Towards Hybrid 2D Nanomaterials: Covalent Functionalization of Boron Nitride Nanosheets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Oxidized BNNSs
2.3. Preparation of the Functionalized BNNSs
2.4. Characterization
3. Results and Discussion
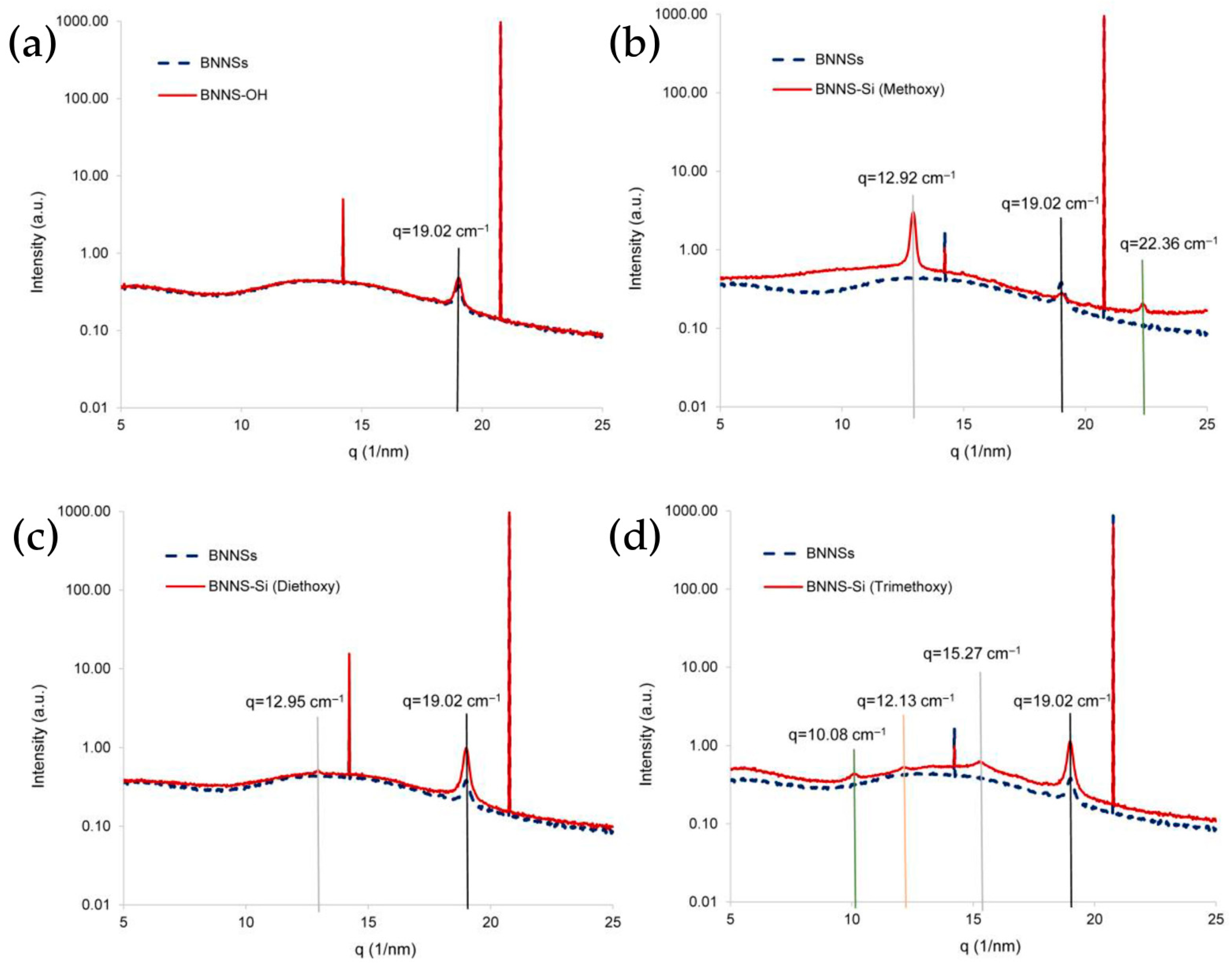
3.1. Oxidation
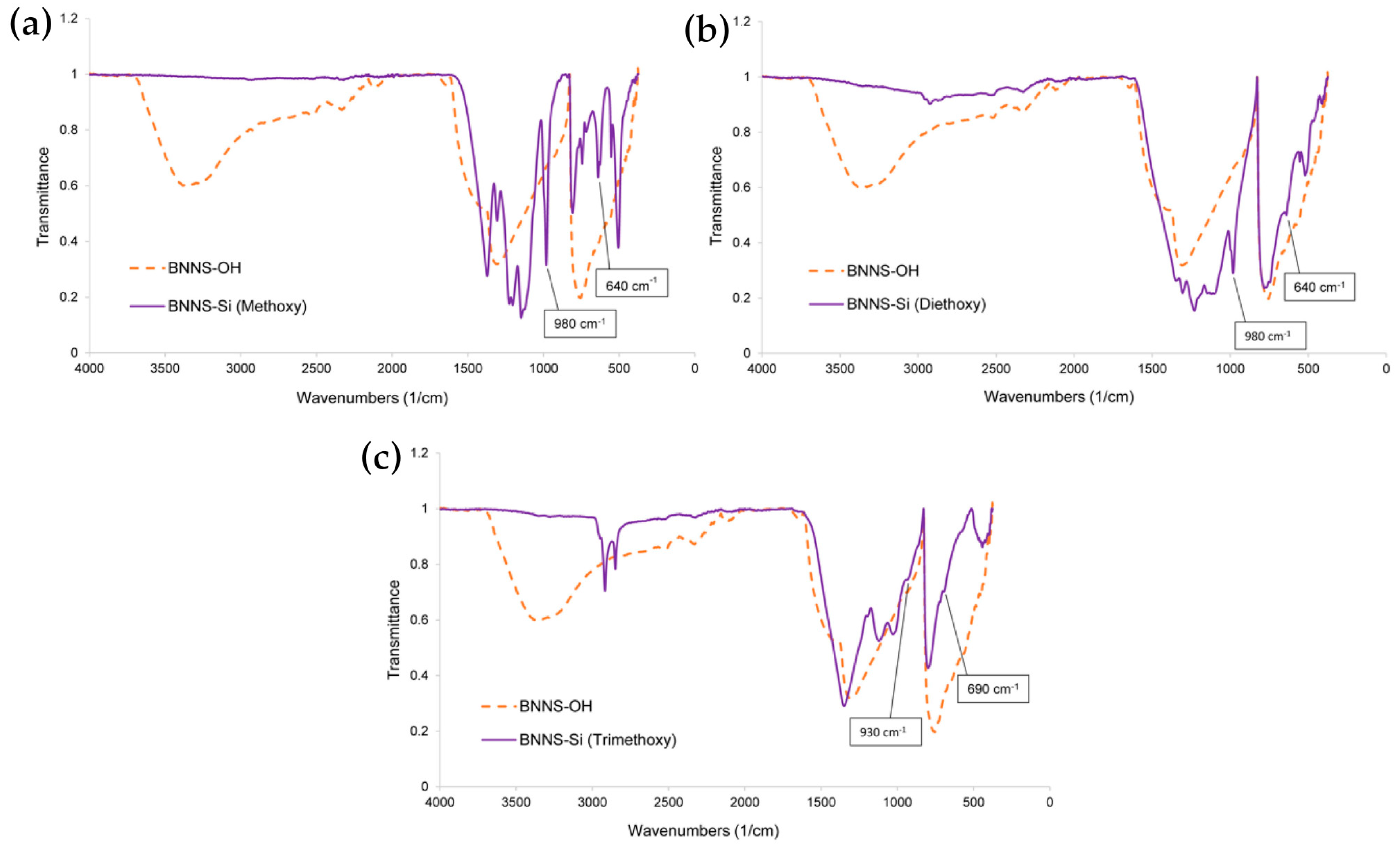
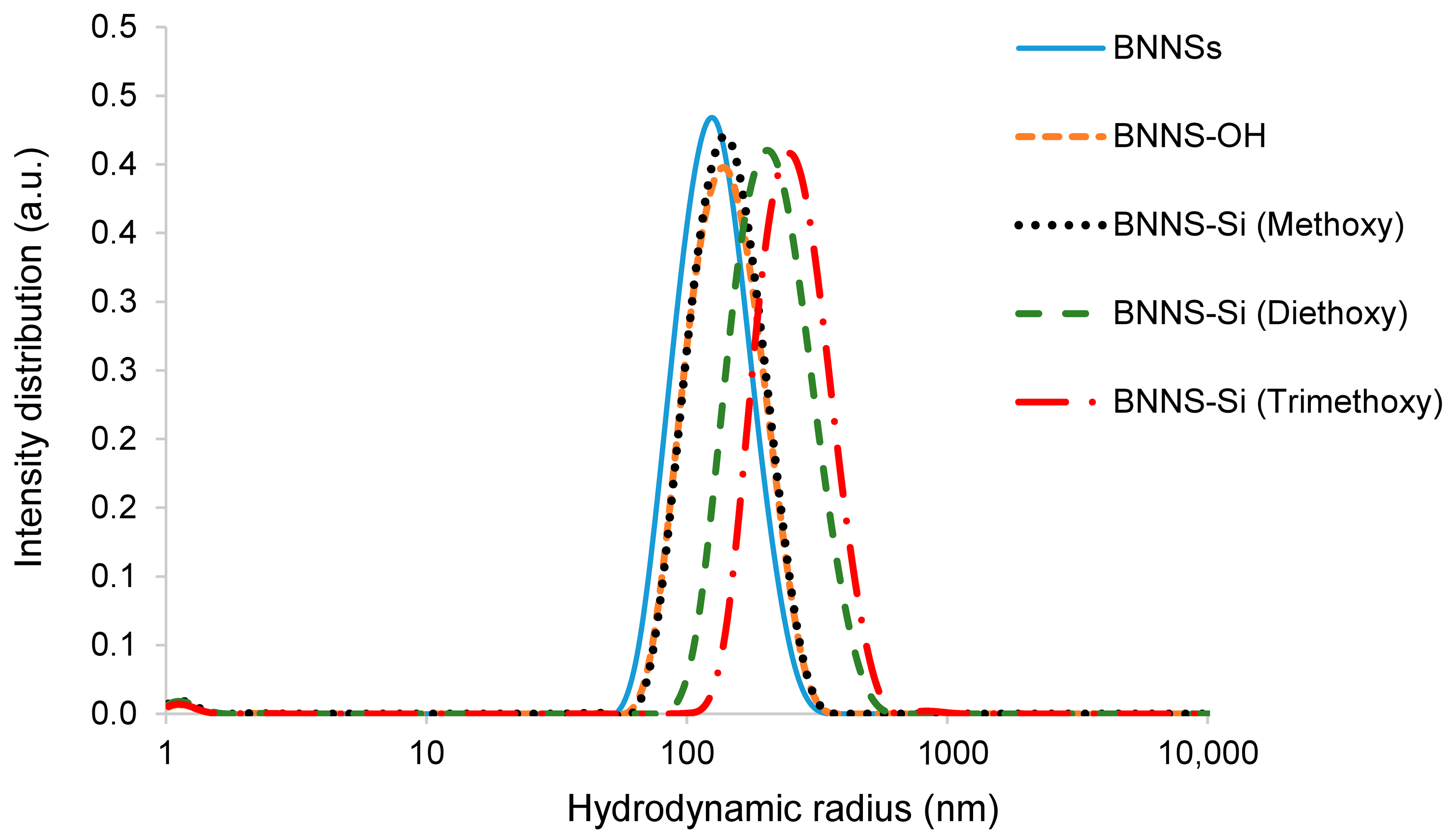
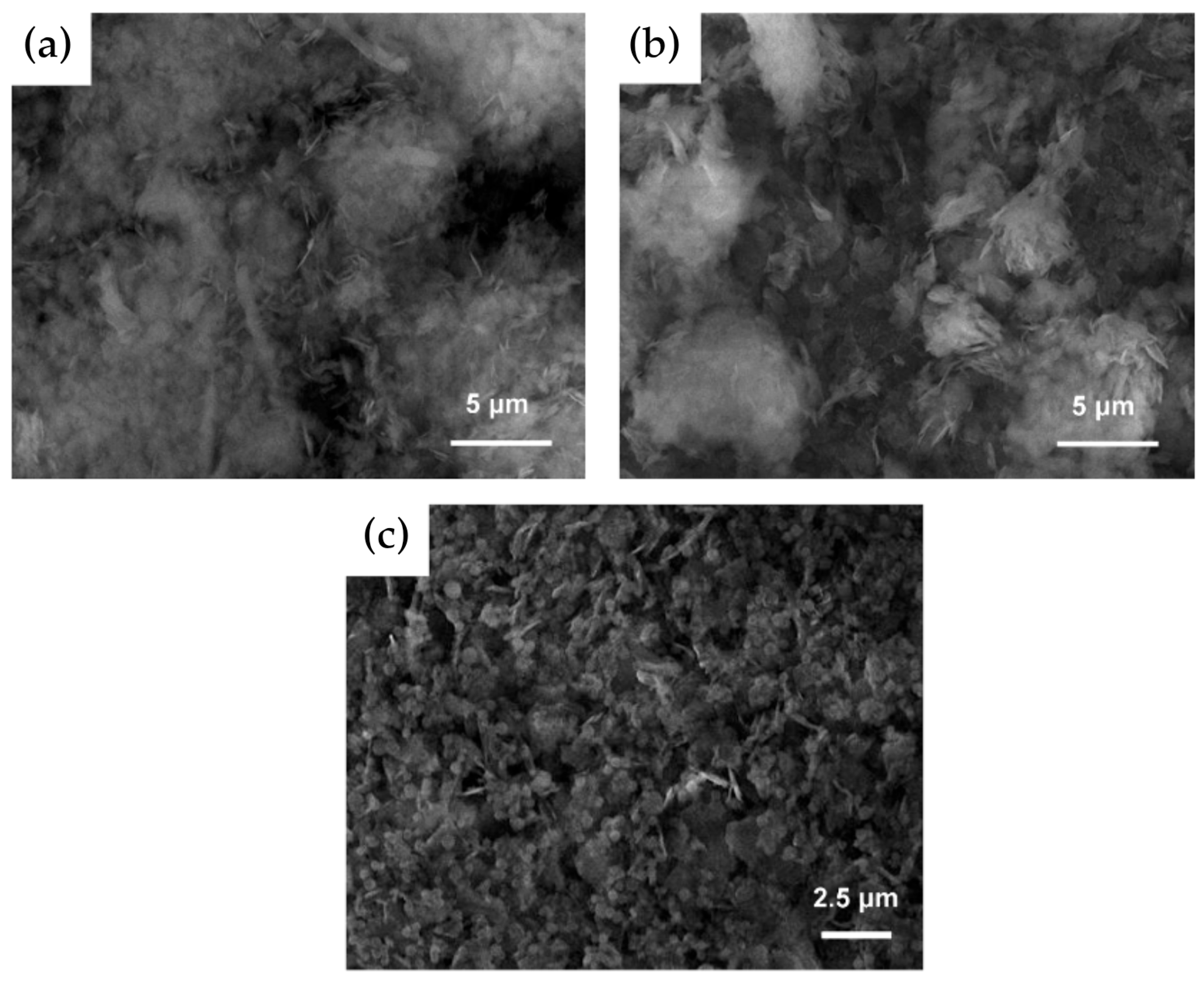
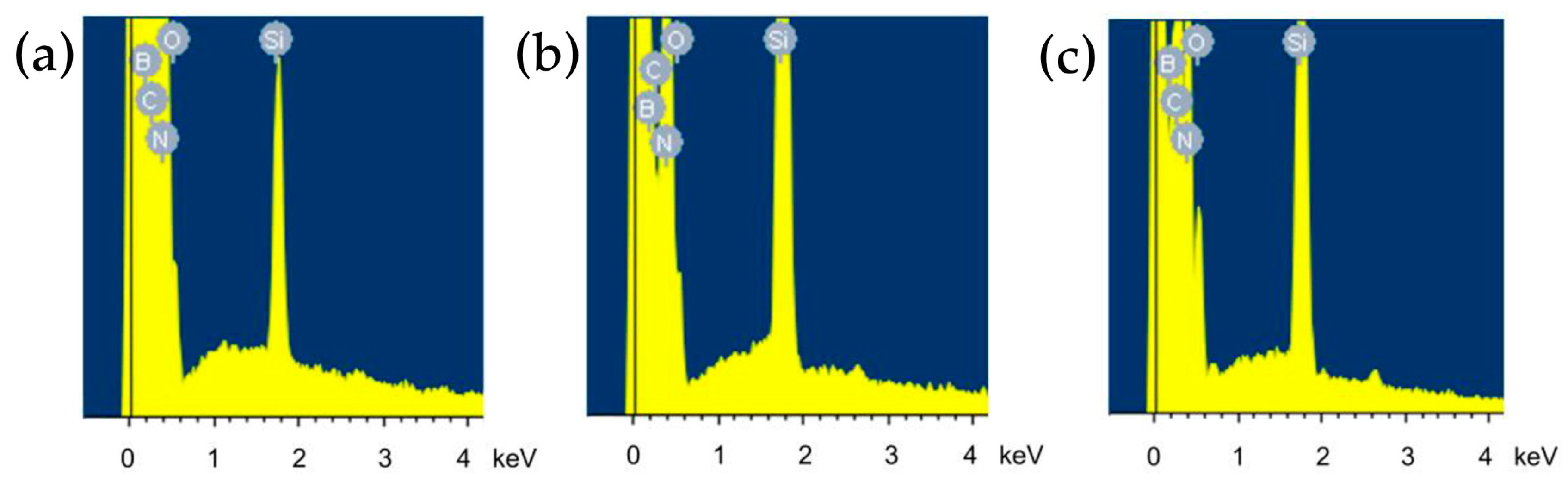
3.2. Silanization
- (a)
- (3-aminopropyl)dimethylmethoxysilane (also called Methoxy for simplicity reasons),
- (b)
- (3-aminopropyl)diethoxymethylsilane (also called Diethoxy),
- (c)
- (3-aminopropyl)trimethoxysilane (also called Trimethoxy).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topsakal, M.; Aktürk, E.; Ciraci, S. First-principles study of two- and one-dimensional honeycomb structures of boron nitride. Phys. Rev. B 2009, 79, 115442. [Google Scholar] [CrossRef]
- Goriachko, A.; He, Y.; Knapp, M.; Over, H.; Corso, M.; Brugger, T.; Berner, S.; Osterwalder, J.; Greber, T. Self-assembly of a hexagonal boron nitride nanomesh on Ru(0001). Langmuir 2007, 23, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Cervenka, J.; Watanabe, K.; Taniguchi, T.; Chen, Y. Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 2014, 8, 1457–1462. [Google Scholar] [CrossRef]
- Cai, Q.; Scullion, D.; Gan, W.; Falin, A.; Zhang, S.; Watanabe, K.; Taniguchi, T.; Chen, Y.; Santos, E.J.G.; Li, L.H. High thermal conductivity of high-quality monolayer boron nitride and its thermal expansion. Sci. Adv. 2019, 5, eaav0129. [Google Scholar] [CrossRef]
- Lindsay, L.; Broido, D.A. Enhanced thermal conductivity and isotope effect in single-layer hexagonal boron nitride. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 84, 155421. [Google Scholar] [CrossRef]
