Visual Detection of Biomolecules Using Concentration Dependent Induced Aggregation of Plasmonic Gold Nanoparticles
Abstract
1. Introduction
2. Procedure and Methodology
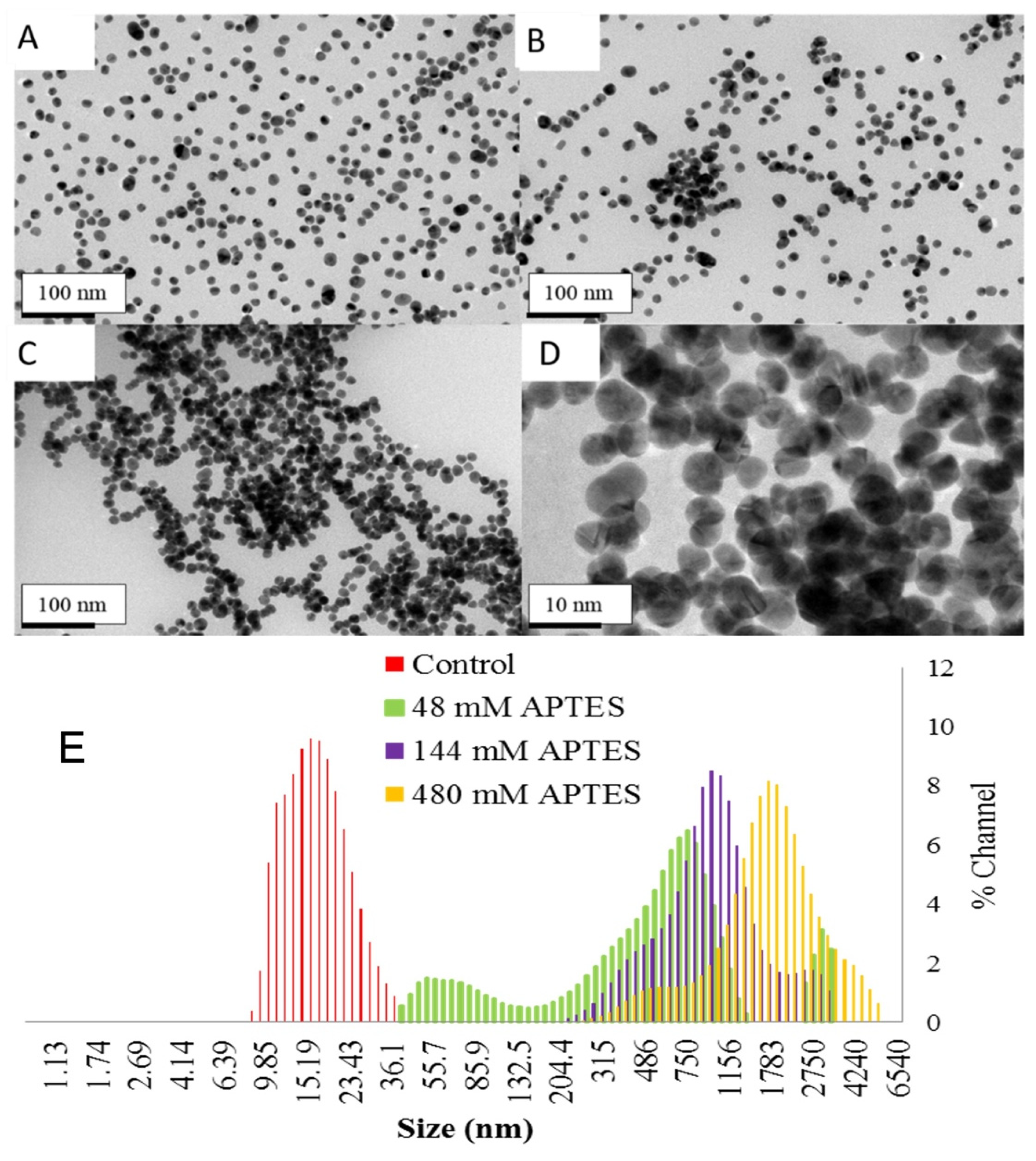
3. Results and Discussion
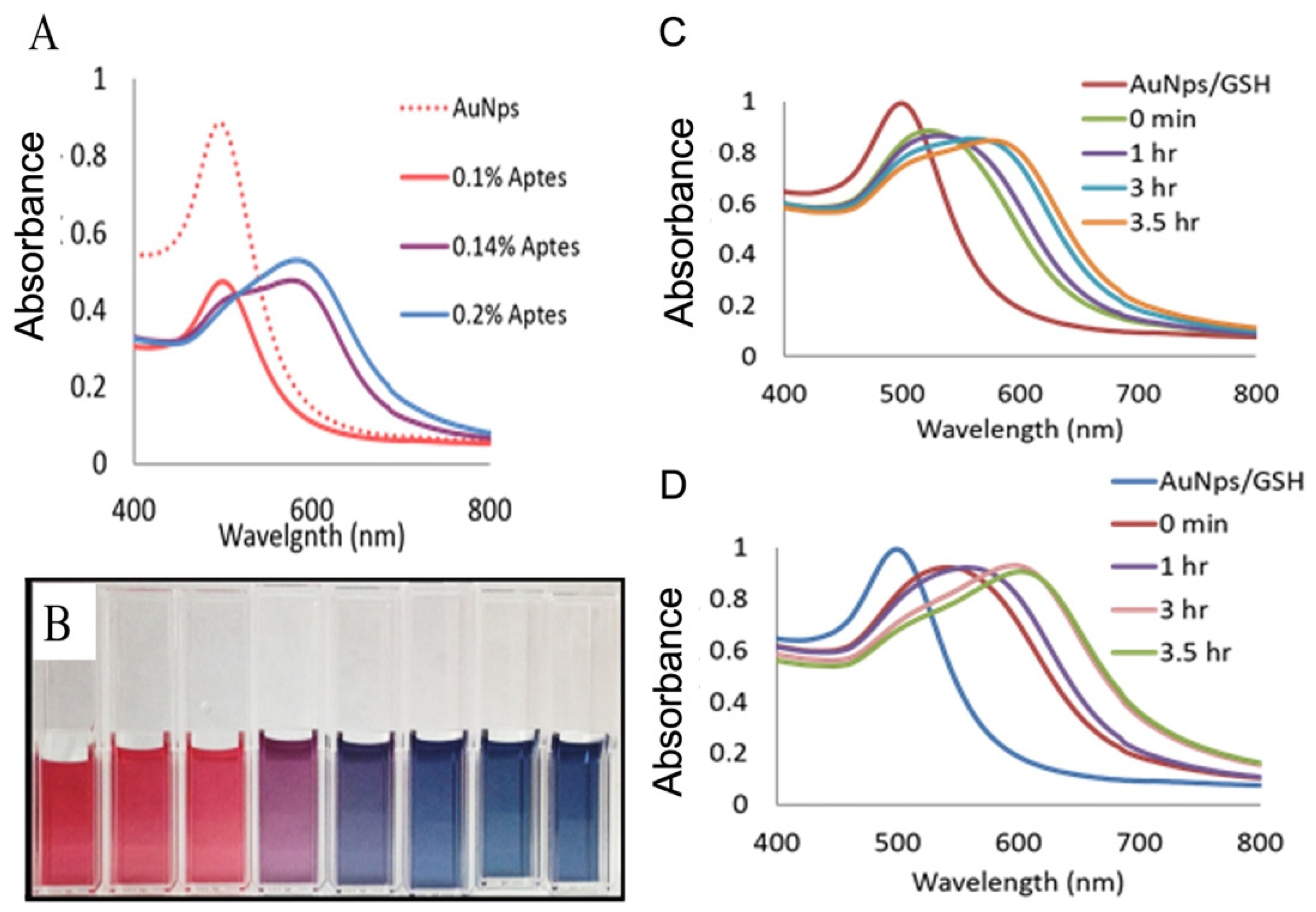
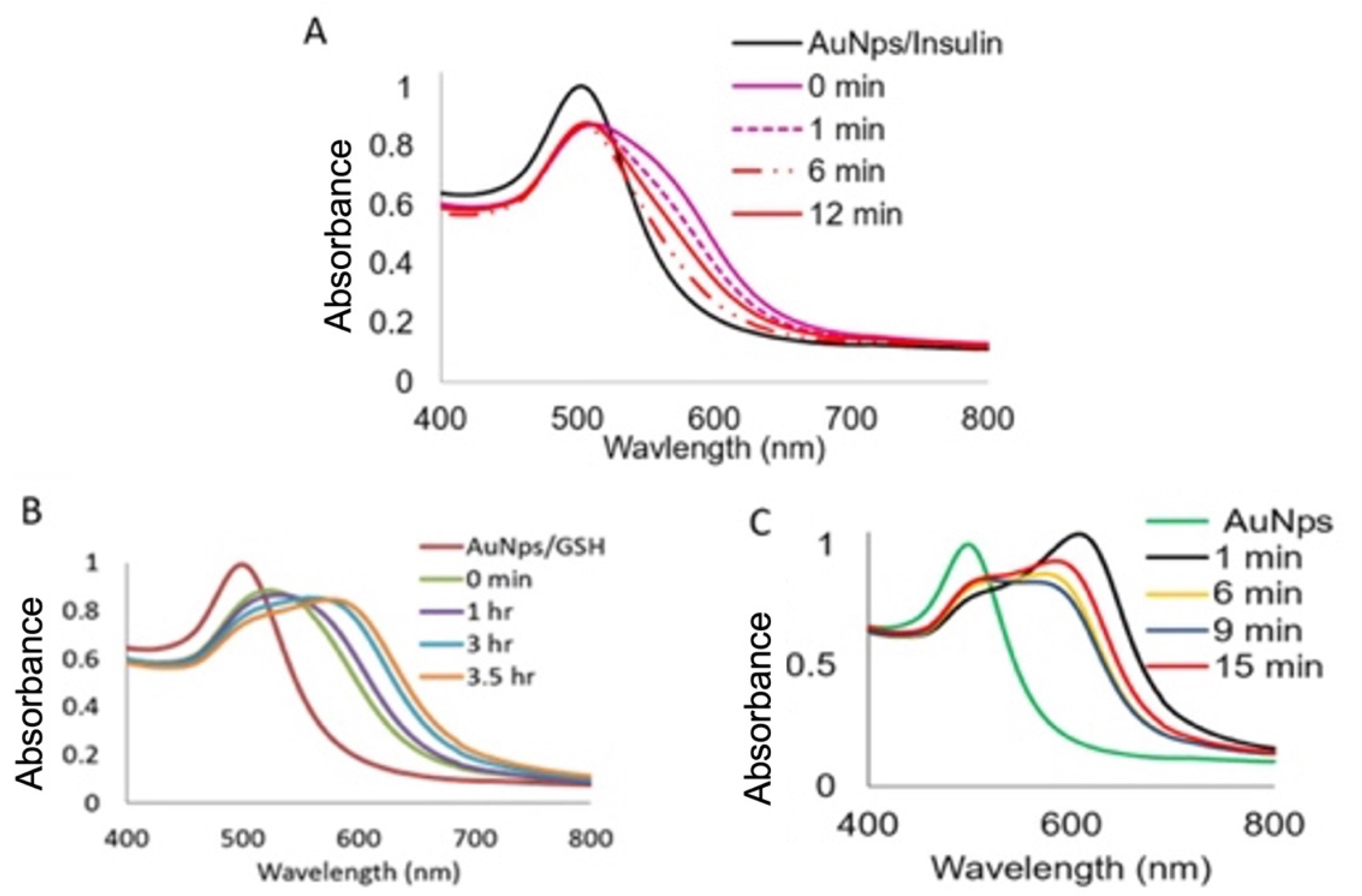
3.1. Visual Detection of Denatured Glutathione Peptides: A Facile Method to Visibly Detect Heat Stressed Biomolecules
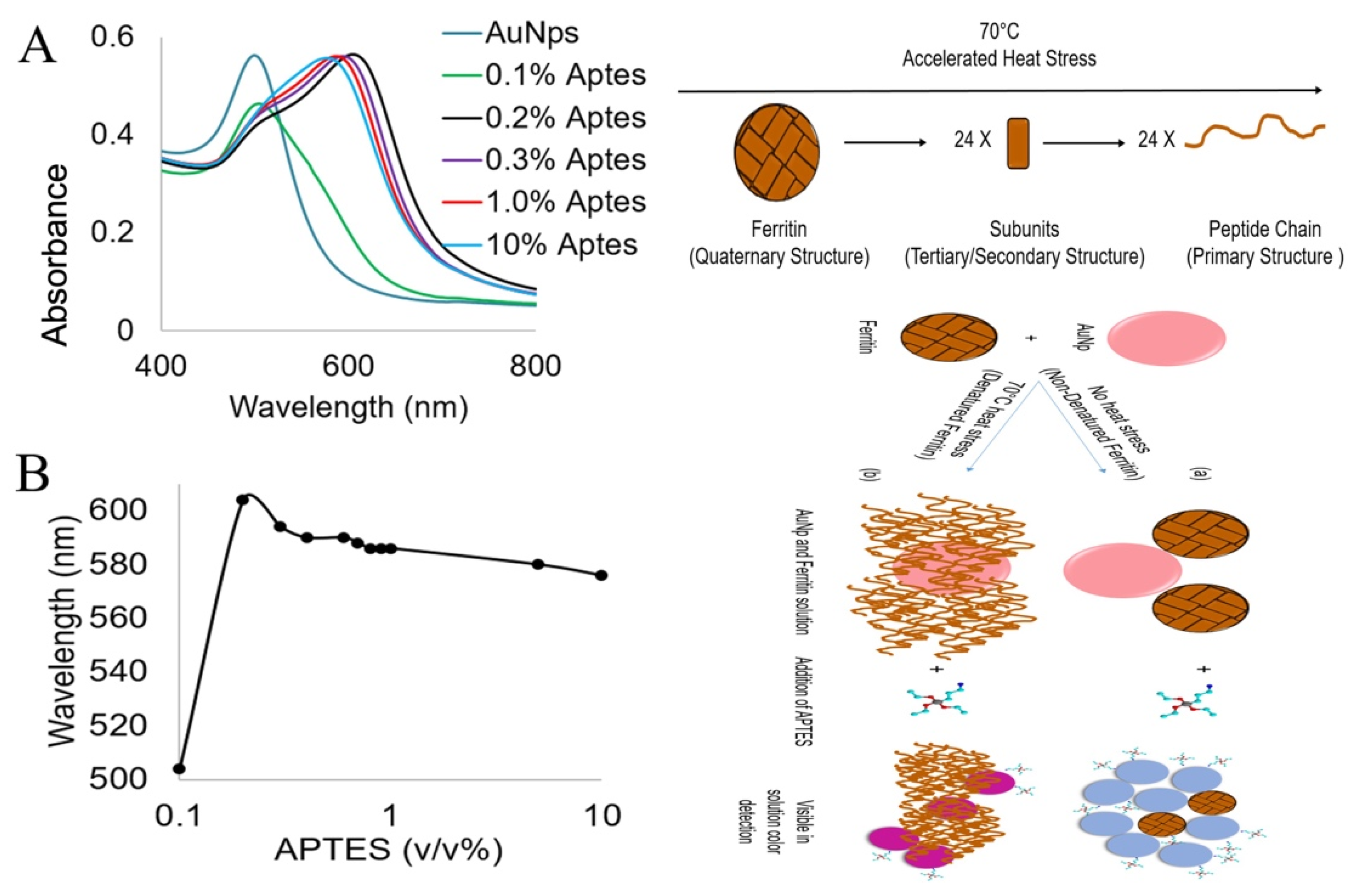
3.2. Visual Detection of Denatured Ferritin Protein from Horse Spleen
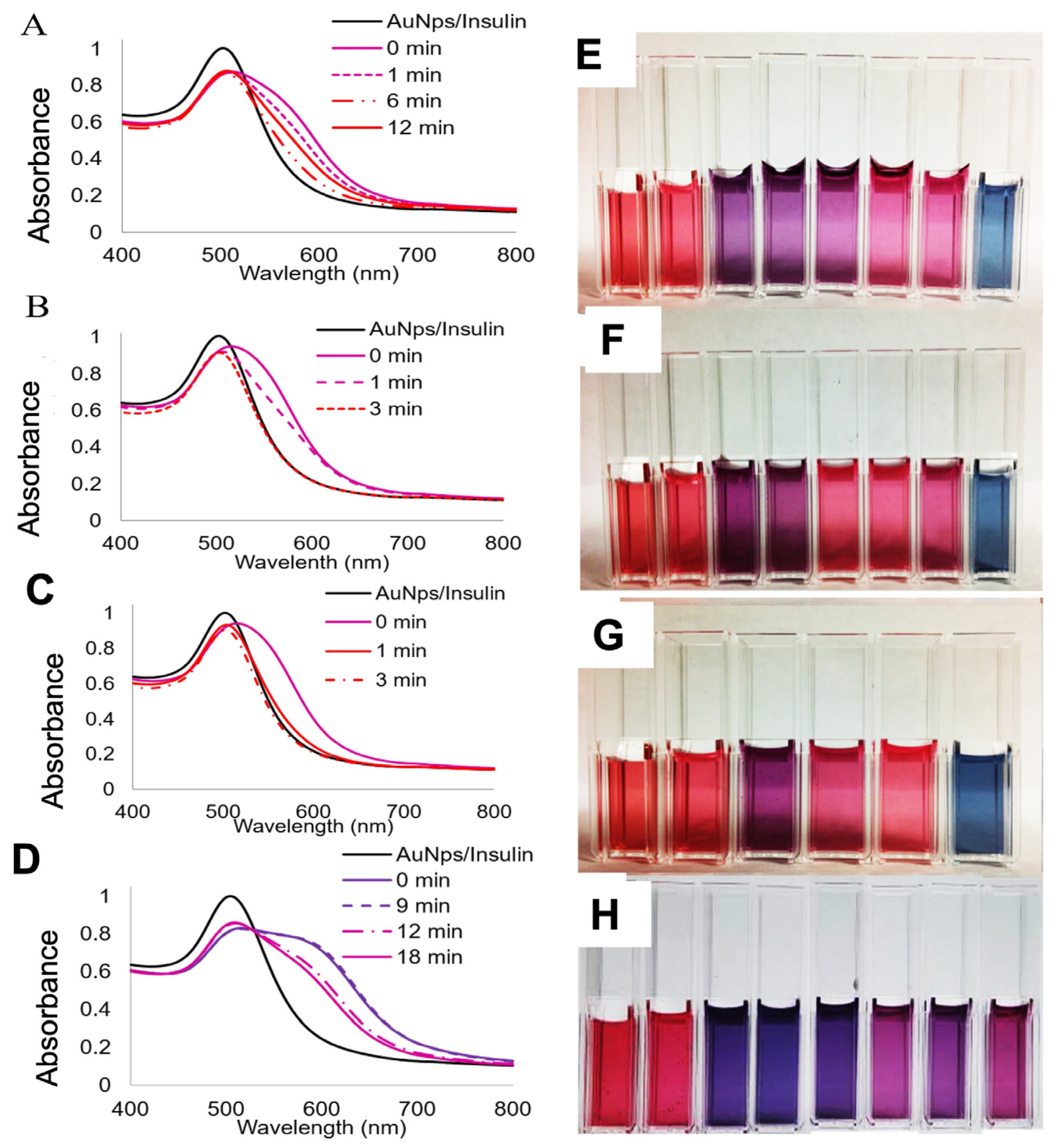
3.3. Visual in Solution Detection of Denatured Insulin Coupled to Gold Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, J.; Zhang, X.; Yonzon, C.R.; Haes, A.J.; Van Duyne, R.P. Localized surface plasmon resonance biosensors. Nanomedicine 2006, 1, 219–228. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Size and Temperature Dependence of the Plasmon Absorption of Colloidal Gold Nanoparticles. J. Phys. Chem. B 1999, 103, 4212. [Google Scholar] [CrossRef]
