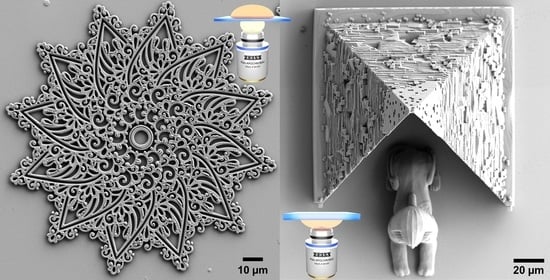
Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System
Abstract
:1. Introduction
2. Materials and Methods
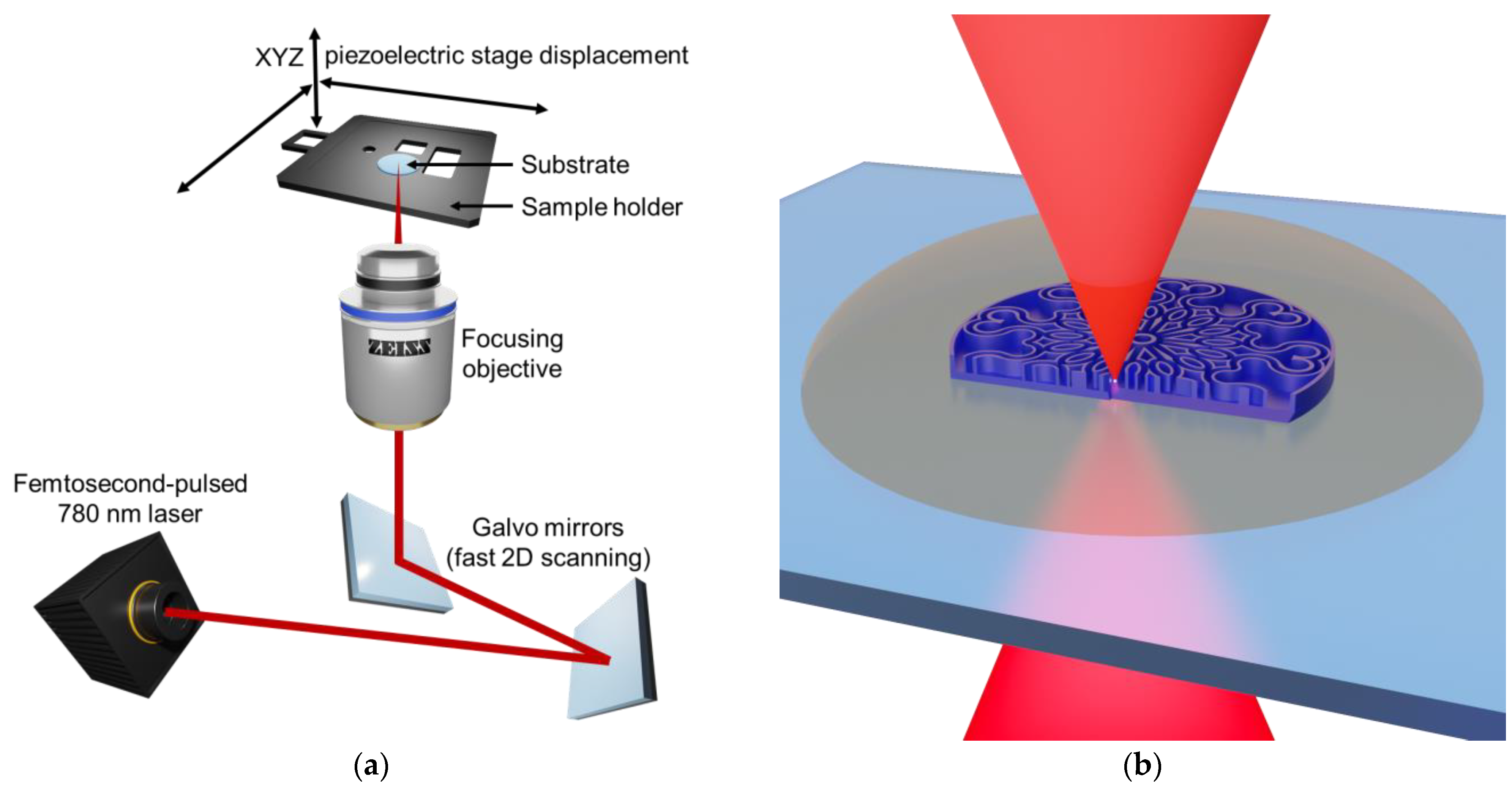
2.1. Fabrication by Two-Photon Polymerization Direct Laser Writing
2.2. Structure Characterization by Scanning Electron Microscopy
2.3. Structure Characterization by Optical Profilometry
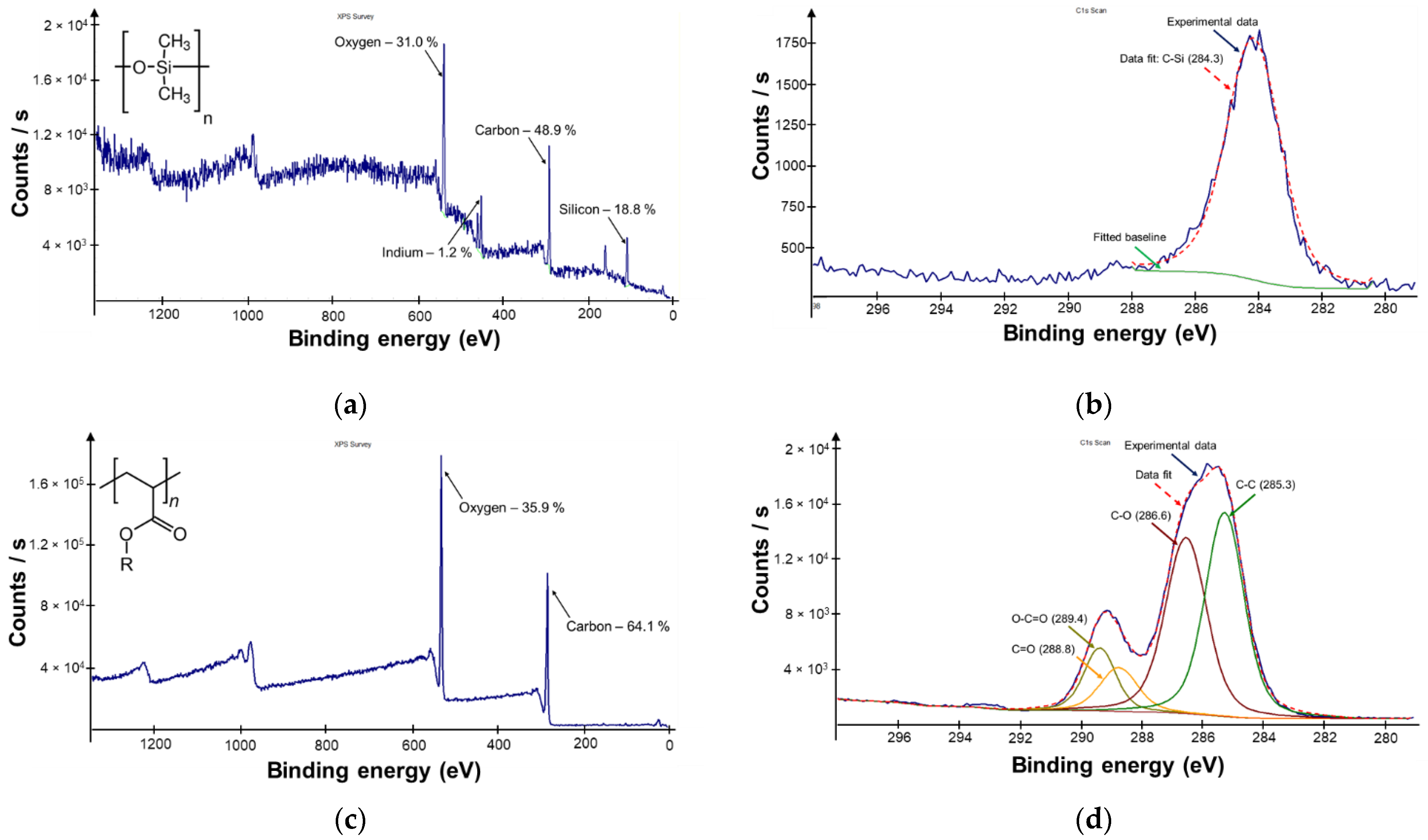
2.4. Structure Characterization by X-ray Photoelectron Spectroscopy
3. Results
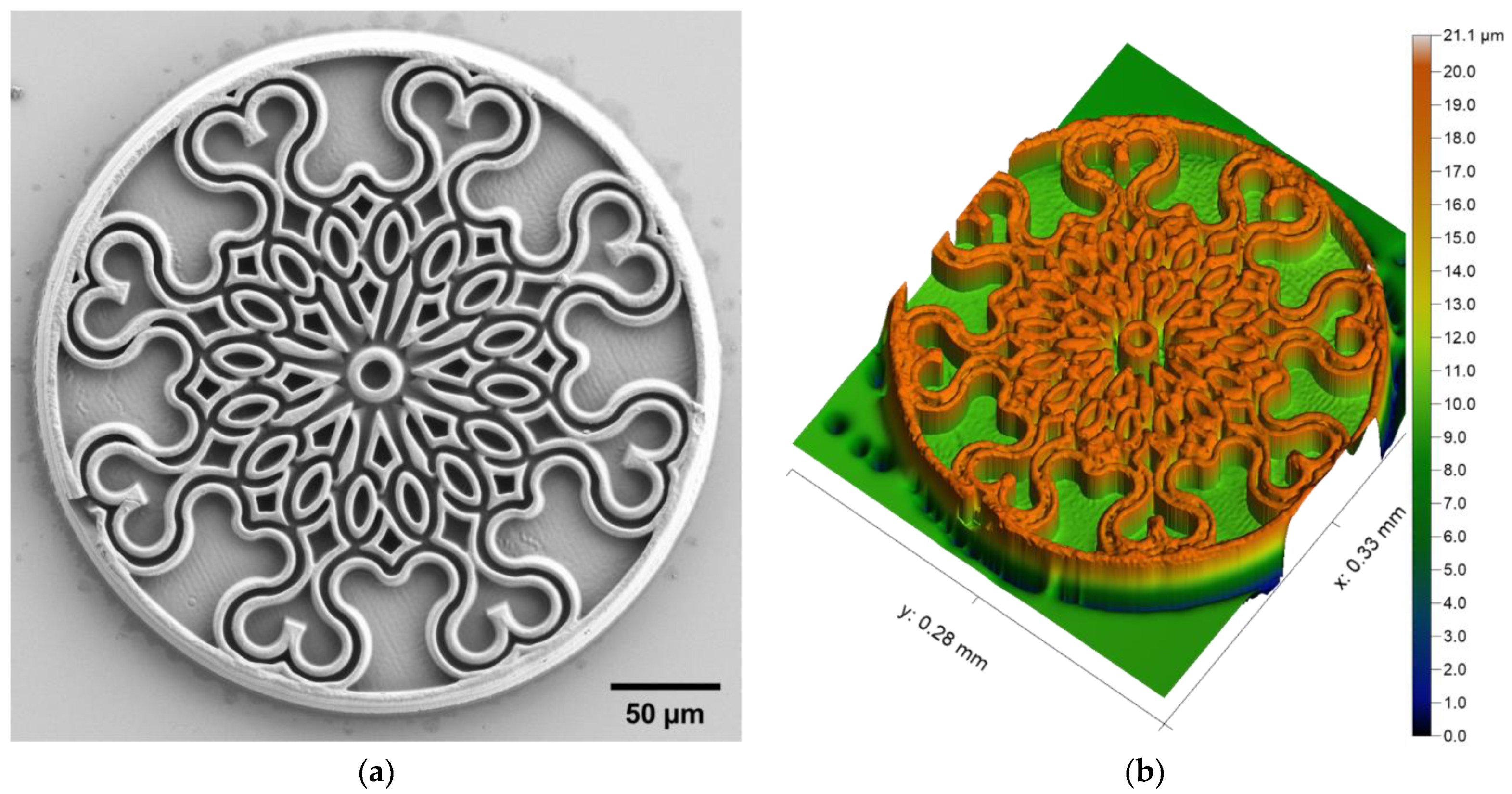
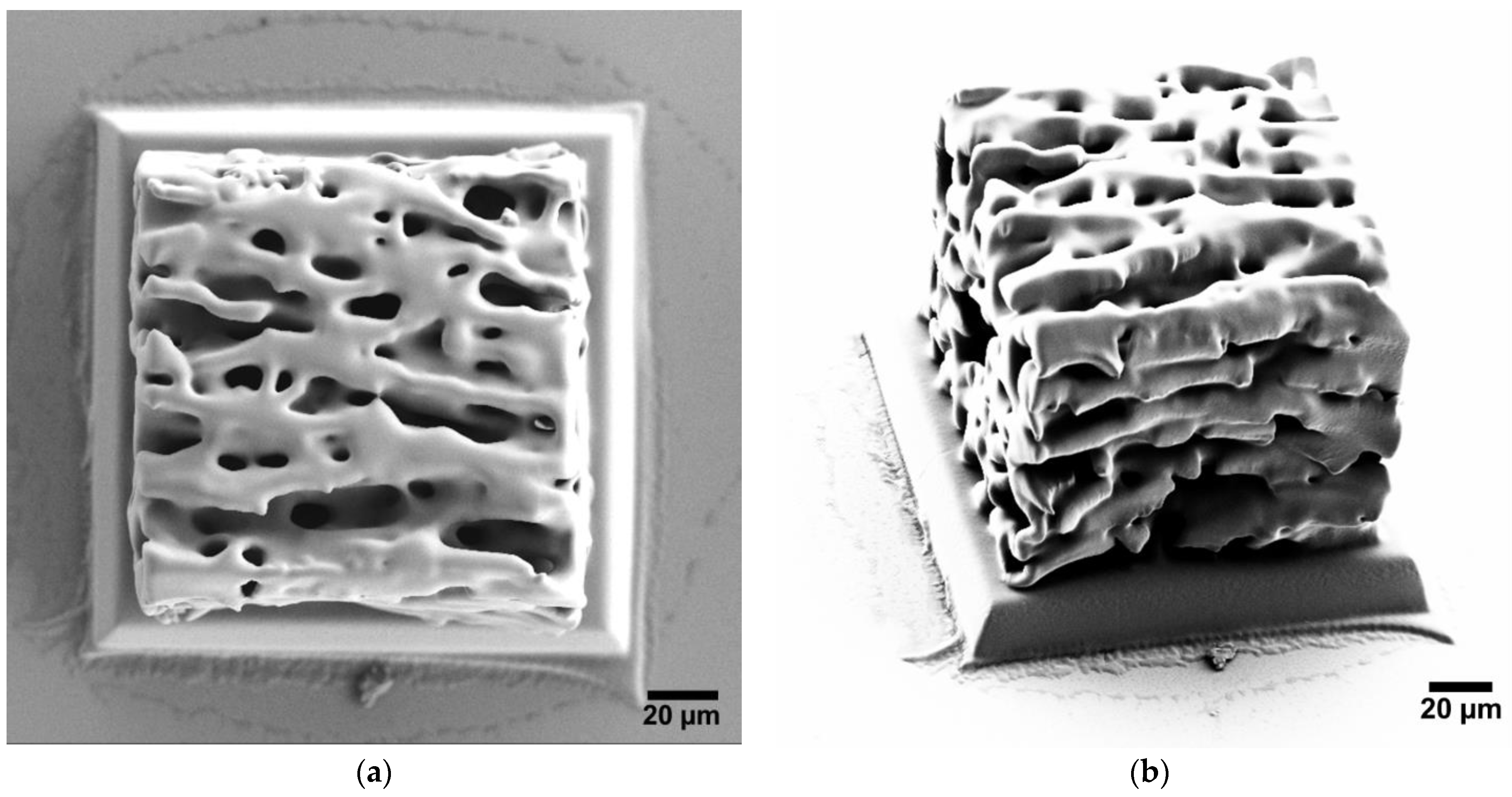
3.1. Soft Structures
3.2. High-Resolution Structures
3.3. Macroscale Structure
3.4. Material Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebhardt, A. Understanding Additive Manufacturing; Carl Hanser Verlag GmbH & Co. KG: Munich, Germany, 2011; ISBN 978-3-446-43162-1. [Google Scholar]
- Nadagouda, M.N.; Rastogi, V.; Ginn, M. A review on 3D printing techniques for medical applications. Curr. Opin. Chem. Eng. 2020, 28, 152–157. [Google Scholar] [CrossRef]
- El-Sayegh, S.; Romdhane, L.; Manjikian, S. A critical review of 3D printing in construction: Benefits, challenges, and risks. Arch. Civ. Mech. Eng. 2020, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Mantihal, S.; Kobun, R.; Lee, B.B. 3D food printing of as the new way of preparing food: A review. Int. J. Gastron. Food Sci. 2020, 22, 100260. [Google Scholar] [CrossRef]
- Kawata, S.; Sun, H.B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Wegener, M. Three-dimensional optical laser lithography beyond the diffraction limit. Laser Photonics Rev. 2013, 7, 22–44. [Google Scholar] [CrossRef]
