A Basic Approach to Equations of States for Studying the Real Behavior of Noble Gases
Abstract
1. Introduction
2. General Intermolecular Interactions
3. Argon
4. Further Noble Gases
5. Exceeding Limiting Concentrations
6. The Two-Phasic Region
7. Isotherms of Other Noble Gases
8. The Temperature Dependence of Parameters
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eucken, A.; Wicke, E. Grundriss der Physikalischen Chemie, 10th ed.; Akademische Verlagsgesellschaft Geest & Portig: Leipzig, Germany, 1959. [Google Scholar]
- Woodcock, L.V. On Failures of van der Waals’ Equation at the Gas–Liquid Critical Point. Int. J. Thermophys. 2018, 39, 120. [Google Scholar] [CrossRef]
- Clausius, R. Über das Verhalten der Kohlensäure in Bezug auf Druck, Volumen und Temperatur. Ann. Phys. Chem. 1880, 9, 337–357. [Google Scholar] [CrossRef]
- Redlich, O.; Kwong, J.N.S. On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions. Chem. Rev. 1949, 44, 233–244. [Google Scholar] [CrossRef]
- Soave, G. Equilibrium Constants from a Modified Redlich-Kwong Equation of State. Chem. Eng. Sci. 1972, 27, 1197–1203. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Benedict, M.; Webb, G.B.; Rubin, L.C. An Empirical Equation for Thermodynamic Properties of Light Hydrocarbons and Their Mixtures: I. Methane, Ethane, Propane, and n-Butane. J. Chem. Phys. 1940, 8, 334–345. [Google Scholar] [CrossRef]
- Ree, F.H. Thermodynamic Functions at Liquid–Vapor Transition Range of the van der Waals, the Berthelot, and the Dieterici Equations of State. J. Chem. Phys. 1962, 36, 3373–3378. [Google Scholar] [CrossRef]
- Wohl, A. Untersuchungen über die Zustandsgleichung ΙII. Die Hauptzustandsgleichung und die Zustandsgleichungen der Einzelstoffe. Z. Phys. Chem. 1921, 99, 226–233. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. Equation-of-state solution model for associating fluids. Fluid Phase Equilib. 1989, 52, 31–38. [Google Scholar] [CrossRef]
- Nezbeda, I. On Molecular-Based Equations of State: Perturbation Theories, Simple Models, and SAFT Modeling. Front. Phys. 2020, 8, 287. [Google Scholar] [CrossRef]