- Kim, Y.; Johnson, R.C.; Hupp, J.T. Gold Nanoparticle-Based Sensing of ‘Spectroscopically Silent’ Heavy Metal Ions. Nano Lett. 2001, 1, 165. [Google Scholar] [CrossRef]
- Huanga, X.; El-Sayeda, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13. [Google Scholar] [CrossRef]
- Nik, B.; El Sayed, A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed—Mediated Growth Method. Chem. Mater. 2003, 16, 1957. [Google Scholar]
- Kozek, K.; Kozek, K.M.; Wu, W.-C.; Mishra, S.R.; Tracy, J.B. Large-Scale Synthesis of Gold Nanorods through Continuous Secondary Growth. Chem. Mater. 2013, 25, 4537. [Google Scholar] [CrossRef]
- Vigderman, L.; Khanal, B.P.; Zubarev, E.R. Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications. Adv. Mater. 2012, 24, 4811. [Google Scholar] [CrossRef]
- Sato, K.; Hosokawa, K.; Maeda, M. Rapid aggregation of gold nanoparticles induced by non-cross-linking DNA hybridization. J. Am. Chem. Soc. 2003, 125, 8102. [Google Scholar] [CrossRef]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective Colorimetric Detection of Polynucleotides Based on the Distance-Dependent Optical Properties of Gold Nanoparticles. Science 1997, 277, 1078. [Google Scholar] [CrossRef]
- Ai, K.; Liu, Y.; Lu, L. Hydrogen-Bonding Recognition-Induced Color Change of Gold Nanoparticles for Visual Detection of Melamine in Raw Milk and Infant Formula. J. Am. Chem. Soc. 2009, 131, 9496. [Google Scholar]
- Liu, J.; Lu, Y. Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2+ detection. J. Am. Chem. Soc. 2004, 126, 12298. [Google Scholar] [CrossRef]
- Kim, N.; Rosenzweig, Z. Development of an Aggregation-Based Immunoassay for Anti-Protein a Using Gold Nanoparticles. Anal. Chem. 2002, 74, 1624–1628. [Google Scholar]