- Wang, X.; Pakdel, A.; Zhang, J.; Weng, Q.; Zhai, T.; Zhi, C.; Golberg, D.; Bando, Y. Large-surface-area BN nanosheets and their utilization in polymeric composites with improved thermal and dielectric properties. Nanoscale Res. Lett. 2012, 7, 662. [Google Scholar] [CrossRef]
- Chen, Y.; Fitz Gerald, J.; Williams, J.; Bulcock, S. Synthesis of boron nitride nanotubes at low temperatures using reactive ball milling. Chem. Phys. Lett. 1999, 299, 260–264. [Google Scholar] [CrossRef]
- Song, L.; Ci, L.; Lu, H.; Sorokin, P.B.; Jin, C.; Ni, J.; Kvashnin, A.G.; Kvashnin, D.G.; Lou, J.; Yakobson, B.I.; et al. Large scale growth and characterization of atomic hexagonal boron nitride layers. Nano Lett. 2010, 10, 3209–3215. [Google Scholar] [CrossRef]
- Falin, A.; Cai, Q.; Santos, E.J.G.; Scullion, D.; Qian, D.; Zhang, R.; Yang, Z.; Huang, S.; Watanabe, K.; Taniguchi, T.; et al. Mechanical properties of atomically thin boron nitride and the role of interlayer interactions. Nat. Commun. 2017, 8, 15815. [Google Scholar] [CrossRef]
- Cai, Q.; Du, A.; Gao, G.; Mateti, S.; Cowie, B.C.C.; Qian, D.; Zhang, S.; Lu, Y.; Fu, L.; Taniguchi, T.; et al. Molecule-Induced Conformational Change in Boron Nitride Nanosheets with Enhanced Surface Adsorption. Adv. Funct. Mater. 2016, 26, 8202–8210. [Google Scholar] [CrossRef]
- Uosaki, K.; Elumalai, G.; Noguchi, H.; Masuda, T.; Lyalin, A.; Nakayama, A.; Taketsugu, T. Boron nitride nanosheet on gold as an electrocatalyst for oxygen reduction reaction: Theoretical suggestion and experimental proof. J. Am. Chem. Soc. 2014, 136, 6542–6545. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Fang, Z.; Xu, J.; Cao, F.; Wan, J.; Preston, C.; Yang, B.; Hu, L. Highly Thermally Conductive Papers with Percolative Layered Boron Nitride Nanosheets. ACS Nano 2014, 8, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777. [Google Scholar] [CrossRef]