- Göppert-Mayer, M. Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 1931, 401, 273–294. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C.G.B. Two-photon excitation in CaF2: Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Baldacchini, T. Three-Dimensional Microfabrication Using Two-Photon Polymerization: Fundamentas, Technology and Applications; Elsevier: Waltham, MA, USA, 2016; ISBN 978-0-323-35321-2. [Google Scholar]
- Zhou, X.; Hou, Y.; Lin, J. A review on the processing accuracy of two-photon polymerization. AIP Adv. 2015, 5, 30701. [Google Scholar] [CrossRef]
- Maruo, S.; Nakamura, O.; Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 1997, 22, 132. [Google Scholar] [CrossRef] [Green Version]
- Engay, E.; Bunea, A.-I.; Chouliara, M.; Bañas, A.; Glückstad, J. Natural convection induced by an optically fabricated and actuated microtool with a thermoplasmonic disk. Opt. Lett. 2018, 43, 3870–3873. [Google Scholar] [CrossRef]
- Bunea, A.; Martella, D.; Nocentini, S.; Parmeggiani, C.; Taboryski, R.; Wiersma, D.S. Light-Powered Microrobots: Challenges and Opportunities for Hard and Soft Responsive Microswimmers. Adv. Intell. Syst. 2021, 3, 2000256. [Google Scholar] [CrossRef]
- Kim, J.A.; Wales, D.J.; Thompson, A.J.; Yang, G. Fiber-Optic SERS Probes Fabricated Using Two-Photon Polymerization For Rapid Detection of Bacteria. Adv. Opt. Mater. 2020, 8, 1901934. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Nguyen, D.V.; Kang, L. Immobilized nanoneedle-like structures for intracellular delivery, biosensing and cellular surgery. Nanomedicine 2021, 16, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Sabaté Rovira, D.; Nielsen, H.M.; Taboryski, R.; Bunea, A.I. Additive manufacturing of polymeric scaffolds for biomimetic cell membrane engineering. Mater. Des. 2021, 201, 109486. [Google Scholar] [CrossRef]
