Structural Consequences of Post-Synthetic Modification of Cu2P3I2
Abstract
1. Introduction
2. Experimental
2.1. Cu2P3I2 Synthesis
2.2. Post-Synthetic Modification
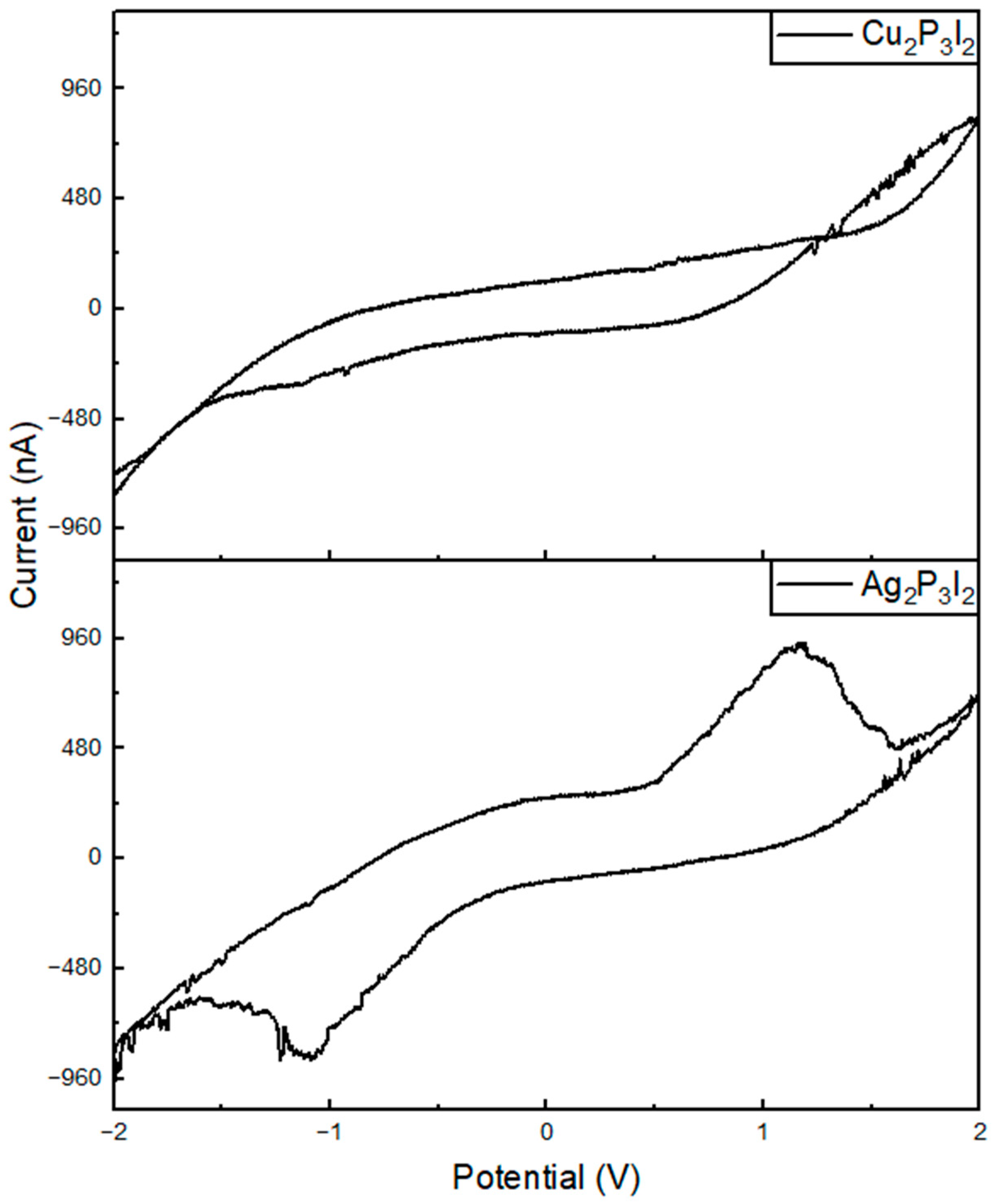
2.3. Electrical Characterization
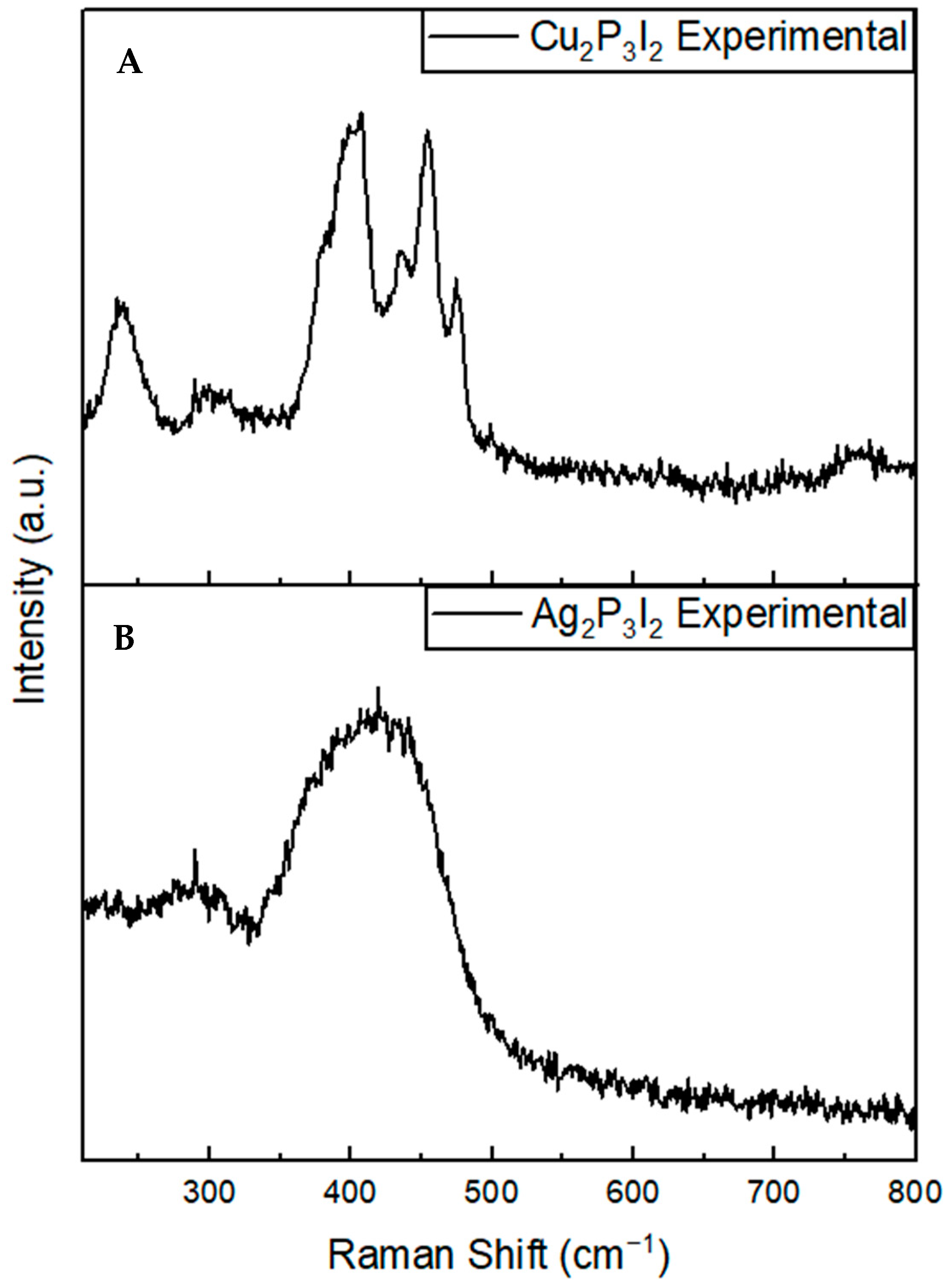
2.4. Raman Spectroscopy
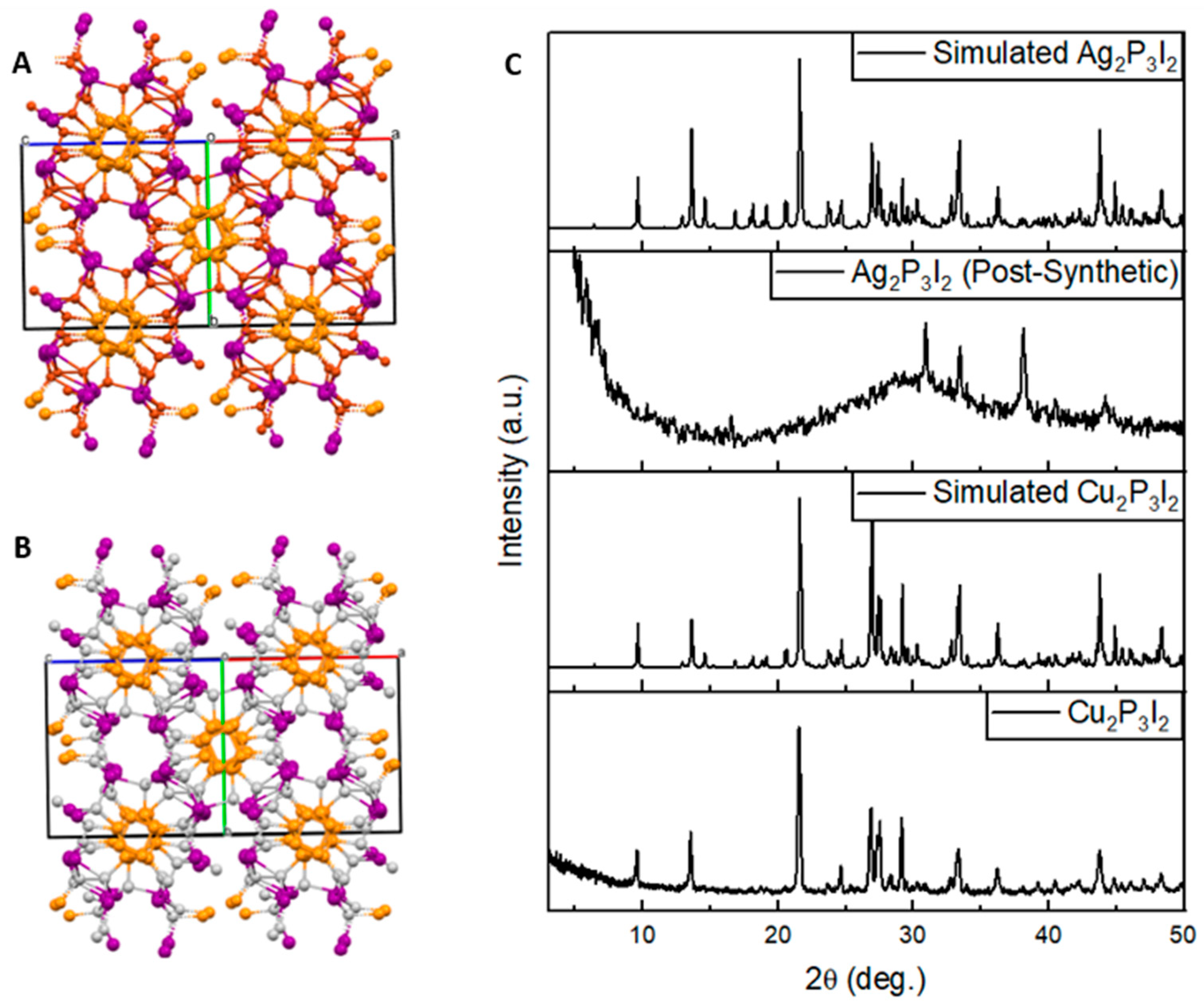
2.5. Powder X-ray Diffraction (P-XRD)
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.L.; DeWitt, T.W.; Smith, A.J. Polymorphism of Red Phosphorus. J. Am. Chem. Soc. 1947, 69, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.E.M.; Nieman, G.P.; Schwenk, G.R.; Jing, H.; Zhang, R.; Cerkez, E.B.; Strongin, D.; Ji, H. High Electron Mobility of Amorphous Red Phosphorus Thin Films. Angew. Chem. Int. Ed. 2019, 58, 6766–6771. [Google Scholar] [CrossRef] [PubMed]
- Zhaojian, S.; Zhang, B.; Yan, Q. Solution Phase Synthesis of the Less-Known Form II Crystalline Red Phosphorus. Inorg. Chem. Front. 2022, 9, 4385–4393. [Google Scholar] [CrossRef]
- Smith, J.B.; Hagaman, D.; DiGuiseppi, D.; Schweitzer-Stenner, R.; Ji, H. Ultra-Long Crystalline Red Phosphorus Nanowires from Amorphous Red Phosphorus Thin Films. Angew. Chem. Int. Ed. 2016, 55, 11829–11833. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.E.M.; Hall, D.C.; Pai, R.; Król, J.E.; Kalra, V.; Ehrlich, G.D.; Ji, H.-F. Fibrous Phosphorus Quantum Dots for Cell Imaging. ACS Appl. Nano Mater. 2020, 3, 752–759. [Google Scholar] [CrossRef]
- Schusteritsch, G.; Uhrin, M.; Pickard, C.J. Single-Layered Hittorf’s Phosphorus: A Wide-Bandgap High Mobility 2D Material. Nano Lett. 2016, 16, 2975–2980. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-L.; Dong, S.; He, H.; Li, J.; Wang, X.; Zhao, H.; Wu, P. Enhanced Photocatalytic Activity of Single-Layered Hittorf’s Violet Phosphorene by Isoelectronic Doping and Mechanical Strain: A First-Principles Research. Comput. Mater. Sci. 2019, 163, 209–217. [Google Scholar] [CrossRef]
- Amaral, P.E.M.; Ji, H.-F. Stable Copper Phosphorus Iodide (Cu2P3I2) Nano/Microwire Photodetectors. ChemNanoMat 2018, 4, 1083–1087. [Google Scholar] [CrossRef]
- Möller, M.H.; Jeitschko, W. Preparation, Properties, and Crystal Structure of the Solid Electrolytes Cu2P3I2 and Ag2P3I2. J. Solid State Chem. 1986, 65, 178–189. [Google Scholar] [CrossRef]
- Pfitzner, A.; Bräu, M.F.; Zweck, J.; Brunklaus, G.; Eckert, H. Phosphorus Nanorods—Two Allotropic Modifications of a Long-Known Element. Angew. Chem. Int. Ed. 2004, 43, 4228–4231. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, G.R.; Walters, J.T.; Ji, H.-F. Stable Cu2P3I2 and Ag2P3I2 Single-Wire and Thin Film Devices for Humidity Sensing. Micro 2022, 2, 183–190. [Google Scholar] [CrossRef]