- Liu, D.; Lei, W.; Qin, S.; Chen, Y. Template-free synthesis of functional 3D BN architecture for removal of dyes from water. Sci. Rep. 2014, 4, 4453. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, Y. Atomically Thin Boron Nitride: Unique Properties and Applications. Adv. Funct. Mater. 2016, 26, 2594–2608. [Google Scholar] [CrossRef]
- Li, L.H.; Santos, E.J.G.; Xing, T.; Cappelluti, E.; Roldán, R.; Chen, Y.; Watanabe, K.; Taniguchi, T. Dielectric screening in atomically thin boron nitride nanosheets. Nano Lett. 2015, 15, 218–223. [Google Scholar] [CrossRef]
- Wang, X.-B.; Weng, Q.; Wang, X.; Li, X.; Zhang, J.; Liu, F.; Jiang, X.-F.; Guo, H.; Xu, N.; Golberg, D.; et al. Biomass-Directed Synthesis of 20 g High-Quality Boron Nitride Nanosheets for Thermoconductive Polymeric Composites. ACS Nano 2014, 8, 9081–9088. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Kelly, P.J.; Brink, J.v.D. Substrate-induced band gap in graphene on hexagonal boron nitride: Ab. initio density functional calculations. Phys. Rev. B 2007, 76, 073103. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, M.; Jiang, D.; Zhou, X.; He, J. Amino functionalized boron nitride and enhanced thermal conductivity of epoxy composites via combining mixed sizes of fillers. Ceram. Int. 2021, 48, 2763–2770. [Google Scholar] [CrossRef]
- Kostoglou, N.; Tampaxis, C.; Charalambopoulou, G.; Constantinides, G.; Ryzhkov, V.; Doumanidis, C.; Matovic, B.; Mitterer, C.; Rebholz, C. Boron nitride nanotubes versus carbon nanotubes: A thermal stability and oxidation behavior study. Nanomaterials 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kutty, R.G.; Zheng, Q.; Eswariah, V.; Sreejith, S.; Liu, Z. Hexagonal Boron Nitride Nanosheets as High-Performance Binder-Free Fire-Resistant Wood Coatings. Small 2017, 13, 1602456. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, J.; Campbell, S.J.; Le Caer, G. Boron nitride nanotubes: Pronounced resistance to oxidation. Appl. Phys. Lett. 2004, 84, 2430–2432. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, H.; Rhee, K.Y. Surface functionalization of boron nitride platelets via a catalytic oxidation/silanization process and thermomechanical properties of boron nitride-epoxy composites. Compos. Part B Eng. 2019, 157, 276–282. [Google Scholar] [CrossRef]
- He, J.; Liu, S.; Li, Y.; Zeng, S.; Qi, Y.; Cui, L.; Dai, Q.; Bai, C. Fabrication of boron nitride nanosheet/polymer composites with tunable thermal insulating properties. New J. Chem. 2019, 43, 4878–4885. [Google Scholar] [CrossRef]
- Yang, N.; Xu, C.; Hou, J.; Yao, Y.; Zhang, Q.; Grami, M.E.; He, L.; Wang, N.; Qu, X. Preparation and properties of thermally conductive polyimide/boron nitride composites. RSC Adv. 2016, 6, 18279–18287. [Google Scholar] [CrossRef]
- Lee, D.; Lee, B.; Park, K.H.; Ryu, H.J.; Jeon, S.; Hong, S.H. Scalable exfoliation process for highly soluble boron nitride nanoplatelets by hydroxide-assisted ball milling. Nano Lett. 2015, 15, 1238–1244. [Google Scholar] [CrossRef]
- Han, G.; Zhao, X.; Feng, Y.; Ma, J.; Zhou, K.; Shi, Y.; Liu, C.; Xie, X. Highly flame-retardant epoxy-based thermal conductive composites with functionalized boron nitride nanosheets exfoliated by one-step ball milling. Chem. Eng. J. 2021, 407, 127099. [Google Scholar] [CrossRef]
- Yadav, V.; Rajput, A.; Rathod, N.H.; Kulshrestha, V. Enhancement in proton conductivity and methanol cross-over resistance by sulfonated boron nitride composite sulfonated poly (ether ether ketone) proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 17017–17028. [Google Scholar] [CrossRef]
- Yu, B.; Xing, W.; Guo, W.; Qiu, S.; Wang, X.; Lo, S.; Hu, Y. Thermal exfoliation of hexagonal boron nitride for effective enhancements on thermal stability, flame retardancy and smoke suppression of epoxy resin nanocomposites via sol–gel process. J. Mater. Chem. A 2016, 4, 7330–7340. [Google Scholar] [CrossRef]
