Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study
Abstract
1. Introduction
2. Materials and Methods
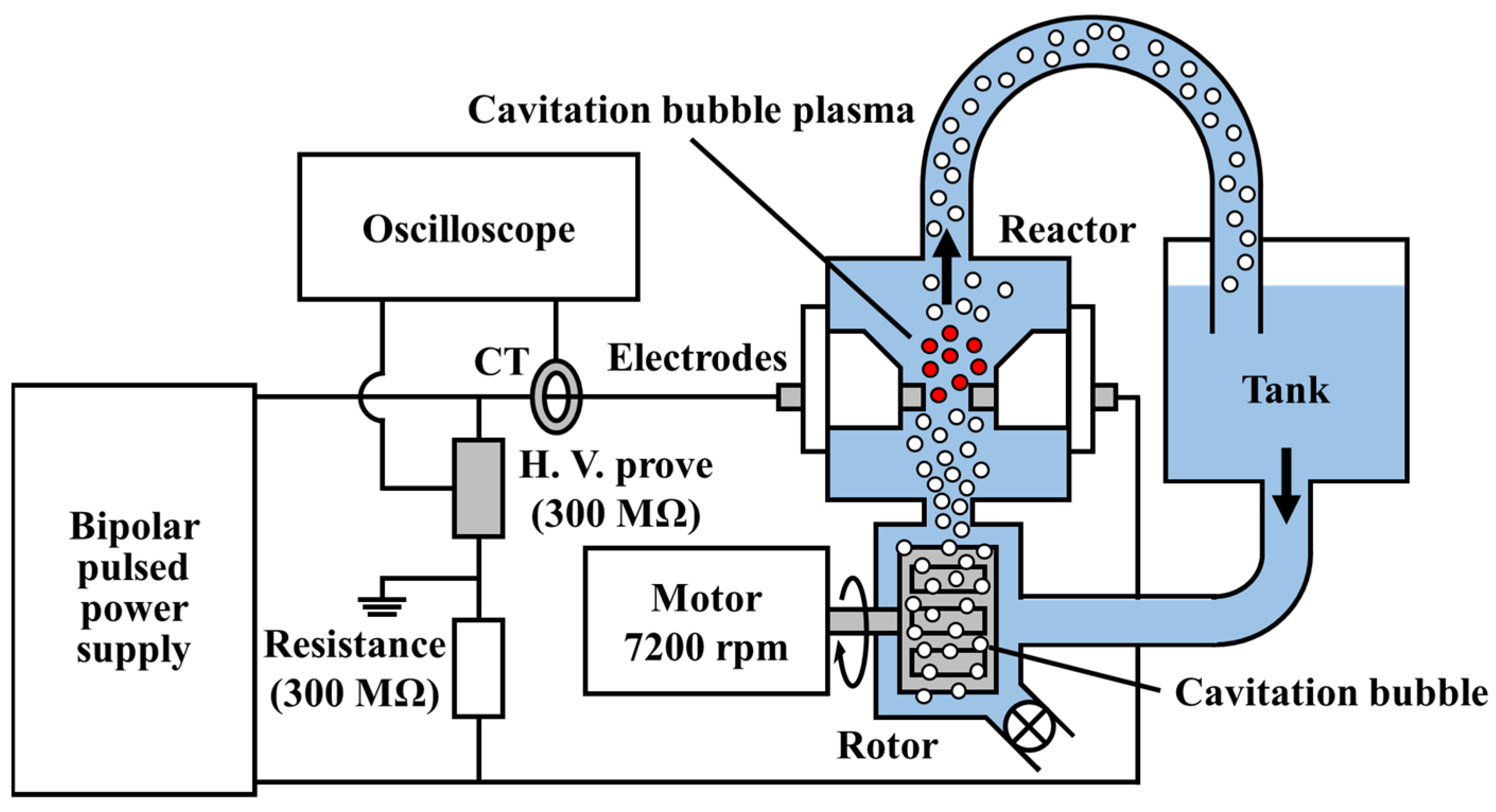
2.1. Preparation of CBPTW
2.2. Measurement of Metal Ion and H2O2 Concentrations in CBPTW
2.3. Investigation of OH Radical Generation
3. Results and Discussion
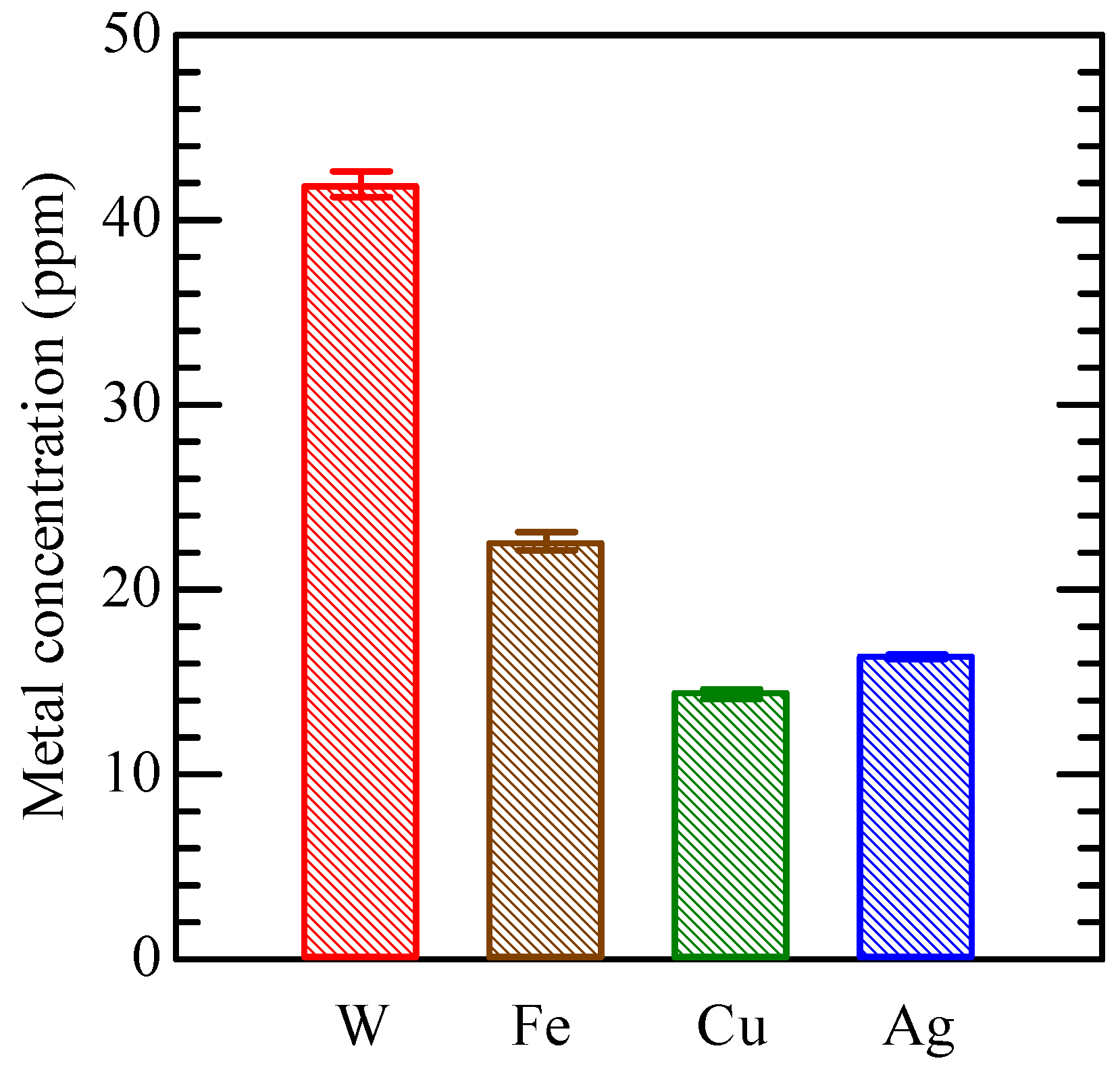
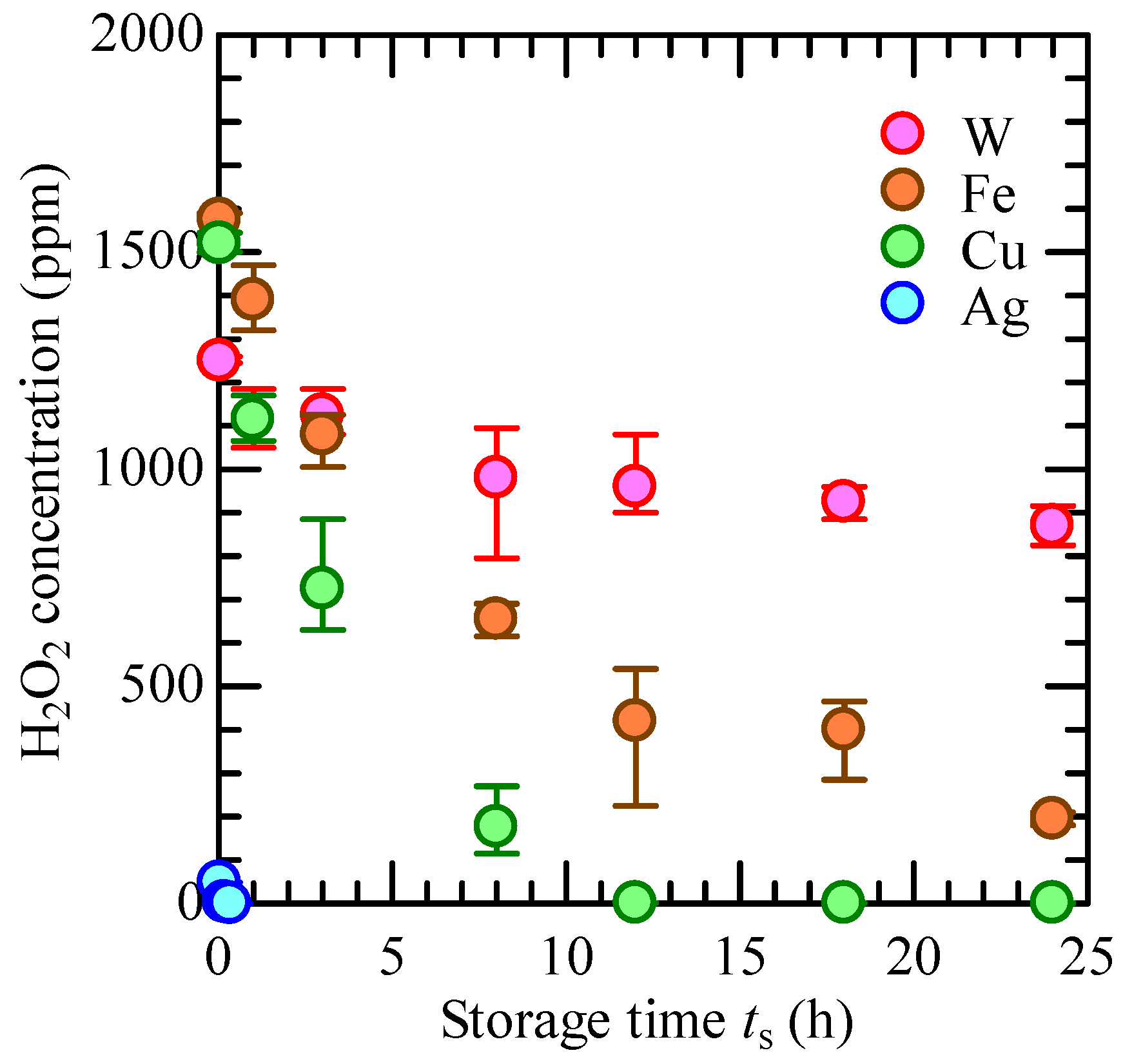
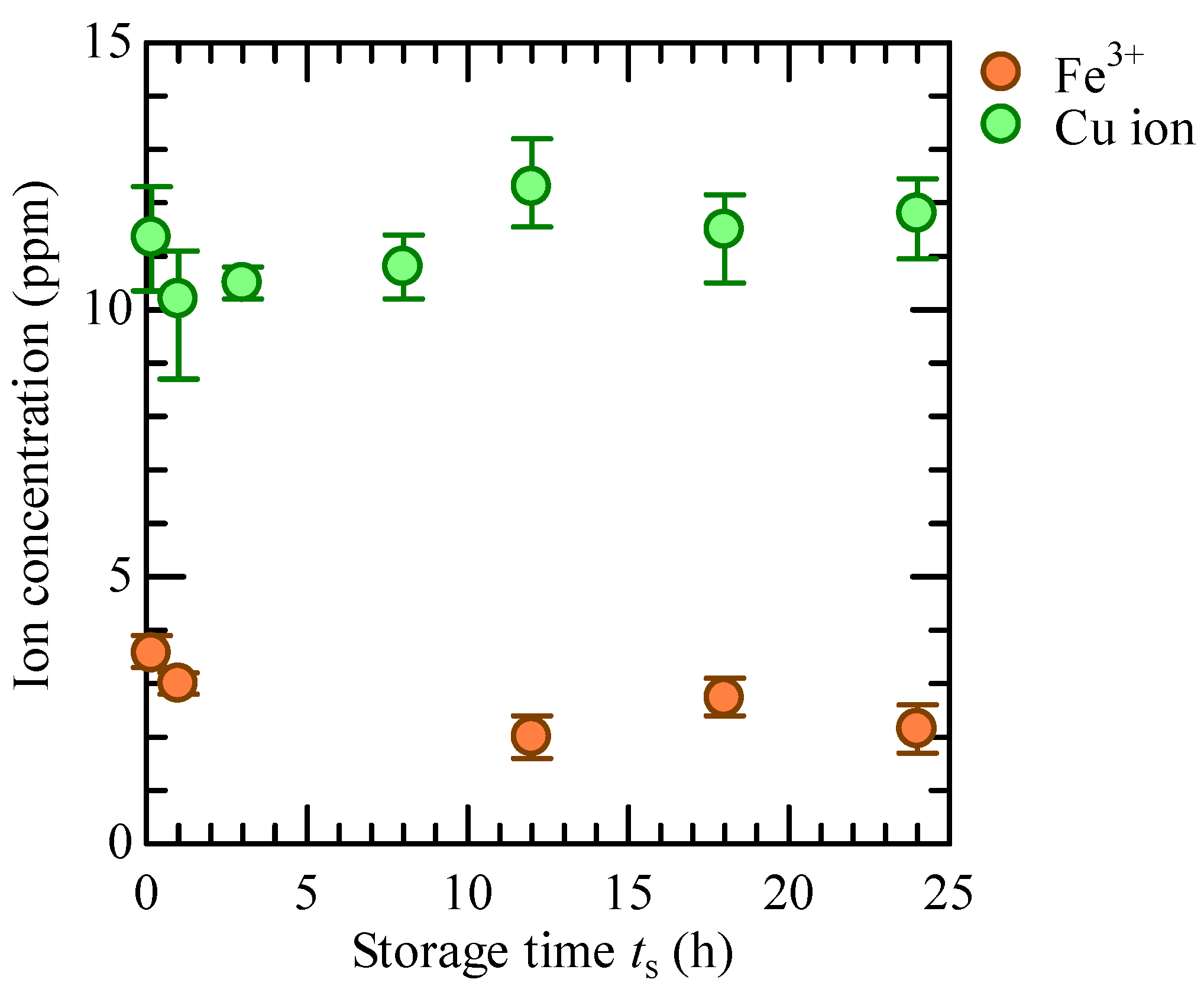
3.1. Constituents of CBPTW
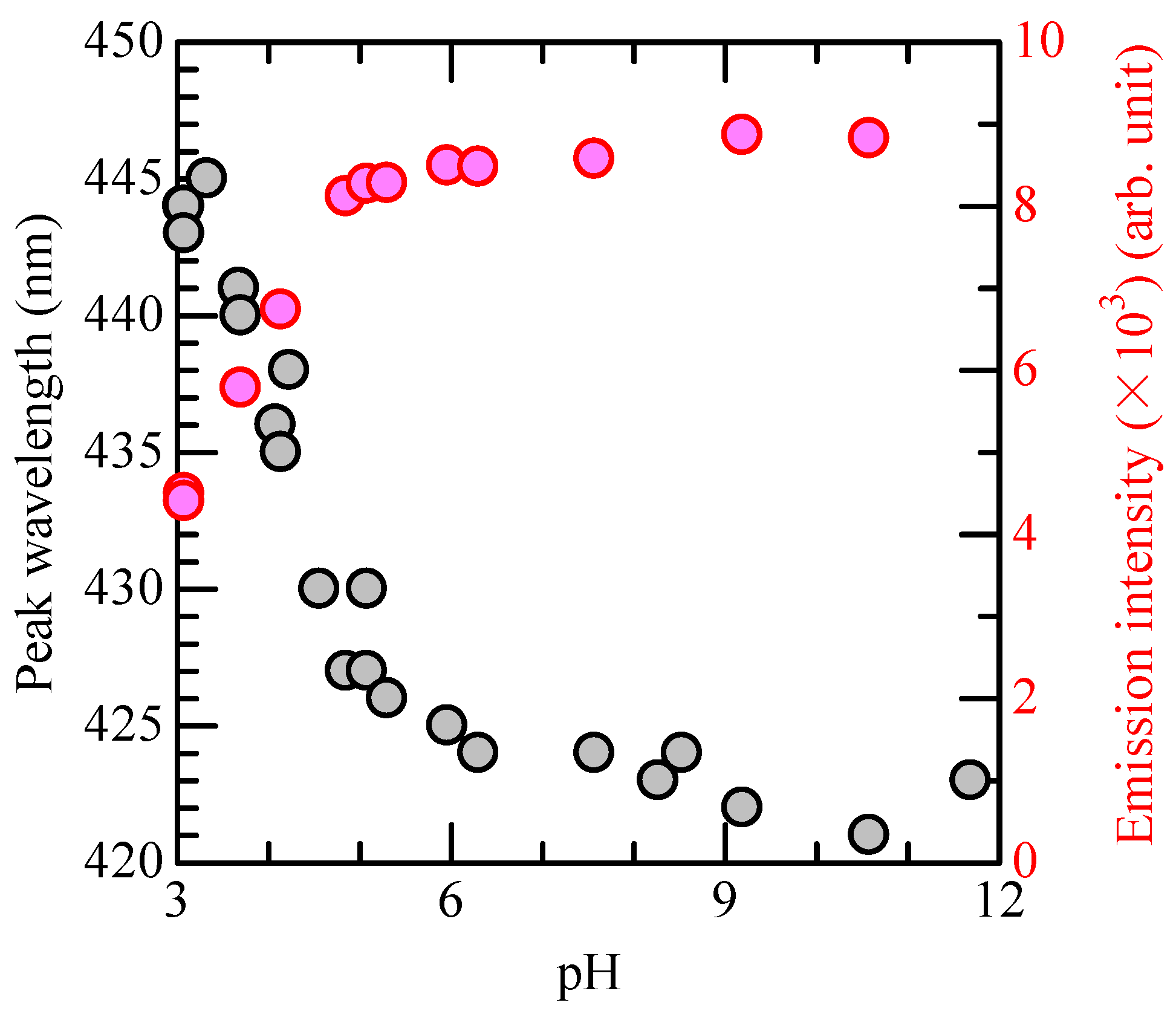
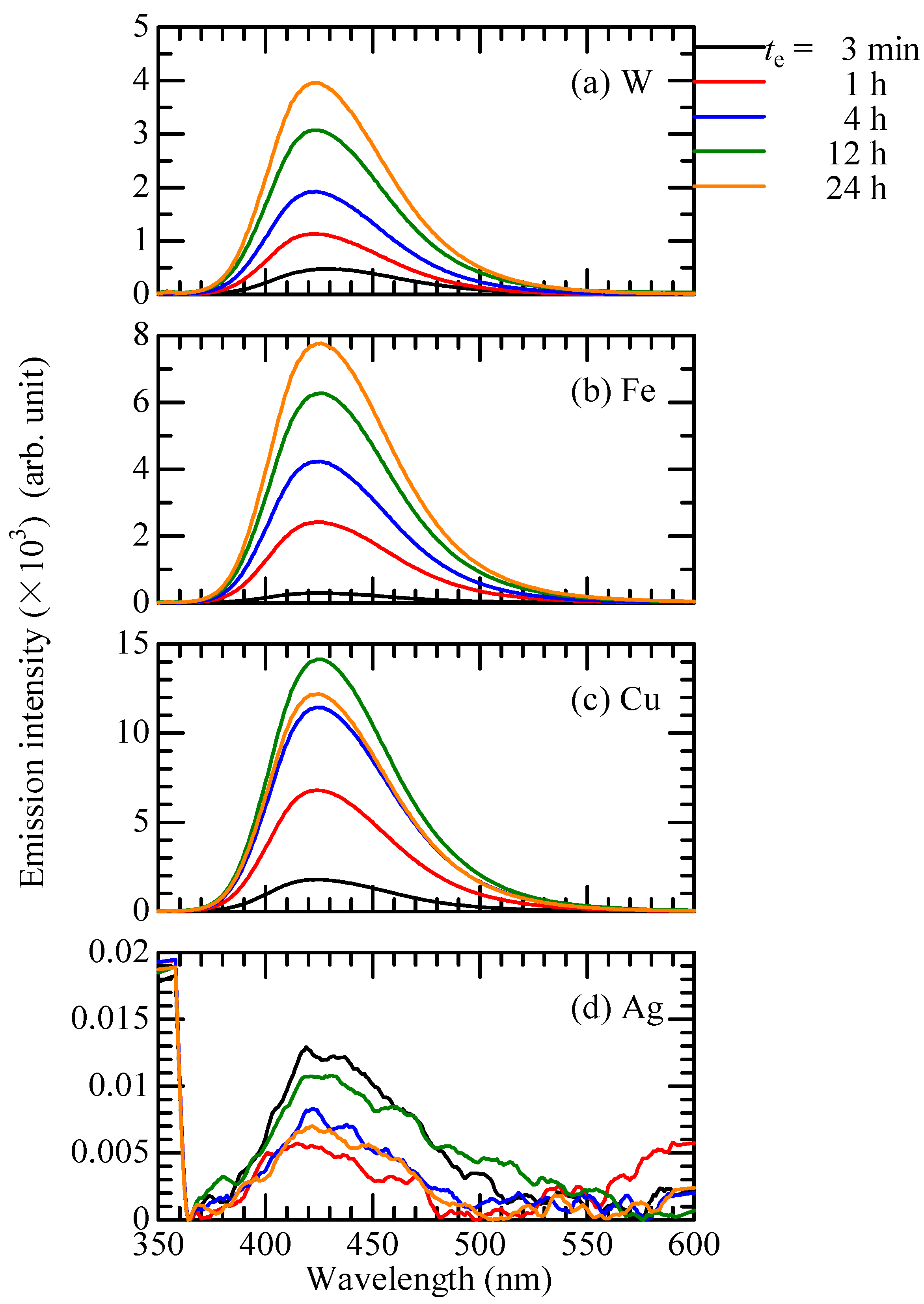
3.2. Preparing for HTA Concentration Measurement
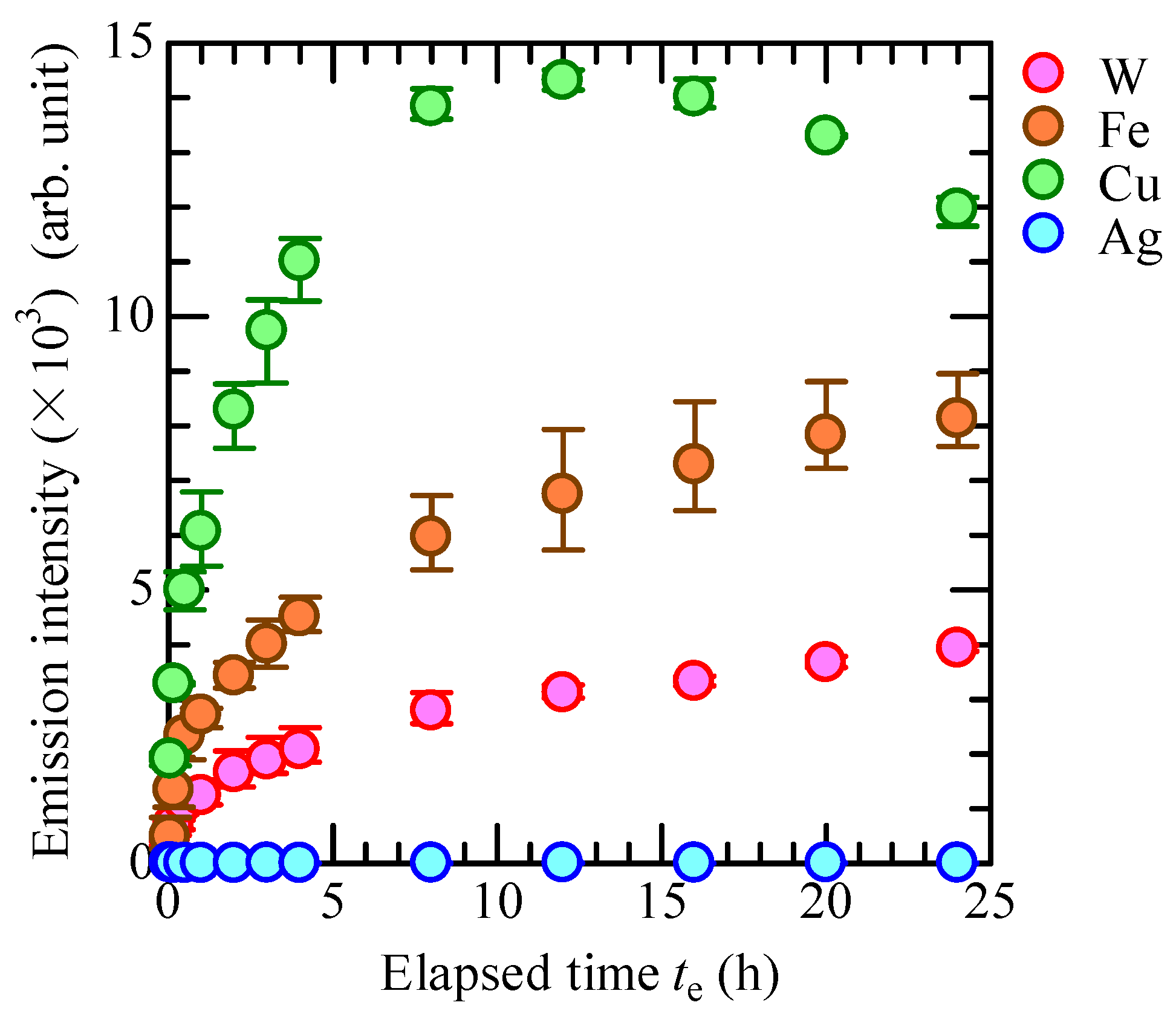
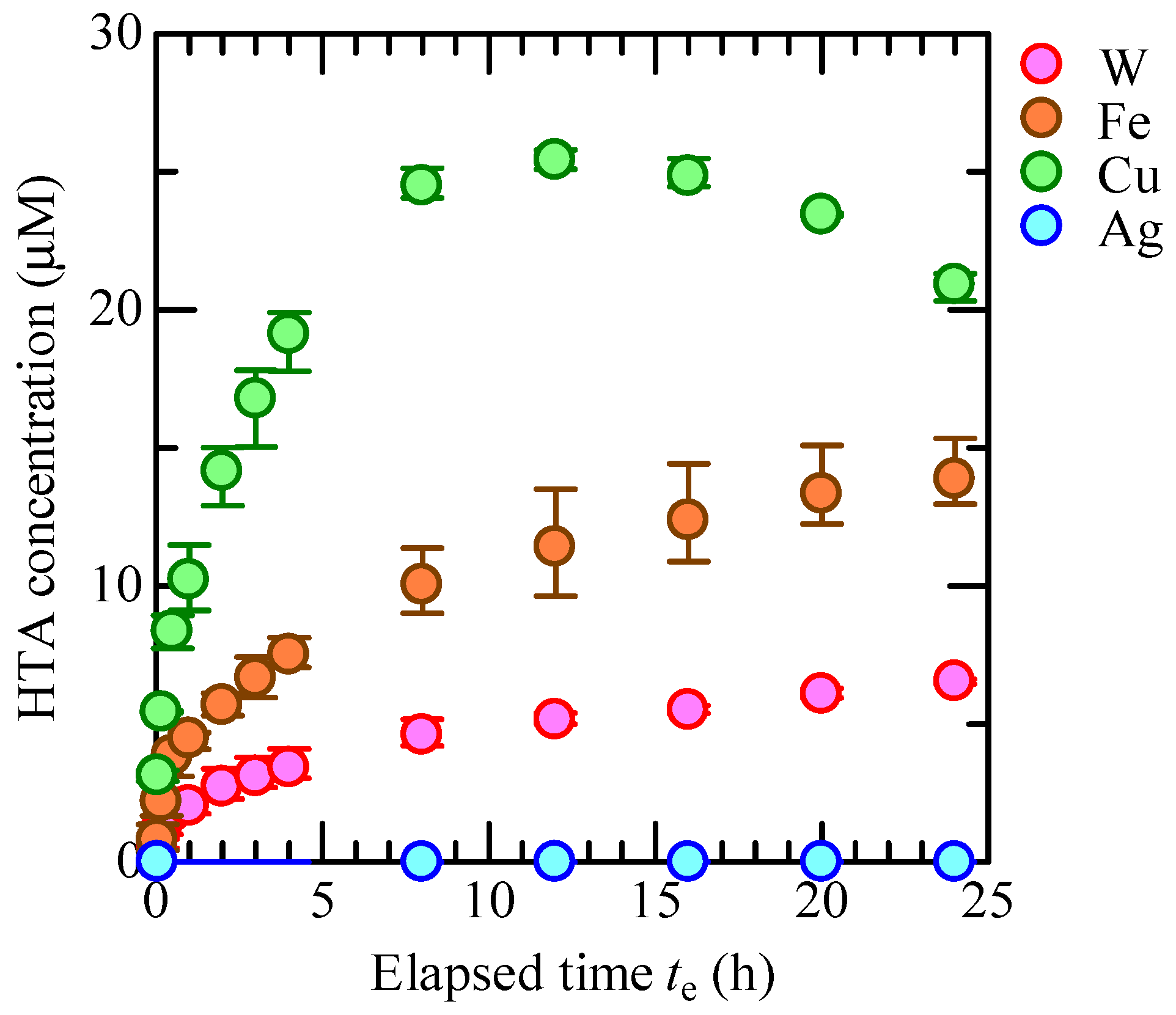
3.3. OH Radical Generation in CBPTW