- Bunea, A.I.; Jakobsen, M.H.; Engay, E.; Bañas, A.R.; Glückstad, J. Optimization of 3D-printed microstructures for investigating the properties of the mucus biobarrier. Micro Nano Eng. 2019, 2, 41–47. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Li, J.; Fejes, P.; Lorenser, D.; Quirk, B.C.; Noble, P.B.; Kirk, R.W.; Orth, A.; Wood, F.M.; Gibson, B.C.; Sampson, D.D.; et al. Two-photon polymerisation 3D printed freeform micro-optics for optical coherence tomography fibre probes. Sci. Rep. 2018, 8, 14789. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Werdehausen, D.; König, P.; Thiele, S.; Schmid, M.; Decker, M.; De Oliveira, P.W.; Herkommer, A.; Giessen, H. Tailored nanocomposites for 3D printed micro-optics. Opt. Mater. Express 2020, 10, 2345. [Google Scholar] [CrossRef]
- Wetzel, A.E.; del Iniesta, N.C.; Engay, E.; Mandsberg, N.K.; Dinesen, C.S.; Hanif, B.R.; Berg-Sørensen, K.; Bunea, A.-I.; Taboryski, R. Bioinspired Microstructured Polymer Surfaces with Antireflective Properties. Nanomaterials 2021, 11, 2298. [Google Scholar] [CrossRef]
- Soukoulis, C.M.; Wegener, M. Past achievements and future challenges in the development of three-dimensional photonic metamaterials. Nat. Photonics 2011, 5, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Von Freymann, G.; Ledermann, A.; Thiel, M.; Staude, I.; Essig, S.; Busch, K.; Wegener, M. Three-Dimensional Nanostructures for Photonics. Adv. Funct. Mater. 2010, 20, 1038–1052. [Google Scholar] [CrossRef]
- Bückmann, T.; Stenger, N.; Kadic, M.; Kaschke, J.; Frölich, A.; Kennerknecht, T.; Eberl, C.; Thiel, M.; Wegener, M. Tailored 3D Mechanical Metamaterials Made by Dip-in Direct-Laser-Writing Optical Lithography. Adv. Mater. 2012, 24, 2710–2714. [Google Scholar] [CrossRef]