- Huang, S.H.; Radosz, M. Equation of State for Small, Large, Polydisperse, and Associating Molecules. Ind. Eng. Chem. Res. 1990, 29, 2284–2294. [Google Scholar] [CrossRef]
- Dufal, S.; Lafitte, T.; Haslam, A.J.; Galindo, A.; Clark, G.N.I.; Vega, C.; Jackson, G. The A in SAFT: Developing the contribution of association to the Helmholtz free energy within a Wertheim TPT1 treatment of generic Mie fluids. Mol. Phys. 2015, 113, 948–984. [Google Scholar] [CrossRef]
- Langhals, H. How the Concept of Solvent Polarity Investigated with Solvatochromic Probes Helps Studying Intermolecular Interactions. Liquids 2023, 3, 481–511. [Google Scholar] [CrossRef]
- Michels, A.; Levelt, J.M.; De Graaff, W. Compressibility Isotherms of Argon at Temperatures Between −25 °C and −155 °C, and at Densities up to 640 Amagat (Pressures up to 1050 Atmospheres). Physica 1958, 24, 659–671. [Google Scholar] [CrossRef]
- Michels, A.; Wijker, H.; Wijker, H.K. Isotherms of Argon Between 0 °C and 150° C and Pressures up to 2900 Atmospheres. Physica 1949, 15, 627–633. [Google Scholar] [CrossRef]