- Jiang, X.; Ma, P.; You, F.; Yao, C.; Yao, J.; Liu, F. A facile strategy for modifying boron nitride and enhancing its effect on the thermal conductivity of polypropylene/polystyrene blends. RSC Adv. 2018, 8, 32132–32137. [Google Scholar] [CrossRef]
- Silberzan, P.; Leger, L.; Ausserre, D.; Benattar, J.J. Silanation of Silica Surfaces. A New Method of Constructing Pure or Mixed Monolayers. Langmuir 1991, 7, 1647–1651. [Google Scholar] [CrossRef]
- DePalma, V.; Tillman, N. Friction and wear of self-assembled trichlorosilane monolayer films on silicon. Langmuir 1989, 5, 868–872. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Z.; Meng, G.; Gu, L. Silane functionalized plasma-treated boron nitride nanosheets for anticorrosive reinforcement of waterborne epoxy coatings. Prog. Org. Coat. 2022, 167, 106831. [Google Scholar] [CrossRef]
- Goni, F.; Chemelli, A.; Uhlig, F. High-Yield Production of Selected 2D Materials by Understanding Their Sonication-Assisted Liquid-Phase Exfoliation. Nanomaterials 2021, 11, 3253. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial modification of boron nitride nanoplatelets for epoxy composites with improved thermal properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Schnablegger, H.; Glatter, O. Optical sizing of small colloidal particles: An optimized regularization technique. Appl. Opt. 1991, 30, 4889–4896. [Google Scholar] [CrossRef]
- Kudus, M.H.A.; Zakaria, M.R.; Akil, H.M.; Ullah, F.; Javed, F. Oxidation of graphene via a simplified Hummers’ method for graphene-diamine colloid production. J. King Saud. Univ.-Sci. 2020, 32, 910–913. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Liakos, I.; Athanassiou, A.; Dinucci, D.; Chiellini, F.; Mattoli, V. A simple approach to covalent functionalization of boron nitride nanotubes. J. Colloid Interface Sci. 2012, 374, 308–314. [Google Scholar] [CrossRef]