- Tudor, A.; Delaney, C.; Zhang, H.; Thompson, A.J.; Curto, V.F.; Yang, G.Z.; Higgins, M.J.; Diamond, D.; Florea, L. Fabrication of soft, stimulus-responsive structures with sub-micron resolution via two-photon polymerization of poly(ionic liquid)s. Mater. Today 2018, 21, 807–816. [Google Scholar] [CrossRef]
- Del Pozo, M.; Delaney, C.; Bastiaansen, C.W.M.; Diamond, D.; Schenning, A.P.H.J.; Florea, L. Direct laser writing of four-dimensional structural color microactuators using a photonic photoresist. ACS Nano 2020, 14, 9832–9839. [Google Scholar] [CrossRef]
- Nocentini, S.; Martella, D.; Parmeggiani, C.; Wiersma, D.S. 3D printed photoresponsive materials for photonics. Adv. Opt. Mater. 2019, 7, 1900156. [Google Scholar] [CrossRef]
- Dai, G.; Hu, X.; Hering, J.; Eifler, M.; Seewig, J.; Freymann, G. von Define and measure the dimensional accuracy of two-photon laser lithography based on its instrument transfer function. J. Phys. Photonics 2021, 3, 034002. [Google Scholar] [CrossRef]
- Oakdale, J.S.; Ye, J.; Smith, W.L.; Biener, J. Post-print UV curing method for improving the mechanical properties of prototypes derived from two-photon lithography. Opt. Express 2016, 24, 27077. [Google Scholar] [CrossRef]
- Bauer, J.; Guell Izard, A.; Zhang, Y.; Baldacchini, T.; Valdevit, L. Thermal post-curing as an efficient strategy to eliminate process parameter sensitivity in the mechanical properties of two-photon polymerized materials. Opt. Express 2020, 28, 20362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.J.; Zhou, Y.S.; Xiong, W.; Gao, Y.; Huang, X.; Jiang, L.; Baldacchini, T.; Silvain, J.-F.; Lu, Y.F. Two-photon polymerization: Investigation of chemical and mechanical properties of resins using Raman microspectroscopy. Opt. Lett. 2014, 39, 3034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougdid, Y.; Sekkat, Z. Voxels Optimization in 3D Laser Nanoprinting. Sci. Rep. 2020, 10, 10409. [Google Scholar] [CrossRef] [PubMed]