- Michels, A.; Levelt, J.M.; Wolkers, G.J. Thermodynamic Properties of Argon at Temperatures Between 0 °C and −140 °C and at Densities up to 640 Amagat (Pressures up to 1050 atm). Physica 1958, 24, 769–794. [Google Scholar] [CrossRef]
- McCain, W.D., Jr.; Ziegler, W.T. Critical Temperature, Critical Pressure, and Vapor Pressure of Argon. J. Chem. Eng. Data 1967, 12, 199–202. [Google Scholar] [CrossRef]
- Taylor, J.R. Error Analysis: The Study of Uncertainties in Physical Measurements, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997; ISBN 0-935702-42-3. [Google Scholar]
- Beattie, J.A.; Brierley, J.S.; Barriault, R.J. The compressibility of krypton. I. An equation of State for krypton and the weight of a liter of krypton. J. Chem. Phys. 1952, 20, 1613–1615. [Google Scholar] [CrossRef]
- Beattie, J.A.; Brierley, J.S.; Barriault, R.J. The Compressibility of Gaseous Krypton. II. The Virial Coefficients and Potential Parameters of Krypton. J. Chem. Phys. 1952, 20, 1615–1618. [Google Scholar] [CrossRef]
- Beattie, J.A.; Barriault, R.J.; Brierley, J.S. The Compressibility of Gaseous Xenon. I. An Equation of State for Xenon and the Weight of a Liter of Xenon. J. Chem. Phys. 1951, 19, 1219–1221. [Google Scholar] [CrossRef]
- Michels, A.; Wassenaar, T.; Louwerse, P. Isotherms of Neon at Temperatures Between 0 °C and 150 °C and at Densities up to 1100 Amagat (Pressure up to 2900 Atmospheres). Physica 1960, 26, 539–543. [Google Scholar] [CrossRef]
- Keesom, W.H.; Kraak, H.H. The compressibility of helium gas between 2.6 °K and 4.2 °K. Physica 1935, 2, 37–44. [Google Scholar] [CrossRef]
- Keesom, W.H.; Walstra, W.K. Isotherms of helium at liquid helium temperatures. Physica 1940, 7, 985–991. [Google Scholar] [CrossRef]
- Canfield, F.B.; Leland, T.W.; Kobashy, R. Compressibility Factors for Helium-Nitrogen Mixtures. J. Chem. Eng. Data 1965, 10, 92–96. [Google Scholar] [CrossRef]
- Hertz, P. Über den gegenseitigen durchschnittlichen Abstand von Punkten, die mit bekannter mittlerer Dichte im Raume angeordnet sind. Math. Ann. 1909, 67, 387–398. [Google Scholar] [CrossRef]