- Kumar, C. (Ed.) UV-VIS and Photoluminescence Spectroscopy for Nanomaterials Characterization; Springer: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Gorog, S. Ultraviolet-Visible Spectrophotometry in Pharmaceutical Analysis; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Clark, B.J.; Frost, T. UV Spectroscopy: Techniques, Instrumentation and Data Handling; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Goldstein, A.; Soroka, Y.; Frušić-Zlotkin, M.; Popov, I.; Kohen, R. High resolution SEM imaging of gold nanoparticles in cells and tissues. J. Microsc. 2014, 256, 237. [Google Scholar] [CrossRef]
- Peckys, D.B.; de Jonge, N. Gold Nanoparticle Uptake in Whole Cells in Liquid Examined by Environmental Scanning Electron Microscopy. Microsc. Microanal. 2014, 20, 189. [Google Scholar] [CrossRef]
- Lashkova, N.A.; Permiakov, N.V.; Maximov, A.I.; Spivak, Y.M.; Moshnikov, V.A. Local analysis of semiconductor nanoobjects by scanning tunneling atomic force microscopy. J. Phys. Math. 2015, 1, 15. [Google Scholar] [CrossRef][Green Version]
- Hatch, J.E. Aluminum: Properties and Physical Metallurgy; ASM International: Almere, The Netherlands, 1984. [Google Scholar]
- Goodhew, P.J.; Humphreys, J.; Beanland, R. Electron Microscopy and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Podzimek, S. Light Scattering, Size Exclusion Chromatography and Asymmetric Flow Field Flow Fractionation: Powerful Tools for the Characterization of Polymers, Proteins and Nanoparticles; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gardiner, D.J. Practical Raman Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1989; ISBN 978-0-387-50254-0. [Google Scholar]
- Available online: https://www.hamamatsu.com/jp/en/technology/lifephotonics/environment/SuperiorDetectionOfDiverseChemicals/index.html (accessed on 19 October 2022).