- Gernhardt, M.; Blasco, E.; Hippler, M.; Blinco, J.; Bastmeyer, M.; Wegener, M.; Frisch, H.; Barner-Kowollik, C. Tailoring the Mechanical Properties of 3D Microstructures Using Visible Light Post-Manufacturing. Adv. Mater. 2019, 31, 1901269. [Google Scholar] [CrossRef]
- Purtov, J.; Verch, A.; Rogin, P.; Hensel, R. Improved development procedure to enhance the stability of microstructures created by two-photon polymerization. Microelectron. Eng. 2018, 194, 45–50. [Google Scholar] [CrossRef]
- Seniutinas, G.; Weber, A.; Padeste, C.; Sakellari, I.; Farsari, M.; David, C. Beyond 100 nm resolution in 3D laser lithography—Post processing solutions. Microelectron. Eng. 2018, 191, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Chidambaram, N.; Kirchner, R.; Fallica, R.; Yu, L.; Altana, M.; Schift, H. Selective Surface Smoothening of Polymer Microlenses by Depth Confined Softening. Adv. Mater. Technol. 2017, 2, 1700018. [Google Scholar] [CrossRef]
- Kirchner, R.; Schift, H. Thermal reflow of polymers for innovative and smart 3D structures: A review. Mater. Sci. Semicond. Process. 2019, 92, 58–72. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Tanzi, S.; Ostergaard, P.F.; Matteucci, M.; Christiansen, T.L.; Cech, J.; Marie, R.; Taboryski, R. Fabrication of combined-scale nano- and microfluidic polymer systems using a multilevel dry etching, electroplating and molding process. J. Micromech. Microeng. 2012, 22, 115008. [Google Scholar] [CrossRef]