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, S.; Wu, W.Y.; Ren, F.; Hsu, C.H.; Zhang, X.; Gao, P.; Wuu, D.S.; Huang, C.J.; Lien, S.Y.; Zhu, W. Growth of GaN Thin Films Using Plasma Enhanced Atomic Layer Deposition: Effect of Ammonia-Containing Plasma Power on Residual Oxygen Capture. Int. J. Mol. Sci. 2022, 23, 16204. [Google Scholar] [CrossRef]
- Cardinaud, C.; Peignon, M.C.; Tessier, P.Y. Plasma etching: Principles, mechanisms, application to micro- and nano-technologies. Appl. Surf. Sci. 2000, 164, 72. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Shiratani, M. Antioxidative activity and growth regulation of Brassicaceae induced by oxygen radical irradiation. Jpn. J. Appl. Phys. 2015, 54, 06D01. [Google Scholar] [CrossRef]
- Barjaste, A.; Dehghani, Z.; Dehghani, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Anpilov, A.M.; Barkhudarov, E.M.; Bark, Y.B.; Zadiraka, Y.V.; Christofi, M.; Kozlov, Y.N.; Kossyi, I.A.; Kop’ev, V.A.; Silakov, V.P.; Taktakisvili, M.I.; et al. Electric Discharge in Water as a Source of UV Radiation, Ozone and Hydrogen Peroxide. J. Phys. D Appl. Phys. 2001, 34, 933–999. [Google Scholar] [CrossRef]
- Lukes, P.; Locke, B.R. Plasmachemical Oxidation Processes in a Hybrid Gas-Liquid Electrical Discharge Reactor. J. Phys. D Appl. Phys. 2005, 38, 4074–4081. [Google Scholar] [CrossRef]
- Miyamoto, I.; Maehara, T.; Miyaoka, H.; Onishi, S.; Mukasa, S.; Toyota, H.; Kuramoto, M.; Nomura, S.; Kawashima, A. Effect of the Temperature of