terephthalate hydrolase (PETase)-like enzyme (SM14est) with polycaprolactone (PCL)-degrading activity, from the marine sponge-derived strain Streptomyces sp. SM14. Front. Microbi. 2019, 10, 2187. [Google Scholar] [CrossRef]
- Gao, R.; Liu, R.; Sun, C. A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J. Hazard. Mater. 2022, 431, 128617. [Google Scholar] [CrossRef]
- Gonda, K.E.; Jendrossek, D.; Molitoris, H.P. Fungal degradation of the thermoplastic polymer poly-ß-hydroxybutyric acid (PHB) under simulated deep sea pressure. In Life at Interfaces and Under Extreme Conditions; Springer: Dordrecht, The Netherlands, 2000; Volume 1, pp. 173–183. [Google Scholar]
- Kawai, F.; Oda, M.; Tamashiro, T.; Waku, T.; Tanaka, N.; Yamamoto, M.; Mizushima, H.; Miyakawa, T.; Tanokura, M. A novel Ca2+-activated, thermostabilized polyesterase capable of hydrolyzing polyethylene terephthalate from Saccharomonospora viridis AHK190. Appl. Microbiol. Biotechnol. 2014, 98, 10053–10064. [Google Scholar] [CrossRef]
- Paço, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
| Genus/Species | Type of Plastic | Geographic Location | Comments | Reference(s) |
|---|---|---|---|---|
| Alcanivorax borkumensis | PE | Mediterranean Sea | 5–27 m depth | [143] |
| Alteromonas | PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] |
| Arenibacter | PE | Mediterranean Sea | 5–27 m depth | [145] |
| Bacillus spp. | PE | India | Pelagic water | [144,146,147,148] |
| Worldwide | Marine waters | [149] | ||
| PS | Arabian Sea | Deep sea | [150] | |
| Brevibacillus borstelensis | PE | India | Seawater | [148] |
| Erythrobacter | PS, PE | Baltic Sea | Cold seawater | [151] |
| PU | Pelagic seawater and seawater tanks | [144] | ||
| Halomonas sp. | PE | Marine environment | In vitro tests | [152] |
| Kocuria palustris | PE | Arabian Sea | Pelagic water | [145] |
| Marinobacter | PE | Mediterranean Sea | 5–27 m depth | [143] |
| PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] | |
| Marinomonas sp. | PLA | Mediterranean Sea | Sediment and water | [153] |
| Pseudomonas spp. | PE | Tamil Nadu, India | Coast | [147] |
| PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] | |
| PVC | India | Coastal seawater | [152] | |
| PCL | Japanese coast | Halotolerant strain | [154] | |
| Rhodococcus ruber | PE | Israel | Laboratory isolate (soil in seawater) | [155] |
| PET | Antarctic Ross Sea | Cold-adapted | [155] | |
| PS | Zhangzhou, China | Marine mangrove ecosystem | [156] | |
| Streptomyces sp. | PE | Galway Bay, Ireland | Isolated from marine sponge | [157] |
| PHA | Galway Bay, Ireland | Isolated from marine sponge | [157] | |
| PCL | Japan | Beach | [154] | |
| Thalassospira | PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] |
| Thioclava sp. | PET | Worldwide | Marine waters | [149] |
| Alternaria sp. | PE | Qingdao, China. | Huiquan bay | [158] |
| Aspergillus sp. | PHB | Bay of Bengal | Deep sea isolate | [159] |
| PE | India | Coastal sediment | [147] | |
| Cladosporium | PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] |
| Clonostachys rosea | PCL | Arctic regions | Cold seawater | [134] |
| Penicillium sp. | PU | San Diego, USA | Pelagic seawater and seawater tanks | [144] |
| Saccharomonospora viridis AHK19 | PE | Laboratory culture | Thermophilic strain | [160] |
| Trichoderma sp. | PCL | Arctic regions | Cold seawater | [134] |
| Zalerion maritimum | PE | Portugal | Seawater | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaylarde, C.C.; de Almeida, M.P.; Neves, C.V.; Neto, J.A.B.; da Fonseca, E.M. The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro 2023, 3, 320-337. https://doi.org/10.3390/micro3010022
Gaylarde CC, de Almeida MP, Neves CV, Neto JAB, da Fonseca EM. The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro. 2023; 3(1):320-337. https://doi.org/10.3390/micro3010022
Chicago/Turabian StyleGaylarde, Christine C., Marcelo P. de Almeida, Charles V. Neves, José Antônio Baptista Neto, and Estefan M. da Fonseca. 2023. "The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems" Micro 3, no. 1: 320-337. https://doi.org/10.3390/micro3010022
APA StyleGaylarde, C. C., de Almeida, M. P., Neves, C. V., Neto, J. A. B., & da Fonseca, E. M. (2023). The Importance of Biofilms on Microplastic Particles in Their Sinking Behavior and the Transfer of Invasive Organisms between Ecosystems. Micro, 3(1), 320-337. https://doi.org/10.3390/micro3010022