- Murthy, S.; Matschuk, M.; Huang, Q.; Mandsberg, N.K.; Feidenhans’l, N.A.; Johansen, P.; Christensen, L.; Pranov, H.; Kofod, G.; Pedersen, H.C.; et al. Fabrication of Nanostructures by Roll-to-Roll Extrusion Coating. Adv. Eng. Mater. 2016, 18, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Murthy, S.; Pranov, H.; Feidenhans’L, N.A.; Madsen, J.S.; Hansen, P.E.; Pedersen, H.C.; Taboryski, R. Plasmonic color metasurfaces fabricated by a high speed roll-to-roll method. Nanoscale 2017, 9, 14280–14287. [Google Scholar] [CrossRef] [Green Version]
- Okulova, N.; Johansen, P.; Christensen, L.; Taboryski, R. Effect of Structure Hierarchy for Superhydrophobic Polymer Surfaces Studied by Droplet Evaporation. Nanomaterials 2018, 8, 831. [Google Scholar] [CrossRef] [Green Version]
- Lemma, E.D.; Rizzi, F.; Dattoma, T.; Spagnolo, B.; Sileo, L.; Qualtieri, A.; De Vittorio, M.; Pisanello, F. Mechanical Properties Tunability of Three-Dimensional Polymeric Structures in Two-Photon Lithography. IEEE Trans. Nanotechnol. 2017, 16, 23–31. [Google Scholar] [CrossRef]
- Guimarães, C.F.; Gasperini, L.; Marques, A.P.; Reis, R.L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 2020, 5, 351–370. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, Y.K.; Han, E.D.; Seo, Y.H.; Kim, B.H.; Mofrad, M.R.K. Passive control of cell locomotion using micropatterns: The effect of micropattern geometry on the migratory behavior of adherent cells. Lab. Chip 2012, 12, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wei, Q.; Mettetal, M.R.; Han, J.; Rau, L.; Tie, J.; May, R.M.; Pathe, E.T.; Reddy, S.T.; Sullivan, L.; et al. Surface micropattern reduces colonization and medical device-associated infections. J. Med. Microbiol. 2017, 66, 1692–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Kong, N.; Lu, Y.; Yuan, Y.; Wu, Q.; Shi, M.; Zhang, S.; Wu, Y.; Peng, W.; Huang, P.; et al. Bioinspired Conical Micropattern Modulates Cell Behaviors. ACS Appl. Bio. Mater. 