Water on the Degradation of Methylene Blue by the Generation of Radio Frequency Plasma in Water. J. Plasma Fusions Res. Series. 2009, 8, 627. [Google Scholar]
- Oka, Y.; Kuroshima, T.; Sawachika, K.; Yamashita, M.; Sakao, M.; Ohnishi, K.; Asami, K.; Yatsuzuka, M. Preparation of Silver Nanocolloidal Solution by Cavitation Bubble Plasma. Vacuum 2019, 167, 530–535. [Google Scholar] [CrossRef]
- Čech, J.; Sťahel, P.; Ráheľ, J.; Prokeš, L.; Rudolf, P.; Maršálková, E.; Maršálek, B. Mass Production of Plasma Activated Water: Case Studies of Its Biocidal Effect on Algae and Cyanobacteria. Water 2020, 12, 3167. [Google Scholar] [CrossRef]
- Sahni, M.; Locke, B.R. Quantification of Hydroxyl Radicals Produced in Aqueous Phase Pulsed Electrical Discharge Reactors. Ind. Eng. Chem. Res. 2006, 45, 5819–5825. [Google Scholar] [CrossRef]
- Santos, I.C.M.S.; Paz, F.A.A.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Klinowski, J.; Cavaleiro, A.M.V. Catalytic Homogeneous Oxyfunctionalization with Hydrogen Peroxide in the Presence of a Peroxotungstate. Appl. Catal. A 2008, 351, 166–173. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Grosjean, A.; Soroka, I.; Jonsson, M. Kinetics and Mechanism of the Reaction between H2O2 and Tungsten Powder in Water. J. Phys. Chem. C 2015, 119, 22560–22569. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G. Reaction Kinetics of Hydrogen Peroxide with Copper and Iron in Seawater. Environ. Sci. Technol. 