- Geick, R.; Perry, C.H.; Rupprecht, G. Normal Modes in Hexagonal Boron Nitride. Phys. Rev. B 1966, 146, 543–547. [Google Scholar] [CrossRef]
- Zhi, C.; Bando, Y.; Tang, C.; Kuwahara, H.; Golberg, D. Large-Scale Fabrication of Boron Nitride Nanosheets and Their Utilization in Polymeric Composites with Improved Thermal and Mechanical Properties. Adv. Mater. 2009, 21, 2889–2893. [Google Scholar] [CrossRef]
- Dibandjo, P.; Bois, L.; Chassagneux, F.; Cornu, D.; Letoffe, J.; Toury, B.; Babonneau, F.; Miele, P. Synthesis of Boron Nitride with Ordered Mesostructure. Adv. Mater. 2005, 17, 571–574. [Google Scholar] [CrossRef]
- Shurvell, H.F.; Faniran, J.A. Infrared spectra of triphenylboron and triphenylborate. Can. J. Chem. 1968, 46, 2081–2087. [Google Scholar] [CrossRef]
- Wang, W.; Gai, Y.; Xiao, D.; Zhao, Y. A facile and general approach for production of nanoscrolls with high-yield from two-dimensional nanosheets. Sci. Rep. 2018, 8, 15262. [Google Scholar] [CrossRef]
- McGovern, M.E.; Kallury, K.M.R.; Thompson, M. Role of Solvent on the Silanization of Glass with Octadecyltrichlorosilane. Langmuir 1994, 10, 3607–3614. [Google Scholar] [CrossRef]
- Sagiv, J. Organized Monolayers by Adsorption. 1. Formation and Structure of Oleophobic Mixed Monolayers on Solid Surfaces. J. Am. Chem. Soc. 1980, 102, 92–98. [Google Scholar] [CrossRef]
- Le Grange, J.D.; Markham, J.L.; Kurkjian, C.R. Effects of Surface Hydration on the Deposition of Silane Monolayers on Silica. Langmuir 1993, 9, 1749–1753. [Google Scholar] [CrossRef]
- Yoshida, W.; Castro, R.P.; Jou, J.-D.; Cohen, Y. Multilayer alkoxysilane silylation of oxide surfaces. Langmuir 2001, 17, 5882–5888. [Google Scholar] [CrossRef]