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhänen, T. Ultrafast Graphene Oxide Humidity Sensors. ACS Nano 2013, 7, 11166. [Google Scholar] [CrossRef]
- Segev-Bar, M.; Haick, H. Flexible Sensors Based on Nanoparticles. ACS Nano 2013, 7, 8366. [Google Scholar] [CrossRef]
- Gruber, K.; Horlacher, T.; Castelli, R.; Mader, A.; Seeberger, P.H.; Hermann, B.A. Cantilever array sensors detect specific carbohydrate-protein interactions with picomolar sensitivity. ACS Nano 2011, 5, 3670. [Google Scholar] [CrossRef]
- Hongliang, T.; Liu, B.; Chen, Y. Lanthanide Coordination Polymer Nanoparticles for Sensing of Mercury (II) by Photoinduced Electron Transfer. ACS Nano 2012, 6, 10505. [Google Scholar]
- Seed, B. Silanizing Glassware. In Current Protocols in Cell Biology; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Liu, Y.; Li, Y.; Li, X.M.; He, T. Kinetics of (3-aminopropyl)triethoxylsilane (aptes) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 2013, 29, 15275. [Google Scholar] [CrossRef]
- Opatkiewicz, J.P.; Lemieux, M.C.; Bao, Z. Influence of Electrostatic Interactions on Spin—Assembled Single—Walled Carbon Nanotube Networks on Amine-Functionalized Surfaces. ACS Nano 2010, 4, 1167. [Google Scholar] [CrossRef]
- Sun, X.; Wei, W. Electrostatic-assembly-driven formation of micrometer-scale supramolecular sheets of (3-aminopropyl)triethoxysilane(APTES)-HAuCl4 and their subsequent transformation into stable APTES bilayer-capped gold nanoparticles through a thermal process. Langmuir 2010, 26, 6133. [Google Scholar] [CrossRef]
- Klug, J.; Pérez, L.A.; Coronado, E.A.; Lacconi, G.I. Chemical and electrochemical oxidation of silicon surfaces functionalized with APTES: The role of surface roughness in the AuNPs anchoring kinetics. J. Phys. Chem. C 2013, 117, 11317. [Google Scholar] [CrossRef]
- Sedighi, A.; Li, P.; Pekcevik, I.C.; Gates, B.D. A Proposed Mechanism of the influence of Gold Nanoparticles on DNA Hybridization. ACS Nano 2014, 8, 6765. [Google Scholar] [CrossRef]
- Peretz-Soroka, H.; Pevzner, A.; Davidi, G.; Naddaka, V.; Tirosh, R.; Flaxer, E.; Patolsky, F. Optically-gated self-calibrating nanosensors: Monitoring pH and metabolic activity of living cells. Nano Lett. 2013, 13, 3157. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and Characterization of Au Colloid Monolayers. Anal. Chem. 1995, 67, 735. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wilbur, J.L.; Whitesides, G.M. Using two-stage chemical amplification to determine the density of defects in self-assembled monolayers of alkanethiolates on gold. Langmuir 1996, 12, 3257. [Google Scholar] [CrossRef]
- Sam, F.L.M.; Razali, M.A.; Jayawardena, K.I.; Mills, C.A.; Rozanski, L.J.; Beliatis, M.J.; Silva, S.R.P. Silver grid transparent conducting electrodes for organic light emitting diodes. Org. Electron. 2014, 15, 3492. [Google Scholar] [CrossRef]
- Yong Yanga, B.; Matsubaraa, S.; Nogamia, M.; Shib, J. Controlling the aggregation behavior of gold nanoparticles. Mater. Sci. Eng. B 2006, 140, 172. [Google Scholar] [CrossRef]
- Du, S.; Kendall, K.; Toloueinia, P.; Mehrabadi, Y.; Gupta, G.; Newton, J. Aggregation and adhesion of gold nanoparticles in phosphate buffered saline. J. Nanoparticle Res. 2012, 14, 758. [Google Scholar] [CrossRef]
- Sarkar, A.; Daniels-Race, T. Electrophoretic Deposition of Carbon Nanotubes on 3-Amino-Propyl-Triethoxysilane (APTES) Surface Functionalized Silicon Substrates. Nanomaterials 2013, 3, 272. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Yu, W.; Xu, L.; Ge, C.; Liu, J.; Gu, N. Linear aggregation of gold nanoparticles in ethanol a National Laboratory of Molecular and Biomolecular Electronics. Colloids Surf. A Physicochem. 2003, 223, 177. [Google Scholar] [CrossRef]