- Freudenthaler, E. Copper(I) Halide-Phosphorus Adducts: A New Family of Copper(I) Ion Conductors. Solid State Ion. 1997, 101, 1053–1059. [Google Scholar] [CrossRef]
- Fasol, G.; Cardona, M.; Hönle, W.; von Schnering, H.G. Lattice Dynamics of Hittorf’s Phosphorus and Identification of Structural Groups and Defects in Amorphous Red Phosphorus. Solid State Commun. 1984, 52, 307–310. [Google Scholar] [CrossRef]
- Winchester, R.A.L.; Whitby, M.; Shaffer, M.S.P. Synthesis of Pure Phosphorus Nanostructures. Angew. Chem. 2009, 121, 3670–3675. [Google Scholar] [CrossRef]
- Akahama, Y.; Kobayashi, M.; Kawamura, H. Raman Study of Black Phosphorus up to 13 GPa. Solid State Commun. 1997, 104, 311–315. [Google Scholar] [CrossRef]
- Kornath, A.; Kaufmann, A.; Torheyden, M. Raman Spectroscopic Studies on Matrix-Isolated Phosphorus Molecules P4 and P2. J. Chem. Phys. 2002, 116, 3323–3326. [Google Scholar] [CrossRef]
- Ruan, B.; Wang, J.; Shi, D.; Xu, Y.; Chou, S.; Liu, H.; Wang, J. A Phosphorus/N-Doped Carbon Nanofiber Composite as an Anode Material for Sodium-Ion Batteries. J. Mater. Chem. A 2015, 3, 19011–19017. [Google Scholar] [CrossRef]