- Chandrasekhar, S. Stochastic Problems in Physics and Astronomy. Rev. Mod. Phys. 1943, 15, 1–89. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Langhals, H. Determination of the Concentration of Gases by Measurement of Pressure. Anal. Lett. 1987, 20, 1595–1610. [Google Scholar] [CrossRef][Green Version]
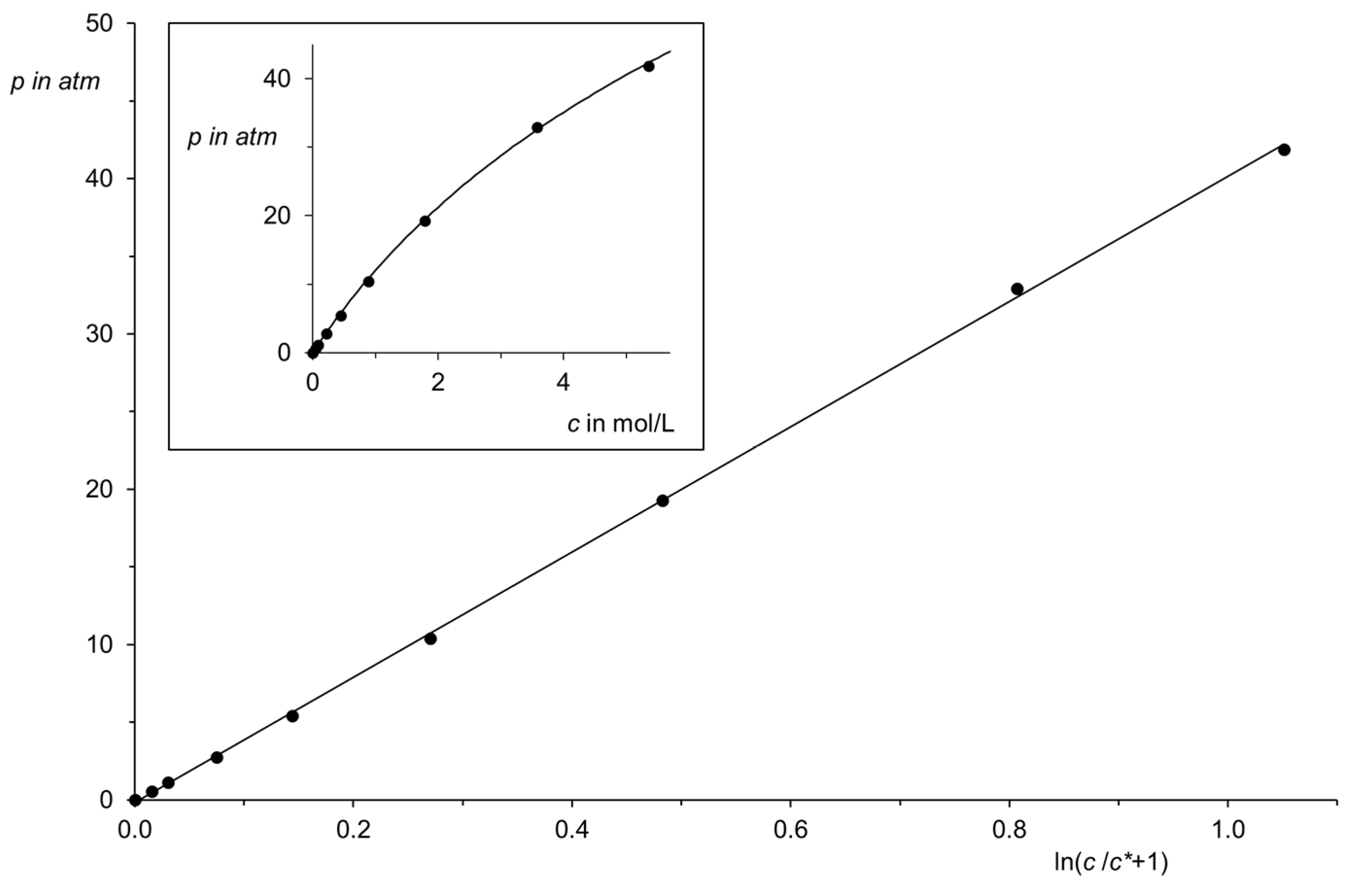

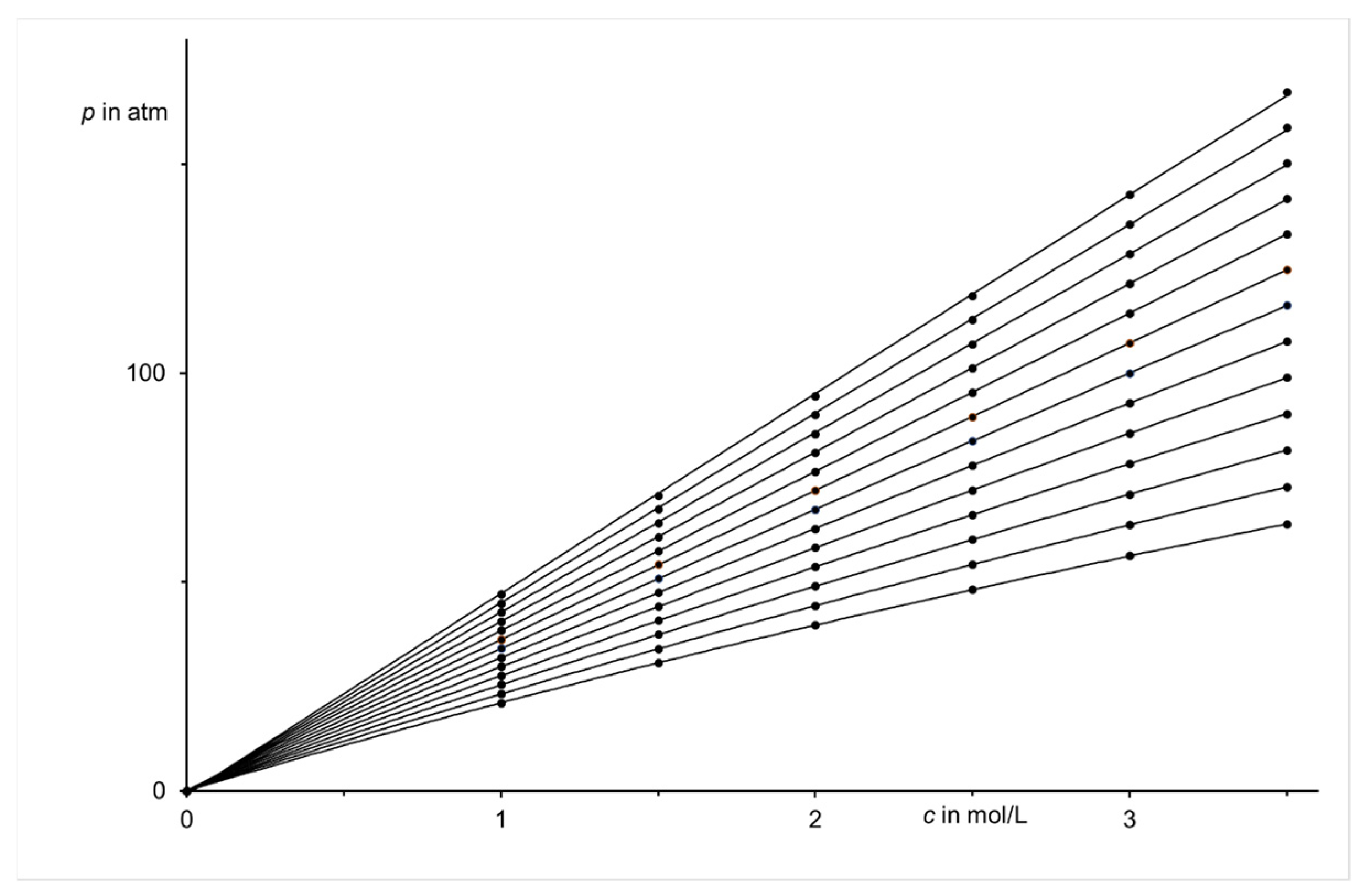
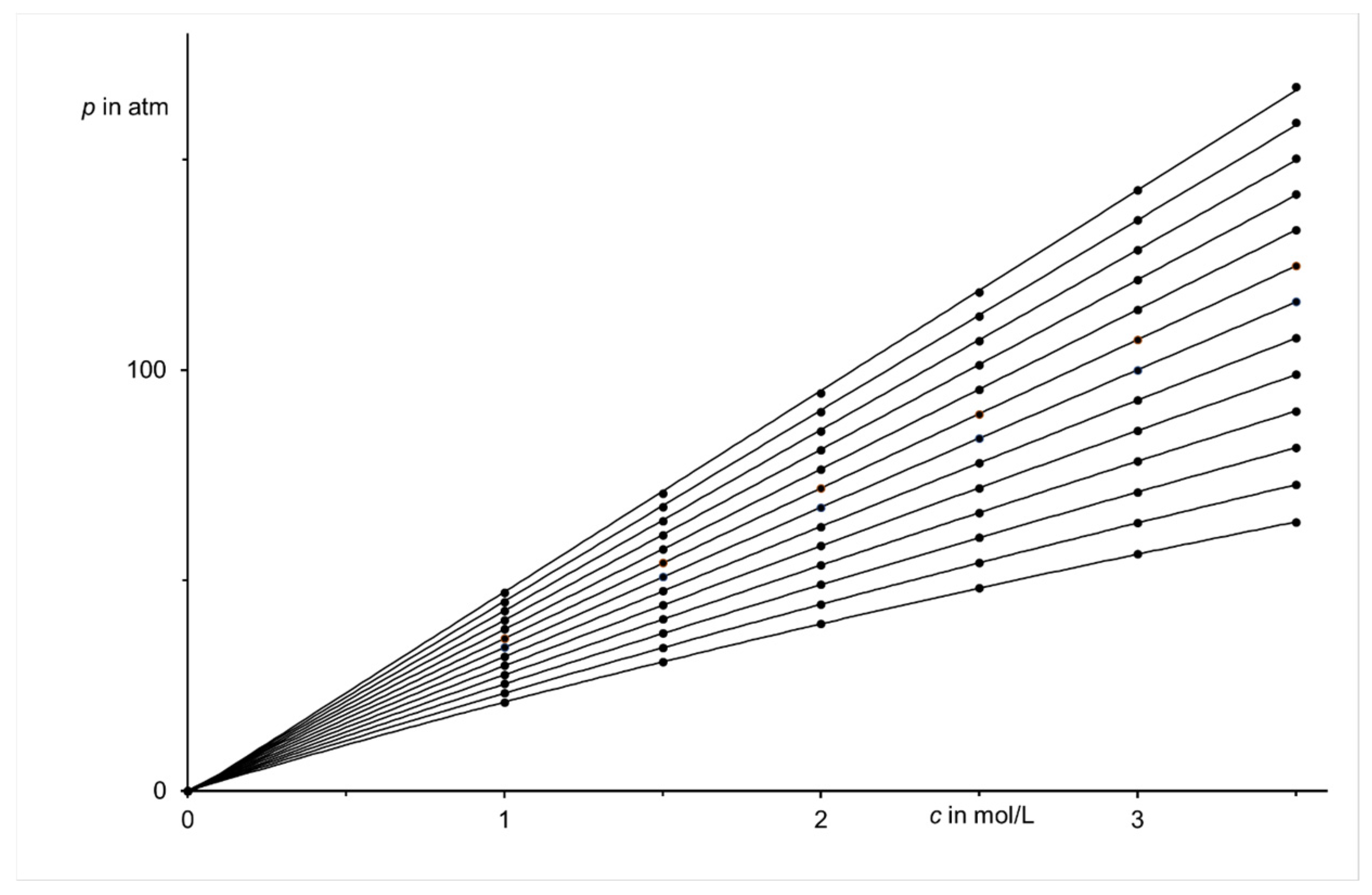


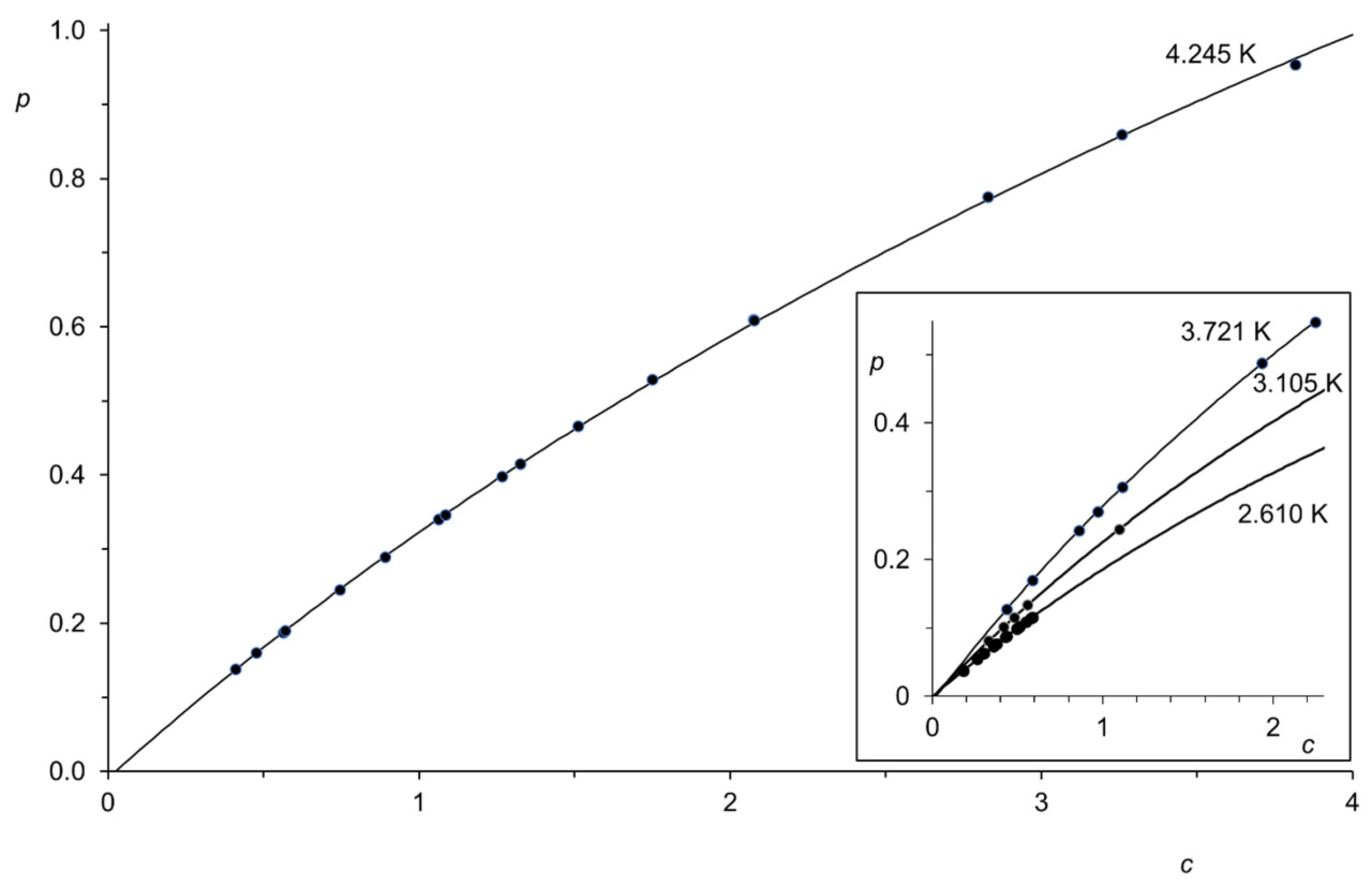
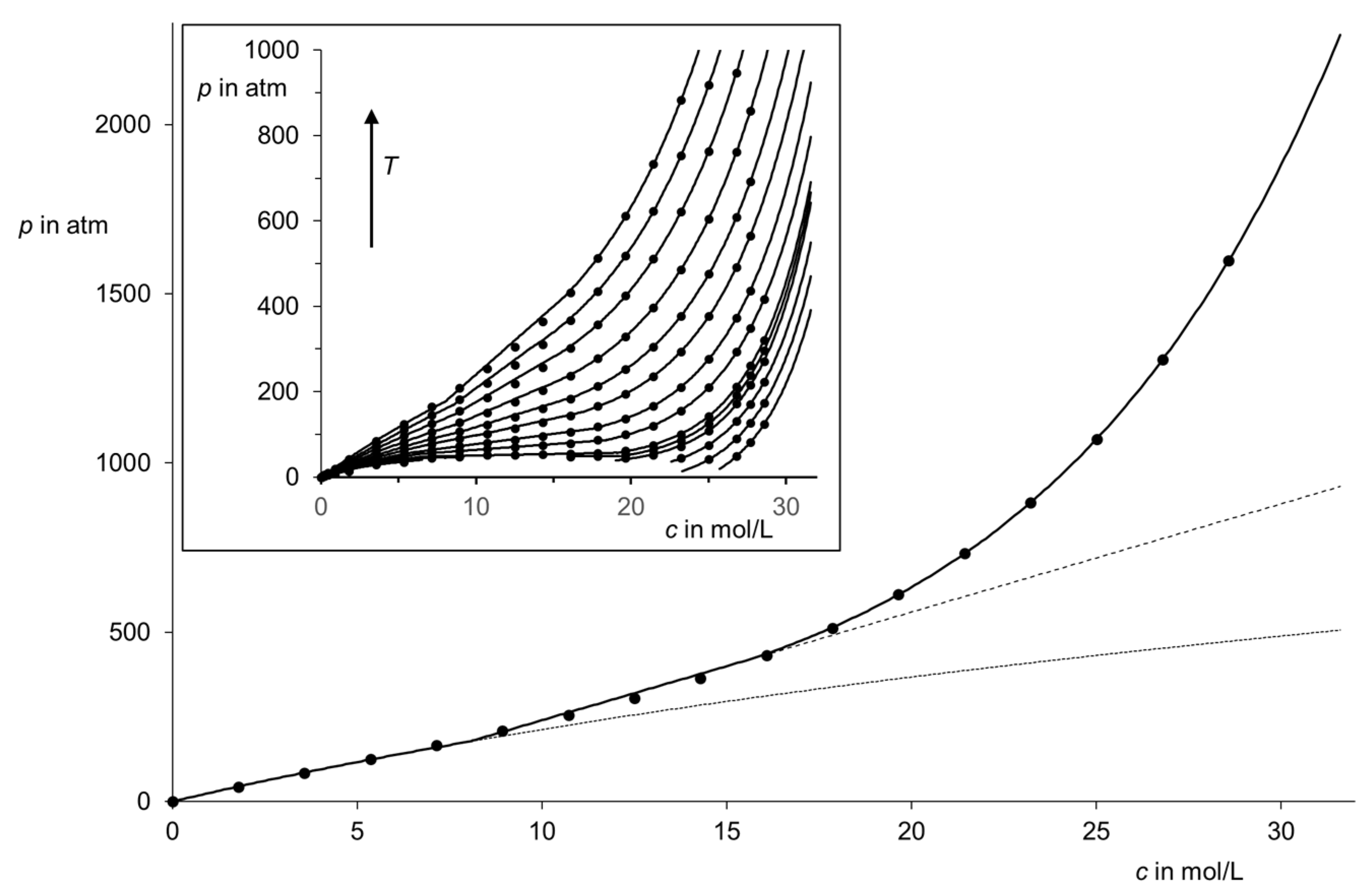
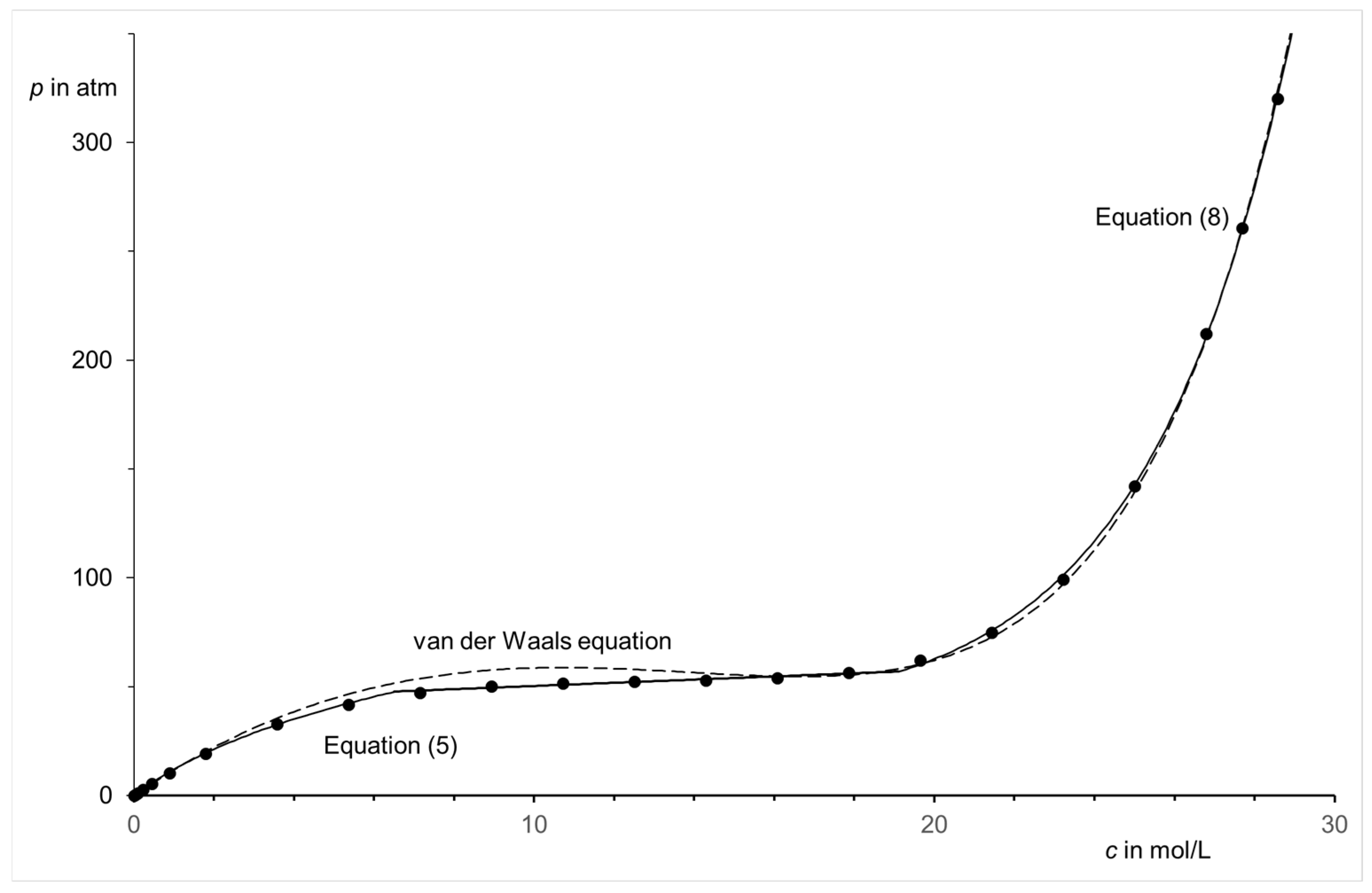

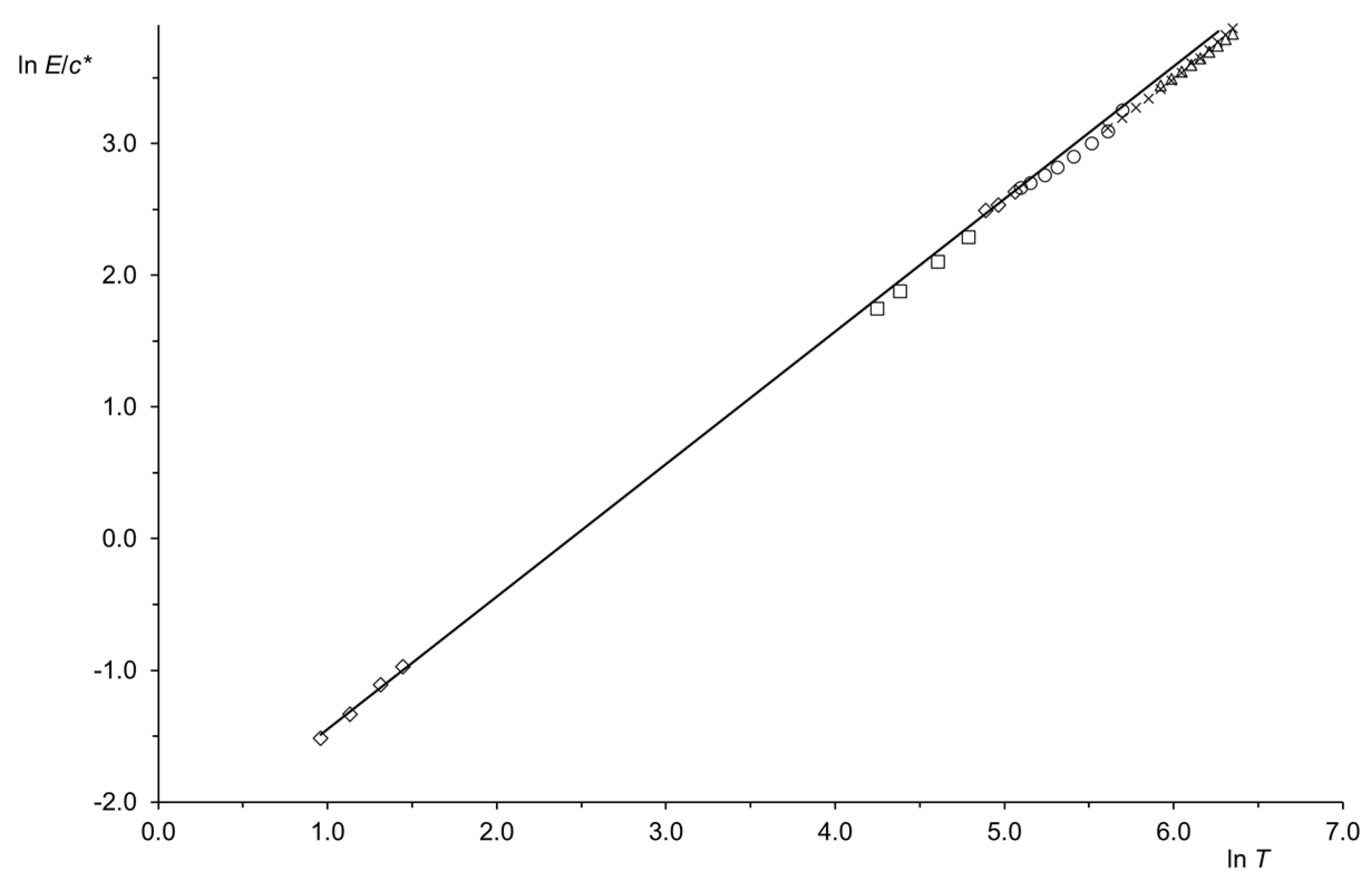
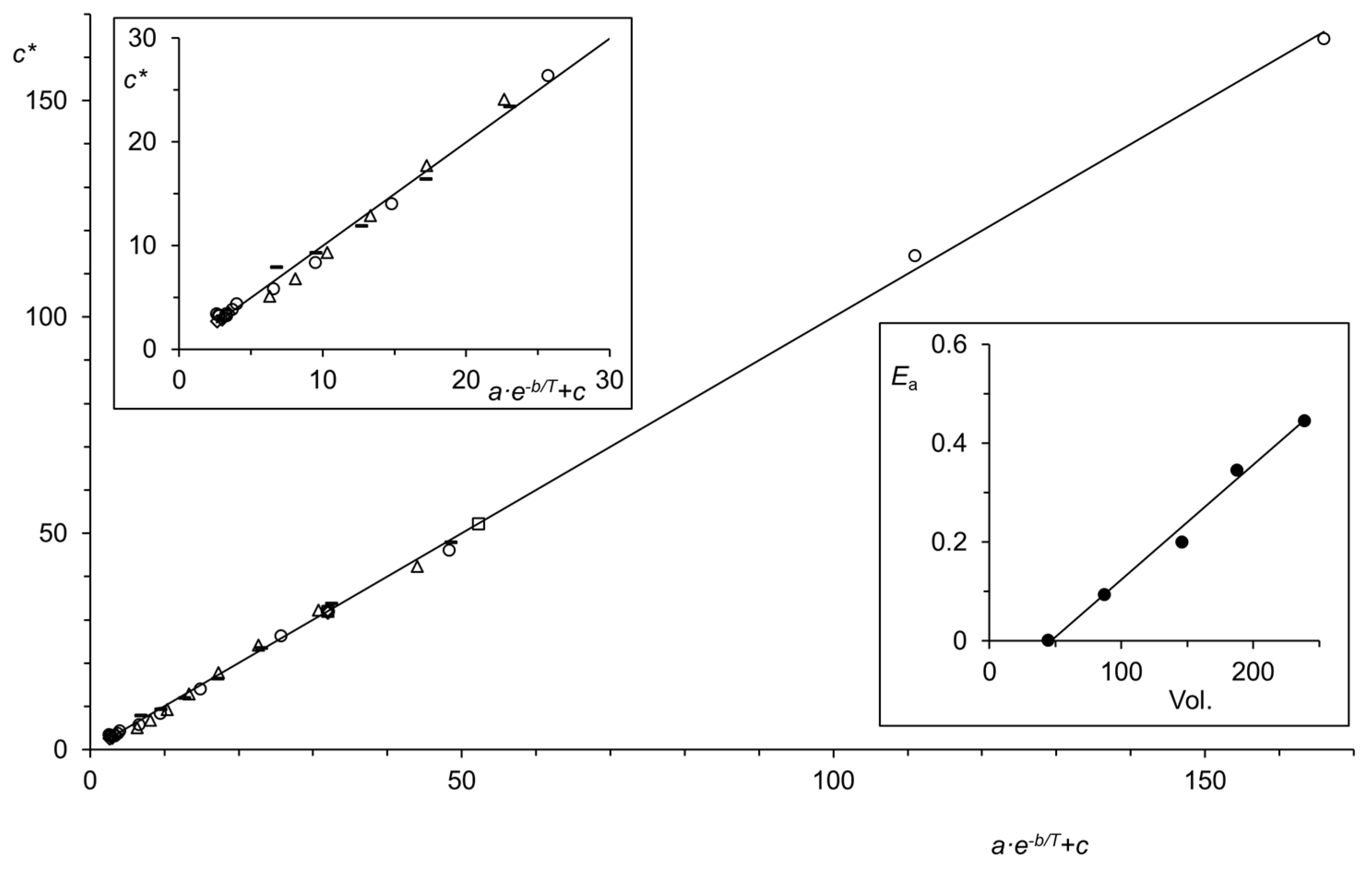
| Region (i) | Region (ii) | Region (iii) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T in °C | E | c* | po | r | s | E | c* | po | r | s | E | p* | po | r | s |
| 25 | 564.9 | 21.8 | −0.931 | 0.9997645 | 1.42 | 907.9 | 28.4 | −79.2 | 0.993 | 15.6 | 43.35 | 0.123 | 121.4 | 0.9999998 | 0.2 |
| 0 | 791.3 | 35.8 | 0.055 | 0.9999980 | 0.08 | 795.6 | 29.9 | −57.5 | 0.993 | 12.9 | 29.75 | 0.131 | 126.4 | 1 | 0.1 |