2018, 1, 1416–1423. [Google Scholar] [CrossRef]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Ristok, S.; Thiele, S.; Toulouse, A.; Herkommer, A.M.; Giessen, H. Stitching-free 3D printing of millimeter-sized highly transparent spherical and aspherical optical components. Opt. Mater. Express 2020, 10, 2370. [Google Scholar] [CrossRef]
- Huang, Z.; Tsui, G.C.-P.; Deng, Y.; Tang, C.-Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136. [Google Scholar] [CrossRef]
- Goseki, R.; Hong, L.; Inutsuka, M.; Yokoyama, H.; Ito, K.; Ishizone, T. Synthesis and surface characterization of well-defined amphiphilic block copolymers composed of polydimethylsiloxane and poly[oligo(ethylene glycol) methacrylate]. RSC Adv. 2017, 7, 25199–25207. [Google Scholar] [CrossRef] [Green Version]
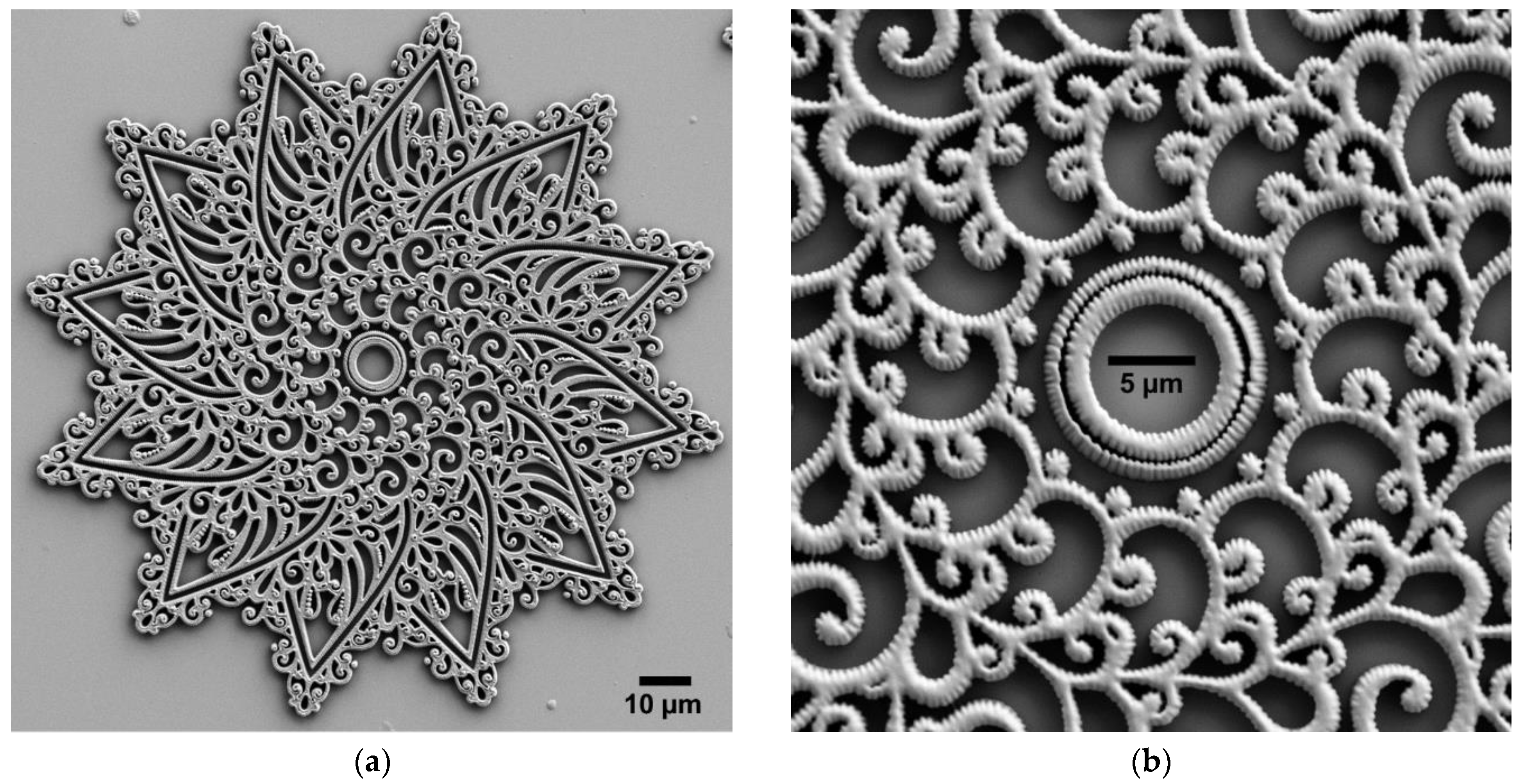

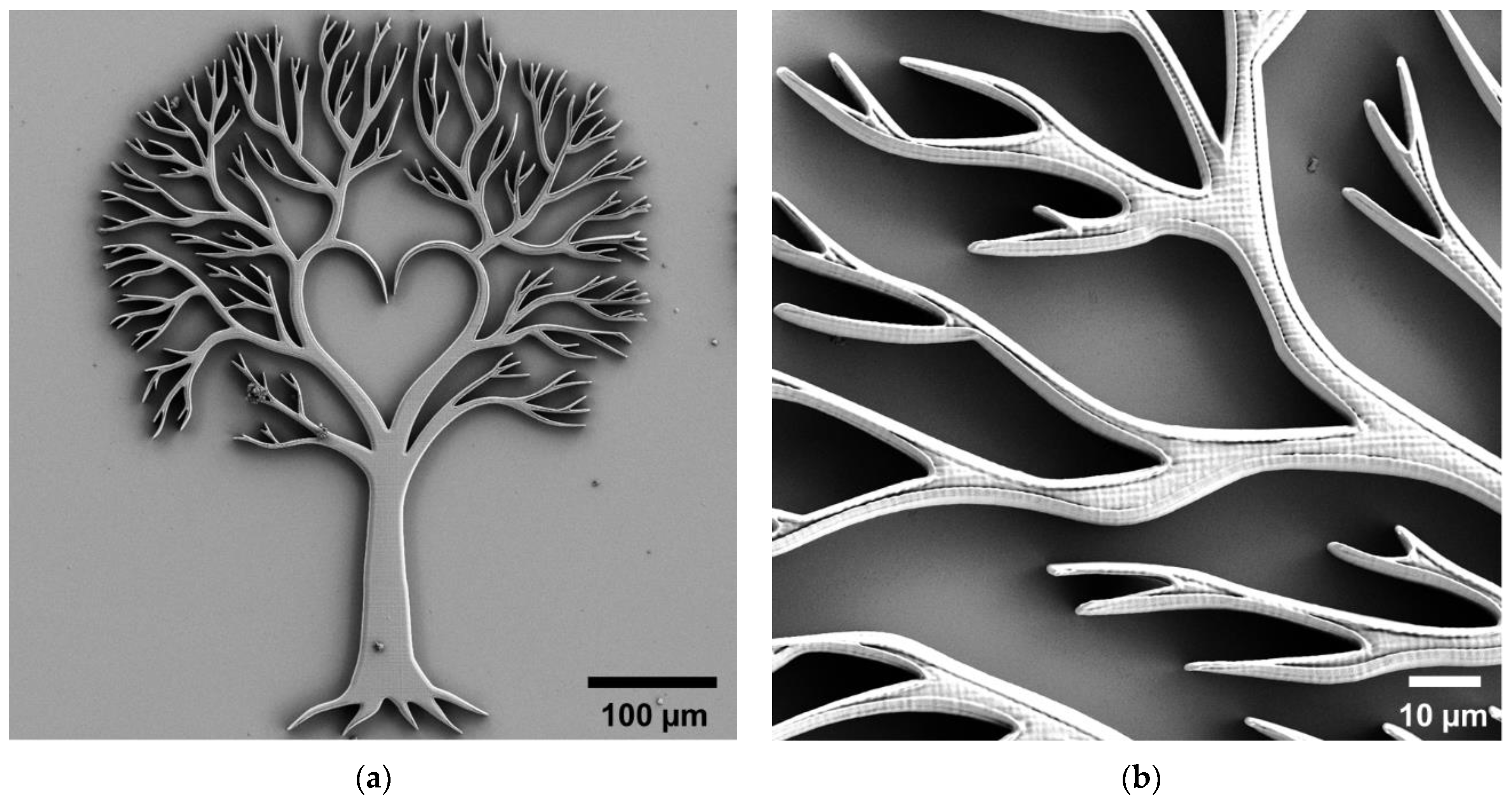
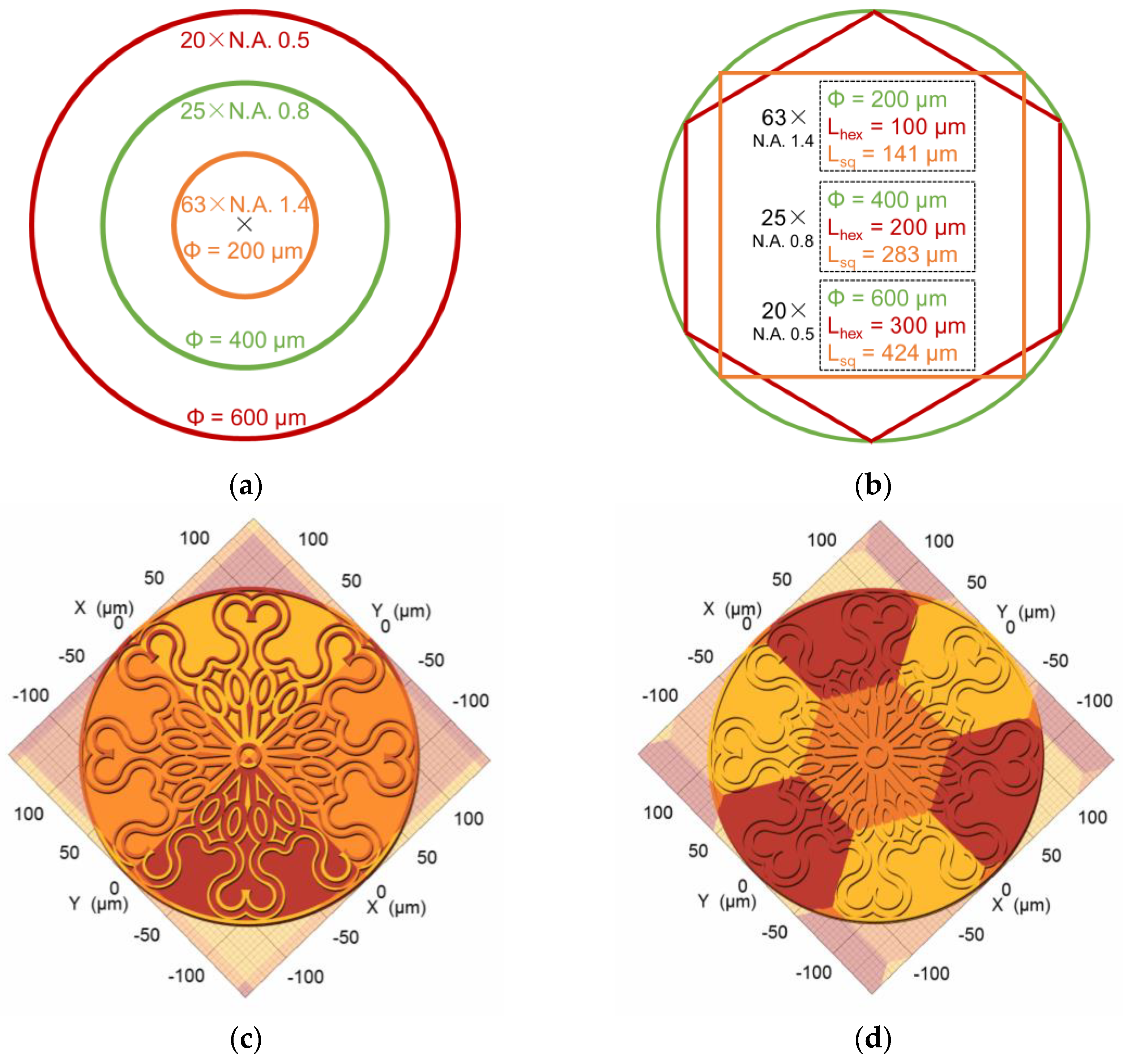
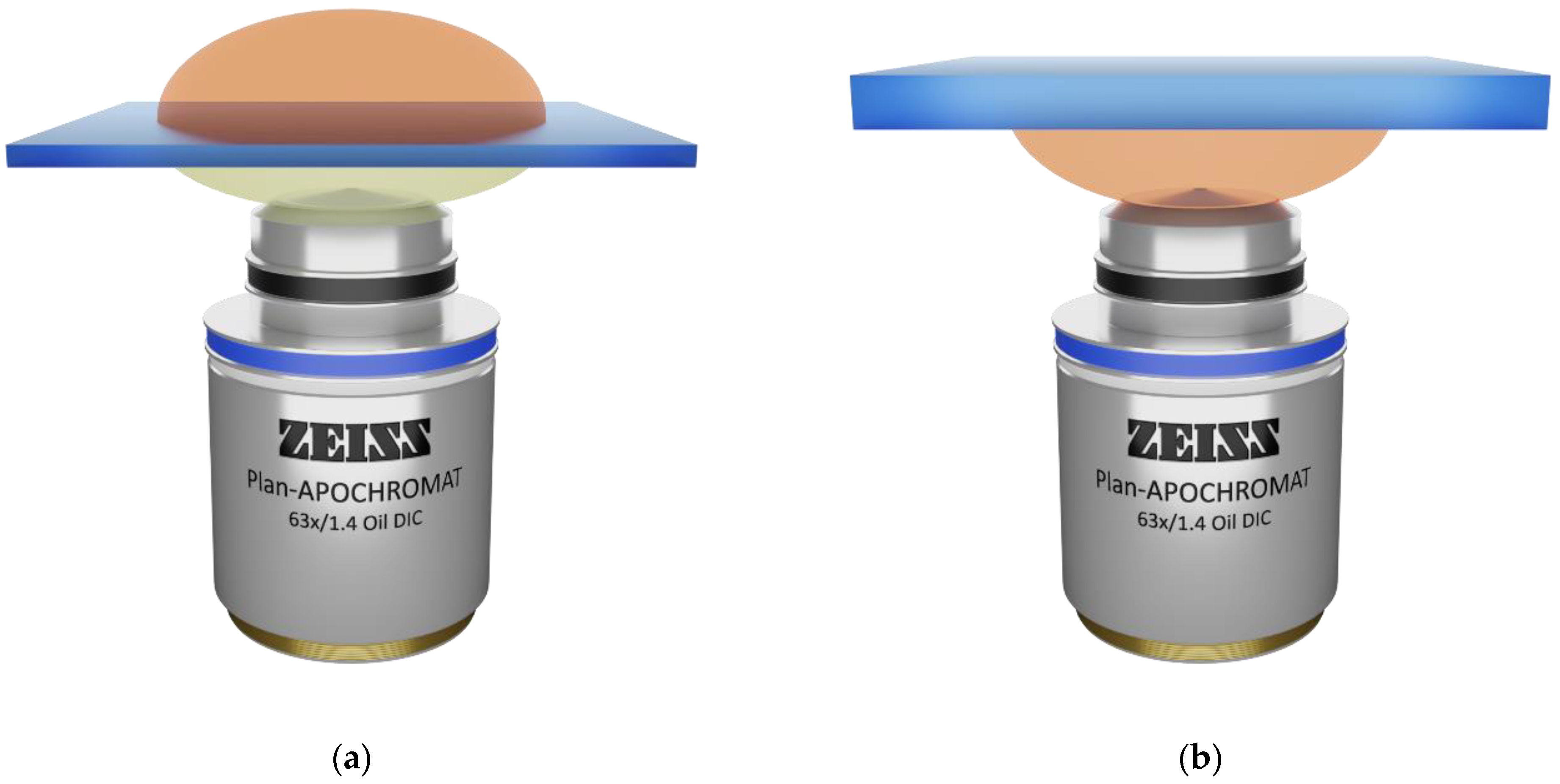

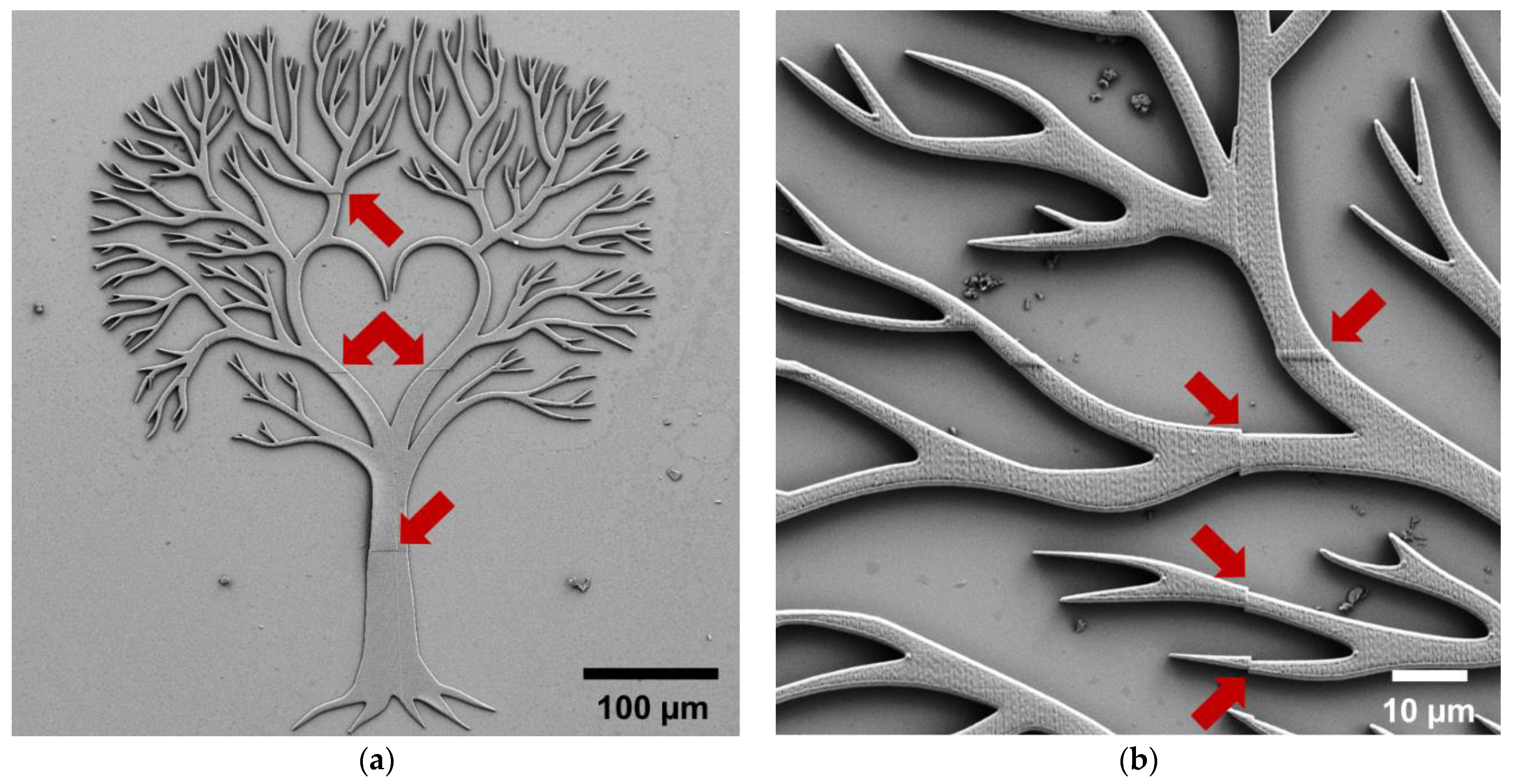
| Type of Structure | Microscope Objective | Configuration | Substrate | Resin | Slicing‖Hatching Distance (nm) | |
|---|---|---|---|---|---|---|
| Soft | 25×/0.80 | DiLL | Activated ITO-coated glass | IP-PDMS | 300 | 300 |
| High resolution, flat | 63×/1.40 | (Oil) Immersion | 170 µm thick borosilicate glass | IP-L 780 | 300 | 200 |
| High resolution, tall | 63×/1.40 | DiLL | Fused silica | IP-Dip | 300 | 200 |
| Large | 20×/0.50 | (Air) Immersion | 170 µm thick borosilicate glass | IP-L 780 * | 500 | 500 |
| Soft Structures | Mandala | Sphynx & Pyramid | Yggdrasil | |||||
|---|---|---|---|---|---|---|---|---|
| Mold | Porous | “Good” | “Bad” | “Good” | “Bad” | “Good” | “Bad” | |