- Shen, Z.; Hu, Z.; Wang, W.; Lee, S.-F.; Chan, D.K.L.; Li, Y.; Gu, T.; Yu, J.C. Crystalline Phosphorus Fibers: Controllable Synthesis and Visible-Light-Driven Photocatalytic Activity. Nanoscale 2014, 6, 14163–14167. [Google Scholar] [CrossRef] [PubMed]
- Nilges, T.; Kersting, M.; Pfeifer, T. A Fast Low-Pressure Transport Route to Large Black Phosphorus Single Crystals. J. Solid State Chem. 2008, 181, 1707–1711. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwenk, G.R.; Walters, J.T.; Ji, H.-F. Structural Consequences of Post-Synthetic Modification of Cu2P3I2. Micro 2023, 3, 256-263. https://doi.org/10.3390/micro3010018
Schwenk GR, Walters JT, Ji H-F. Structural Consequences of Post-Synthetic Modification of Cu2P3I2. Micro. 2023; 3(1):256-263. https://doi.org/10.3390/micro3010018
Chicago/Turabian StyleSchwenk, Gregory R., John T. Walters, and Hai-Feng Ji. 2023. "Structural Consequences of Post-Synthetic Modification of Cu2P3I2" Micro 3, no. 1: 256-263. https://doi.org/10.3390/micro3010018
APA StyleSchwenk, G. R., Walters, J. T., & Ji, H.-F. (2023). Structural Consequences of Post-Synthetic Modification of Cu2P3I2. Micro, 3(1), 256-263. https://doi.org/10.3390/micro3010018