- Kallury, K.M.R.; Macdonald, P.M.; Thompson, M. Effect of Surface Water and Base Catalysis on the Silanization of Silica by (Aminopropyl) alkoxysilanes Studied by X-ray Photoelectron Spectroscopy and 13C Cross-Polarization/Magic Angle Spinning Nuclear Magnetic Resonance. Langmuir 1994, 10, 492–499. [Google Scholar] [CrossRef]
- Launer, P.J. Silicon Compounds: Silanes & Silicones; Gelest Inc.: Morrisville, PA, USA, 2013. [Google Scholar]
- Cai, W.; Wang, B.; Liu, L.; Zhou, X.; Chu, F.; Zhan, J.; Hu, Y.; Kan, Y.; Wang, X. An operable platform towards functionalization of chemically inert boron nitride nanosheets for flame retardancy and toxic gas suppression of thermoplastic polyurethane. Compos. Part B Eng. 2019, 178, 107462. [Google Scholar] [CrossRef]
- Tenney, A.S.; Wong, J. Vibrational Spectra of Vapor-Deposited Binary Borosilicate Glasses. J. Chem. Phys. 1972, 56, 5516–5523. [Google Scholar] [CrossRef]
- Gautam, C.R.; Kumar, D.; Parkash, O.M. IR study of Pb-Sr titanate borosilicate glasses. Bull. Mater. Sci. 2010, 33, 145–148. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Singh, A.K. A Review on Infrared Spectroscopy of Borate Glasses with Effects of Different Additives. ISRN Ceram. 2012, 2012, 428497. [Google Scholar] [CrossRef]
- Alemi, A.A.; Sedghi, H.; Mirmohseni, A.R.; Golsanamlu, V. Synthesis and characterization of cadmium doped lead-borate glasses. Bull. Mater. Sci. 2006, 29, 55–58. [Google Scholar] [CrossRef]
- Lin, Y.; Connell, J.W. Advances in 2D boron nitride nanostructures: Nanosheets, nanoribbons, nanomeshes, and hybrids with graphene. Nanoscale 2012, 4, 6908–6939. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zulhairun, A.; Wong, T.; Alireza, S.; Marzuki, M.; Ismail, A. Water transport properties of boron nitride nanosheets mixed matrix membranes for humic acid removal. Heliyon 2019, 5, e01142. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Shi, X.; Wang, X.; Wu, W. Preparation of functionalized boron nitride nanosheets by high-gravity liquid phase exfoliation technology. Chem. Eng. Process.-Process Intensif. 2021, 169, 108602. [Google Scholar] [CrossRef]