| Microscope objective | 25×/0.80 | 63×/1.40 | 63×/1.40 | 20×/0.50 | 63×/1.40 | |||
| Configuration | DiLL | Oil immersion | DiLL | DiLL | Oil immersion | (Air) Immersion | Oil immersion | |
| Substrate | Activated ITO-coated glass | 170 µm thick borosilicate glass | Fused silica | Fused silica | 170 µm thick borosilicate glass | 170 µm thick borosilicate glass | ||
| Resin | IP-PDMS | IP-L 780 | IP-Dip | IP-Dip | IP-L 780 | IP-L 780 | ||
| Slicing mode | Fixed | Fixed | Fixed | Fixed | ||||
| Slicing distance (µm) | 0.3 | 0.2 | 0.3 | 0.5 | 0.3 | |||
| Contour count | 2 | 1 | 0 | 2 | 3 | |||
| Hatching distance (µm) | 0.2 | 0.3 | 0.2 | 0.3 | 0.5 | 0.2 | ||
| Laser power (%) | 50 | 75 | 50 | 50 | 60 | 50 | ||
| Scan speed (µm·s−1) | 10,000 | 20,000 | 10,000 | 10,000 | 500 | 10,000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunea, A.-I.; del Castillo Iniesta, N.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System. Micro 2021, 1, 164-180. https://doi.org/10.3390/micro1020013
Bunea A-I, del Castillo Iniesta N, Droumpali A, Wetzel AE, Engay E, Taboryski R. Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System. Micro. 2021; 1(2):164-180. https://doi.org/10.3390/micro1020013
Chicago/Turabian StyleBunea, Ada-Ioana, Nuria del Castillo Iniesta, Ariadni Droumpali, Alexandre Emmanuel Wetzel, Einstom Engay, and Rafael Taboryski. 2021. "Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System" Micro 1, no. 2: 164-180. https://doi.org/10.3390/micro1020013
APA StyleBunea, A.-I., del Castillo Iniesta, N., Droumpali, A., Wetzel, A. E., Engay, E., & Taboryski, R. (2021). Micro 3D Printing by Two-Photon Polymerization: Configurations and Parameters for the Nanoscribe System. Micro, 1(2), 164-180. https://doi.org/10.3390/micro1020013