- Hu, H.; Duan, H.; Yang, J.K.W.; Shen, Z.X. Plasmon-modulated photoluminescence of individual gold nanostructures. ACS Nano 2012, 6, 10147. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ming, T.; Wang, X.; Wang, P.; Wang, J.; Chen, J. High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2010, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Bhanu, U.; Islam, M.R.; Tetard, L.; Khondaker, S.I. Photoluminescence quenching in gold—MoS2 hybrid nanoflakes. Sci. Rep. 2014, 4, 5575. [Google Scholar] [CrossRef] [PubMed]
- Lumdee, C.; Yun, B.; Kik, P.G. Gap-Plasmon Enhanced Gold Nanoparticle Photoluminescence. ACS Photonics 2014, 1, 1224. [Google Scholar] [CrossRef]
- Amran, T.S.T.; Hashim, R.; Al-Obaidi, N.K.A.; Yazid, H.; Adnan, R. Optical absorption and photoluminescence studies of gold nanoparticles deposited on porous silicon. Nanoscale Res. Lett. 2013, 8, 35. [Google Scholar] [CrossRef]
- Biagioni, P.; Brida, D.; Huang, J.-S.; Kern, J.; Duò, L.; Hecht, B.; Finazzi, M.; Cerullo, G. Dynamics of four-photon photoluminescence in gold nanoantennas. Nano Lett. 2012, 12, 2941. [Google Scholar] [CrossRef]
- Liao, H.; Wen, W.; Wong, G.K. Photoluminescence from Au nanoparticles embedded in Au:oxide composite films. J. Opt. Soc. Am. B 2006, 23, 2518. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1, 246. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, P.; Wang, Y.; Zhang, J.; Aili, D.; Liedberg, B. Biofunctionalized gold nanoparticles for colorimetric sensing of botulinum neurotoxin A light chain. Anal. Chem. 2014, 86, 2345. [Google Scholar] [CrossRef] [PubMed]
- Jongjinakool, S.; Palasak, K.; Bousod, N.; Teepoo, S. Gold Nanoparticles-based Colorimetric Sensor for Cysteine Detection. Energy Procedia 2014, 56, 10–18. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Medina, S.; McDonough, S.; Swanglap, P.; Landes, C.F.; Link, S. In situ measurement of bovine serum albumin interaction with gold nanospheres. Langmuir 2012, 28, 9131. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Medina, S.; Blankenburg, J.; Olson, J.; Landes, C.F.; Link, S. Adsorption of a protein monolayer via hydrophobic interactions prevents nanoparticle aggregation under harsh environmental conditions. ACS Sustain. Chem. Eng. 2013, 1, 833. [Google Scholar] [CrossRef] [PubMed]
- De Paoli Lacerda, S.H.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623. [Google Scholar] [CrossRef]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir 2005, 21, 9303. [Google Scholar] [CrossRef]
- Cañaveras, F.; Madueño, R.; Sevilla, J.M.; Blázquez, M.; Pineda, T. Role of the functionalization of the gold nanoparticle surface on the formation of bioconjugates with human serum albumin. J. Phys. Chem. C 2012, 116, 10430. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 2009, 81, 9425. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Z.; Li, J.; Zhao, J. Bovine Serum Albumins (BSA) Induced Aggregation and Separation of Gold Colloid Nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 2206. [Google Scholar] [CrossRef] [PubMed]
- Prodan, E.; Radlodd, C.; Halas, N.; Nordlander, P. A Hybridization Model for the Plasmon Response of Complex Nanostructures. Science 2003, 302, 419. [Google Scholar] [CrossRef]
- Farrell, M.; Rutherford, G.; Pradhan, A. Characterization of the Effects of 3-Aminopropyl Triethoxysilane on Stability, Optical Properties and Morphology of Colloidal Gold Nanoparticles. Curr. Phys. Chem. 2016, 6, 145–151. [Google Scholar] [CrossRef]
- Farrell, M.; Hawley, W.; Reaume, R.; Gurrola, A.; Pradhan, A.K. Visual in Solution Detection of Denatured Insulin Coupled to Gold Nanoparticles in the Presence of an Aminosilane. J. Electrochem. Soc. 2016, 164, B3008–B3012. [Google Scholar] [CrossRef]
- Lumelsky, N.; Blondel, O.; Laeng, P.; Velasco, I.; Ravin, R.; McKay, R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 2001, 292, 1389. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A. The rising cost of insulin: Why the price of this lifesaving drug is reaching new heights. Diabetes Forecast. March 2016. Available online: http://www.diabetesforecast.org/2016/mar-apr/rising-costs-insulin.html (accessed on 10 May 2016).