- Hubbard, C.R.; Swanson, H.E.; Mauer, F.A. A silicon powder diffraction standard reference material. J. Appl. Crystallogr. 1975, 8, 45–48. [Google Scholar] [CrossRef]
- Wang, Q. Preparation and surface modification of Mg(OH)2/siloxane nanocomposite flame retardant. J. Polym. Eng. 2015, 35, 113–117. [Google Scholar] [CrossRef]
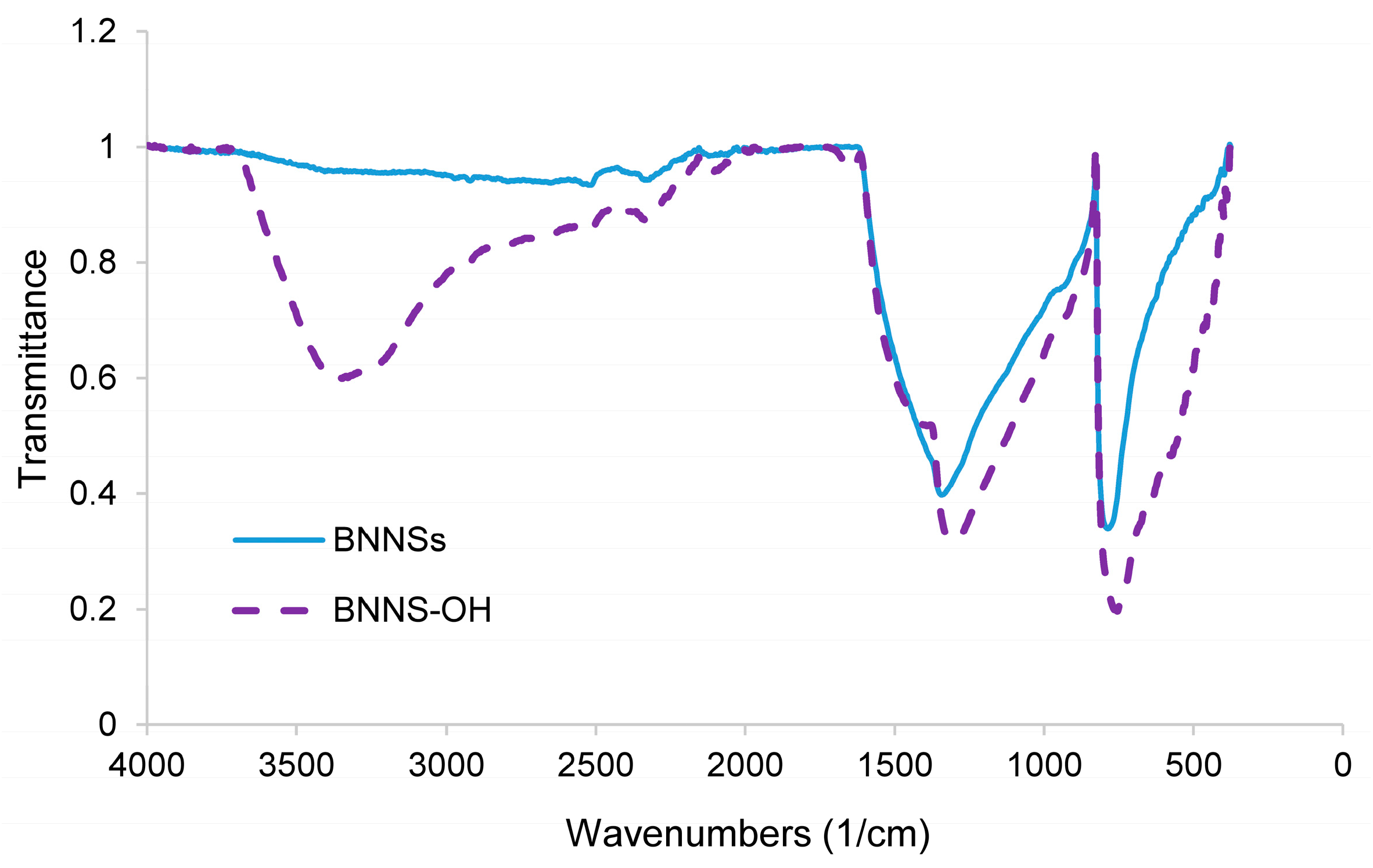
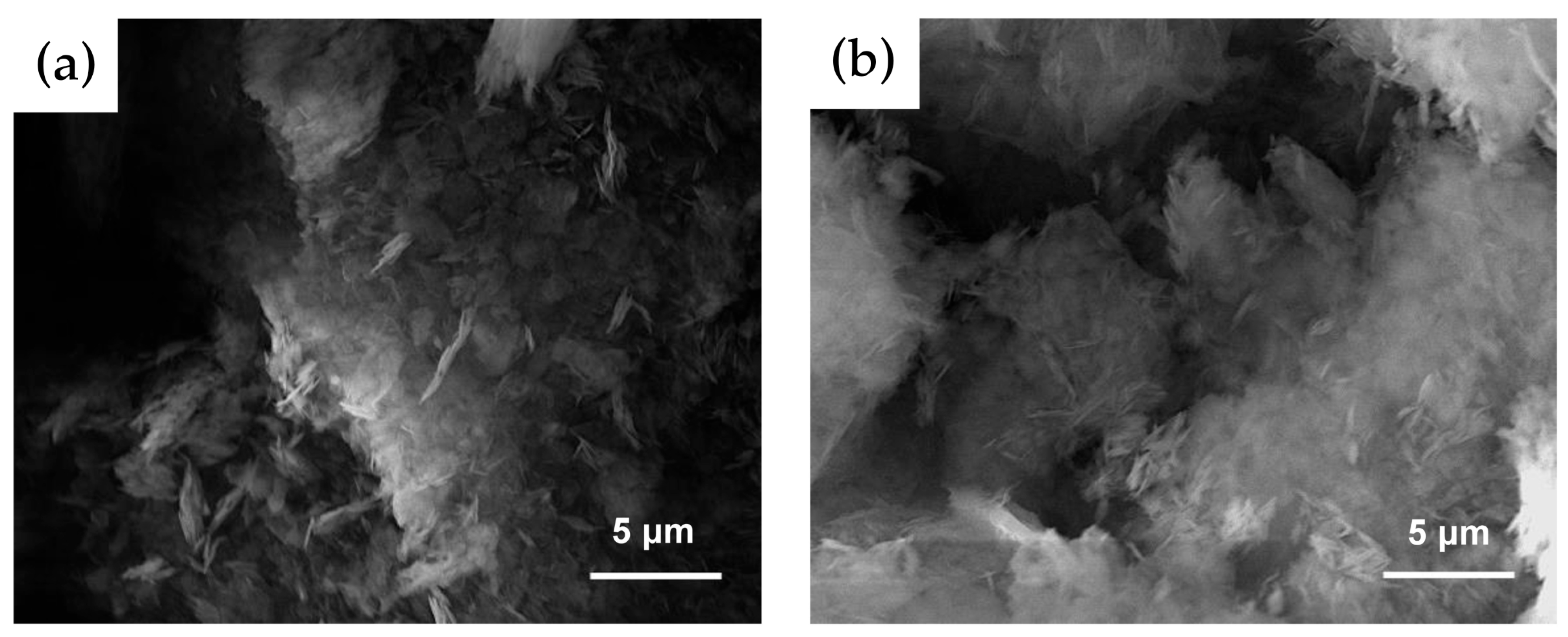
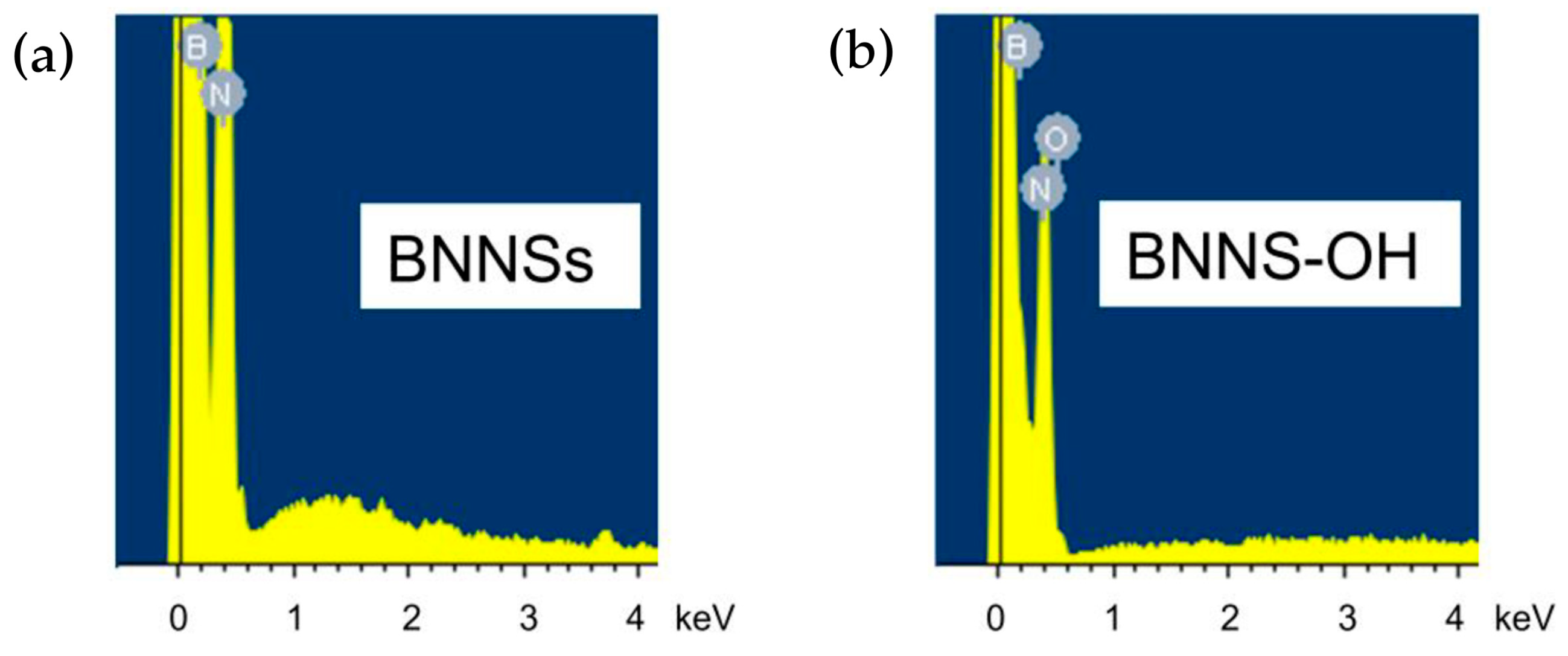
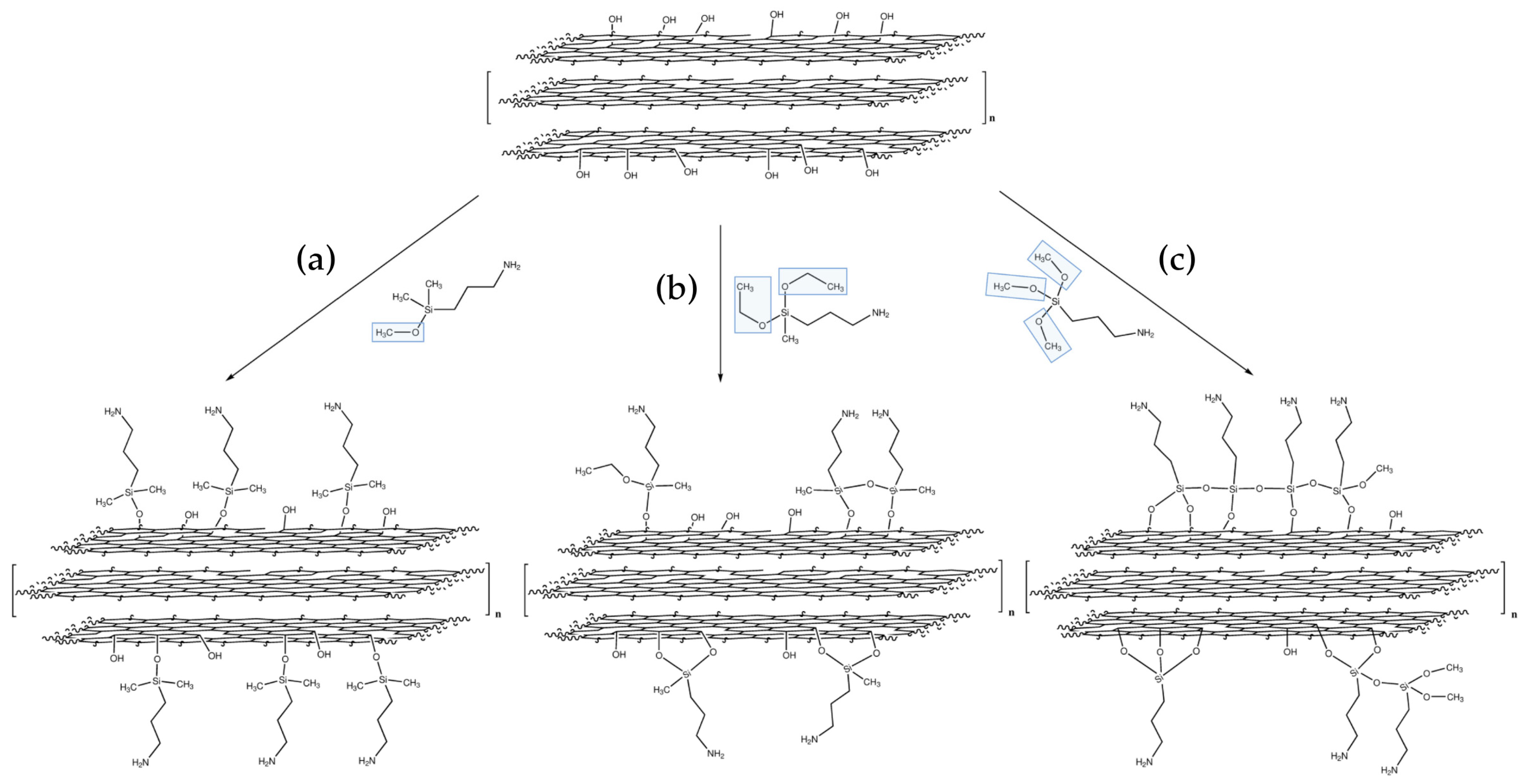
| FT-IR Vibrations/(cm−1) | |||
|---|---|---|---|
| BNNS-Si (Methoxy) | BNNS-Si (Diethoxy) | BNNS-Si (Trimethoxy) | |
| B-N | 1350, 780 | 1350, 780 | 1350, 780 |
| Si-CH3 | 1260, 750 | 1300 | - |
| Si-CH2 | 1220, 1200 | 1220 | - |
| Si-O | 1150 | 1140 | 1150 |
| Si-O-Si | - | 1100, 460 | 1120, 1020, 440 |
| B-O | 530, 505 | 530, 510 | 580 |
| B-O-Si | 980, 640 | 980, 640 | 930, 690 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goni, F.; Chemelli, A.; Uhlig, F. Towards Hybrid 2D Nanomaterials: Covalent Functionalization of Boron Nitride Nanosheets. Liquids 2025, 5, 31. https://doi.org/10.3390/liquids5040031
Goni F, Chemelli A, Uhlig F. Towards Hybrid 2D Nanomaterials: Covalent Functionalization of Boron Nitride Nanosheets. Liquids. 2025; 5(4):31. https://doi.org/10.3390/liquids5040031
Chicago/Turabian StyleGoni, Freskida, Angela Chemelli, and Frank Uhlig. 2025. "Towards Hybrid 2D Nanomaterials: Covalent Functionalization of Boron Nitride Nanosheets" Liquids 5, no. 4: 31. https://doi.org/10.3390/liquids5040031
APA StyleGoni, F., Chemelli, A., & Uhlig, F. (2025). Towards Hybrid 2D Nanomaterials: Covalent Functionalization of Boron Nitride Nanosheets. Liquids, 5(4), 31. https://doi.org/10.3390/liquids5040031