| −25 | 439.2 | 21.8 | 0.037 | 0.9999987 | 0.06 | 601.0 | 28.4 | −35.6 | 0.993 | 10.3 | 18.32 | 0.142 | 127.0 | 0.9999999 | 0.1 |
| −50 | 250.7 | 13.7 | 0.012 | 0.9999997 | 0.02 | 445.6 | 28.4 | −13.5 | 0.994 | 7.5 | 9.79 | 0.156 | 119.0 | 0.9999999 | 0.1 |
| −70 | 158.7 | 9.45 | −0.017 | 0.9999997 | 0.02 | 321.0 | 28.4 | 4.1 | 0.994 | 5.3 | 5.02 | 0.172 | 105.3 | 0.9999993 | 0.2 |
| −85 | 110.1 | 6.97 | −0.049 | 0.9999958 | 0.07 | 240.0 | 29.9 | 17.1 | 0.994 | 3.7 | 2.45 | 0.190 | 92.0 | 0.9999974 | 0.4 |
| −100 | 74.1 | 4.98 | −0.092 | 0.9999773 | 0.14 | 143.1 | 29.9 | 29.4 | 0.995 | 2.1 | 0.88 | 0.217 | 73.6 | 0.9999904 | 0.6 |
| −110 | 55.5 | 3.86 | −0.129 | 0.9999399 | 0.21 | 80.7 | 29.9 | 36.9 | 0.994 | 1.2 | 0.41 | 0.237 | 54.4 | 0.9999529 | 1.3 |
| −120 | 40.3 | 2.88 | −0.178 | 0.9998387 | 0.30 | 22.5 | 30.8 | 43.0 | 0.970 | 0.8 | 0.11 | 0.275 | 35.6 | 0.9998646 | 1.8 |
| −122.5 | 36.9 | 2.65 | −0.193 | 0.9997909 | 0.33 | 9.4 | 30.8 | 43.5 | 0.915 | 0.9 | 0.07 | 0.287 | 30.4 | 0.9998158 | 2.0 |
| −125 | 33.8 | 2.43 | −0.209 | 0.9997282 | 0.36 | 0.04 | 0.302 | 25.1 | 0.9997335 | 2.2 | |||||