- Rosas, P.C.; Nagaraja, G.M.; Kaur, P.; Panossian, A.; Wickman, G.; Garcia, L.R.; Al-Khamis, F.A.; Asea, A.A. Hsp72 (HSPA1A) prevents human islet amyloid polypeptide aggregation and toxicity: A new approach for type 2 diabetes treatment. PLoS ONE 2016, 11, e0149409. [Google Scholar]
- Hassiepen, U.; Federwisch, M.; Mülders, T.; Wollmer, A. The lifetime of insulin hexamers. Biophys. J. 1999, 77, 1638. [Google Scholar] [CrossRef]
- Blundell, T.L.; Cutfield, J.F.; Dodson, G.G.; Dodson, E.; Hodgkin, D.C.; Mercola, D. The structure and biology of insulin. Biochem. J. 1971, 125, 50. [Google Scholar] [CrossRef]
- Huus, K.; Havelund, S.; Olsen, H.B.; van de Weert, M.; Frokjaer, S. Thermal dissociation and unfolding of insulin. Biochemistry 2005, 44, 11171. [Google Scholar] [CrossRef]
- Giger, K.; Vanam, R.P.; Seyrek, E.; Dubin, P.L. Suppression of insulin aggregation by heparin. Biomacromolecules 2008, 9, 2338. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [PubMed]
- Zhang, J.; Oyama, M. Gold Nanoparticle-Attached ITO as a Biocompatible Matrix for Myoglobin Immobilization: Direct Electrochemistry and Catalysis to Hydrogen Peroxide. J. Electroanal. Chem. 2005, 577, 273–279. [Google Scholar] [CrossRef]
- Gorin, D.; Portnov, S.; Inozemtseva, O.; Luklinska, Z.; Yashchenok, A.; Pavlov, A.; Skirtach, A.; Möhwald, H.; Sukhorukovc, G. Magnetic/Gold Nanoparticle Functionalized Biocompatible Microcapsules with Sensitivity to Laser Irradiation. Phys. Chem. Chem. Phys. 2008, 10, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Reaume, R.; Pradhan, A.K. Visual Detection of Denatured Glutathione Peptides: A Facile Method to Visibly Detect Heat Stressed Biomolecules. Sci. Rep. 2017, 7, 2604–2611. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325. [Google Scholar] [CrossRef]
- Zhang, X.; Servos, M.R.; Liu, J. Surface science of DNA adsorption onto citrate-capped gold nanoparticles. Langmuir 2012, 28, 3896. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Liu, A.L.; Hong, L.; Deng, H.H.; Lin, X.H. Comparison of the peroxidase-like activity of unmodified, amino-modified, and citrate-capped gold nanoparticles. ChemPhysChem 2012, 13, 1199. [Google Scholar] [CrossRef]
- Pham, T.; Jackson, J.B.; Halas, N.J.; Lee, T.R. Preparation and Characterization of Gold Nanoshells Coated withSelf-Assembled Monolayers. Langmuir 2002, 18, 4915–4920. [Google Scholar] [CrossRef]
- Farrokh Takin, E.; Ciofani, G.; Puleo, G.; Giuseppe, V.; Filippeschi, C.; Mazzolai, B.; Piazza, V.; Mattoli, V. BariumTitanate Core–Gold Shell Nanoparticles for Hyperthermia Treatments. Int. J. Nanomed. 2013, 8, 2319–2331. [Google Scholar]
- Chithrani, B.; Ghazani, A.; Chan, W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef]
- Eustisa, S.; El-Sayed, M. Why Gold Nanoparticles Are More Precious than Pretty Gold: Noble Metal Surface Plasmon Resonance and its Enhancement of the Radiative and Nonradiative Properties of Nanocrystals of Different Shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Orner, B.P. Self-Assembly in the Ferritin Nano-Cage Protein Superfamily. Int. J. Mol. Sci. 2011, 12, 5406–5421. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Li, X. Optimal Size of Gold Nanoparticles for Surface-Enhanced Raman Spectroscopy under Different Conditions. J. Nanomater. 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Liao, J.; Xu, L.; Ge, C. Self-assembly of length-tunable gold nanoparticle chains in organic solvents. Appl. Phys. A 2003, 76, 541. [Google Scholar] [CrossRef]
- Glick Bernard, R.; Pasternak Jack, J.; Patten Cheryl, L. Molecular Biotechnology: Principles and Applications of Recombinant DNA, 4th ed.; Wiley (USA), ASM Press American Society for Microbiology: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Farrell, M.; Rutherford, G.; Pradhan, A. Visual Biosensing of Bovine Serum Albumin Using Induced Aggregation of Gold Nanoparticles via 3-Aminopropyl Triethoxysilane. J. Nanosci. Nanotechnol. 2016, 16, 7684–7688. [Google Scholar] [CrossRef]
- Hsieh, L.-Z.; Chau, Y.-F.C.; Lim, C.M.; Lin, M.-H.; Huang, H.J.; Lin, C.-T.; Syafi’Ie, I.M.N. Metal nano-particles sizing by thermal annealing for the enhancement of surface plasmon effects in thin-film solar cells application. Opt. Commun. 2016, 370, 85–90. [Google Scholar] [CrossRef]
- Chau, Y.F.C. Mid-infrared sensing properties of a plasmonic metal–insulator–metal waveguide with a single stub including defects. J. Phys. D Appl. Phys. 2020, 53, 115401. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farrell, M.; Pradhan, A. Visual Detection of Biomolecules Using Concentration Dependent Induced Aggregation of Plasmonic Gold Nanoparticles. Micro 2022, 2, 649-662. https://doi.org/10.3390/micro2040043
Farrell M, Pradhan A. Visual Detection of Biomolecules Using Concentration Dependent Induced Aggregation of Plasmonic Gold Nanoparticles. Micro. 2022; 2(4):649-662. https://doi.org/10.3390/micro2040043
Chicago/Turabian StyleFarrell, Monique, and Aswini Pradhan. 2022. "Visual Detection of Biomolecules Using Concentration Dependent Induced Aggregation of Plasmonic Gold Nanoparticles" Micro 2, no. 4: 649-662. https://doi.org/10.3390/micro2040043
APA StyleFarrell, M., & Pradhan, A. (2022). Visual Detection of Biomolecules Using Concentration Dependent Induced Aggregation of Plasmonic Gold Nanoparticles. Micro, 2(4), 649-662. https://doi.org/10.3390/micro2040043