1987, 21, 804–810. [Google Scholar] [CrossRef]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of Hydrogen Peroxide-Kinetics and Review of Chosen Catalysts. Acta Innov. 2018, 26, 45–52. [Google Scholar] [CrossRef]
- Gonzalez, D.H.; Kuang, X.M.; Scott, J.A.; Rocha, G.O.; Paulson, S.E. Terephthalate Probe for Hydroxyl Radicals: Yield of 2-Hydroxy Terephthalic Acid and Transition Metal Interference. Anal. Lett. 2018, 51, 2488–2497. [Google Scholar] [CrossRef]
- Kanazawa, S.; Furuki, T.; Nakaji, T.; Akamine, S.; Ichiki, R. Application of Chemical Dosimetry to Hydroxyl Radical Measurement During Underwater Discharge. J. Phys. Conf. Ser. 2013, 418, 012102. [Google Scholar] [CrossRef]
- Page, S.E.; Arnold, W.A.; McNeill, K. Terephthalate as a Probe for Photochemically Generated Hydroxyl Radical. J. Environ. Monit. 2010, 12, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Hart, D.P. Ultrasound Induced Cavitation and Sonochemical Yields. J. Acoust. Soc. Am. 1998, 104, 2675–2682. [Google Scholar] [CrossRef]
- Sazgarnia, A.; Shanei, A. Evaluation of Acoustic Cavitation in Terephthalic Acid Solutions Containing Gold Nanoparticles by the Spectrofluorometry Method. Int. J. Photoenergy 2012, 2012, 376047. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Sonochemical Formation of Hydrogen Peroxide. Proceedings 2017, 2, 188. [Google Scholar] [CrossRef]
- Weissler, A. Formation of Hydrogen Peroxide by Ultrasonic Waves: Free Radicals. J. Am. Chem. Soc. 1958, 81, 1077–1081. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawano, K.; Oka, Y. Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study. Liquids 2025, 5, 26. https://doi.org/10.3390/liquids5040026
Kawano K, Oka Y. Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study. Liquids. 2025; 5(4):26. https://doi.org/10.3390/liquids5040026
Chicago/Turabian StyleKawano, Kotaro, and Yoshihiro Oka. 2025. "Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study" Liquids 5, no. 4: 26. https://doi.org/10.3390/liquids5040026
APA StyleKawano, K., & Oka, Y. (2025). Hydroxyl Radical Formation and Its Mechanism in Cavitation Bubble Plasma-Treated Water: A Chemical Probe Study. Liquids, 5(4), 26. https://doi.org/10.3390/liquids5040026