| −130 | 27.9 | 2.00 | −0.246 | 0.9995254 | 0.44 | 0.05 | 0.296 | −2.1 | 0.9999351 | 0.9 | |||||
| −135 | 19.5 | 1.67 | −0.005 | 0.9999901 | 0.02 | 0.04 | 0.301 | −27.0 | 0.9999923 | 0.3 | |||||
| −140 | 36.3 | 3.25 | −0.015 | 0.9999958 | 0.02 | 0.03 | 0.300 | −57.5 | 1 | 0.0 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langhals, H. A Basic Approach to Equations of States for Studying the Real Behavior of Noble Gases. Liquids 2025, 5, 2. https://doi.org/10.3390/liquids5010002
Langhals H. A Basic Approach to Equations of States for Studying the Real Behavior of Noble Gases. Liquids. 2025; 5(1):2. https://doi.org/10.3390/liquids5010002
Chicago/Turabian StyleLanghals, Heinz. 2025. "A Basic Approach to Equations of States for Studying the Real Behavior of Noble Gases" Liquids 5, no. 1: 2. https://doi.org/10.3390/liquids5010002
APA StyleLanghals, H. (2025). A Basic Approach to Equations of States for Studying the Real Behavior of Noble Gases. Liquids, 5(1), 2. https://doi.org/10.3390/liquids5010002







