Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography
Abstract
1. Introduction
2. Experimental
2.1. Compounds: Identity and Purity Controls
2.2. Methods
2.3. Thermochemical Method: Vaporization Enthalpies
2.4. Thermochemical Method: Vapor Pressures
2.5. Uncertainties
2.6. Estimations of Vaporization Enthalpy
2.7. Vaporization Enthalpies and Vapor Pressures of the Standards and of 5α-Cholestane
3. Results
3.1. Vaporization Enthalpies
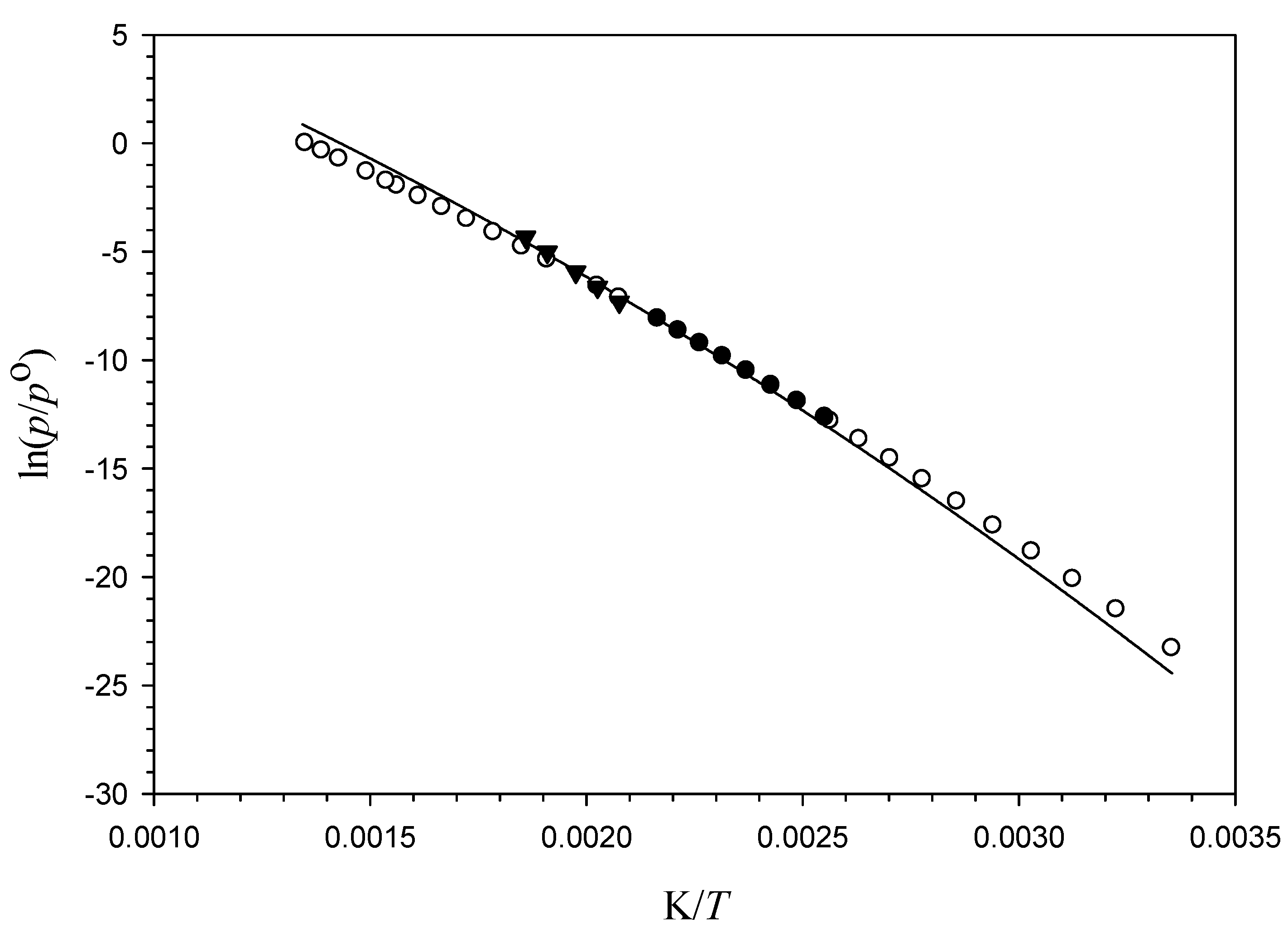
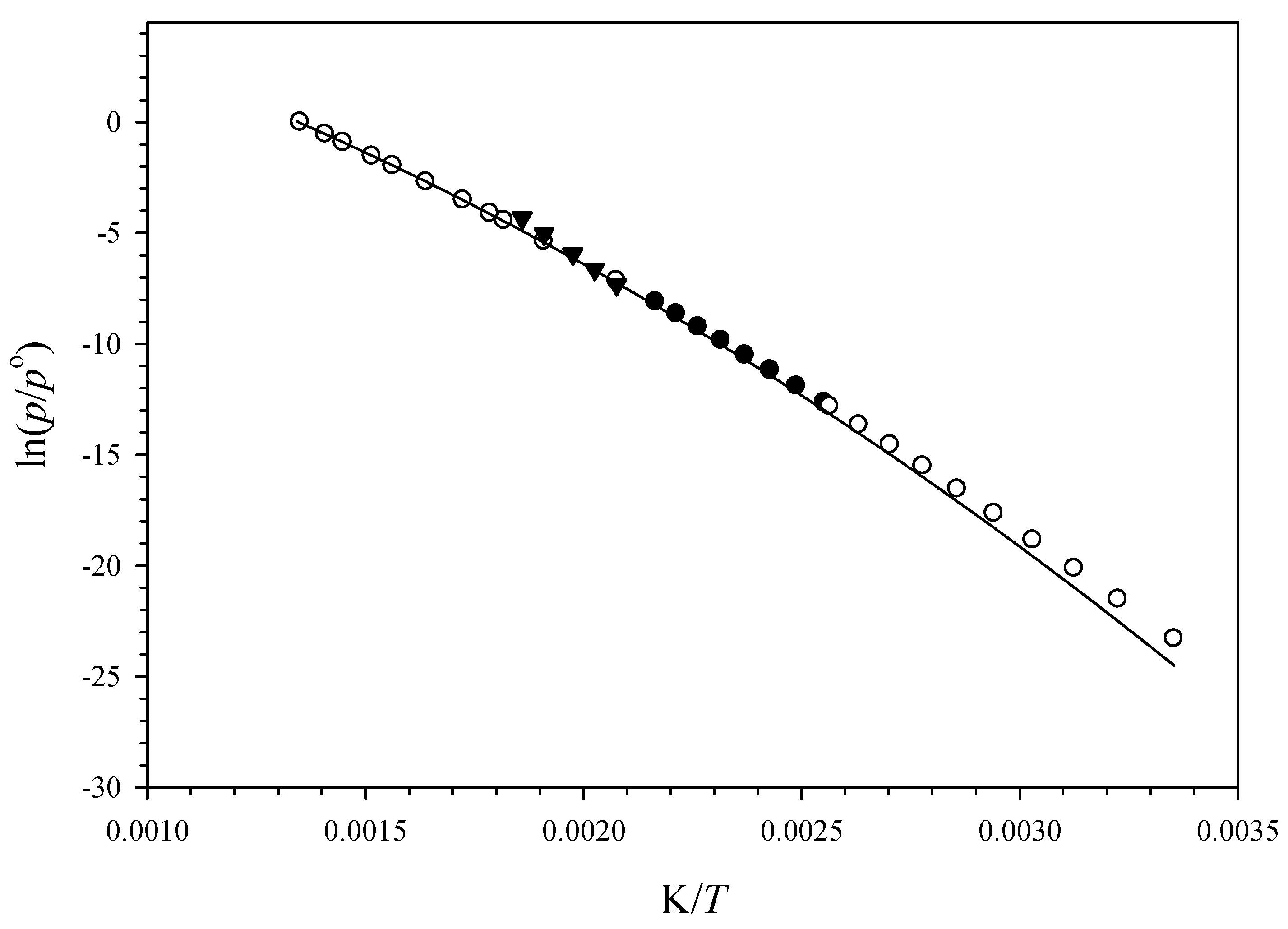
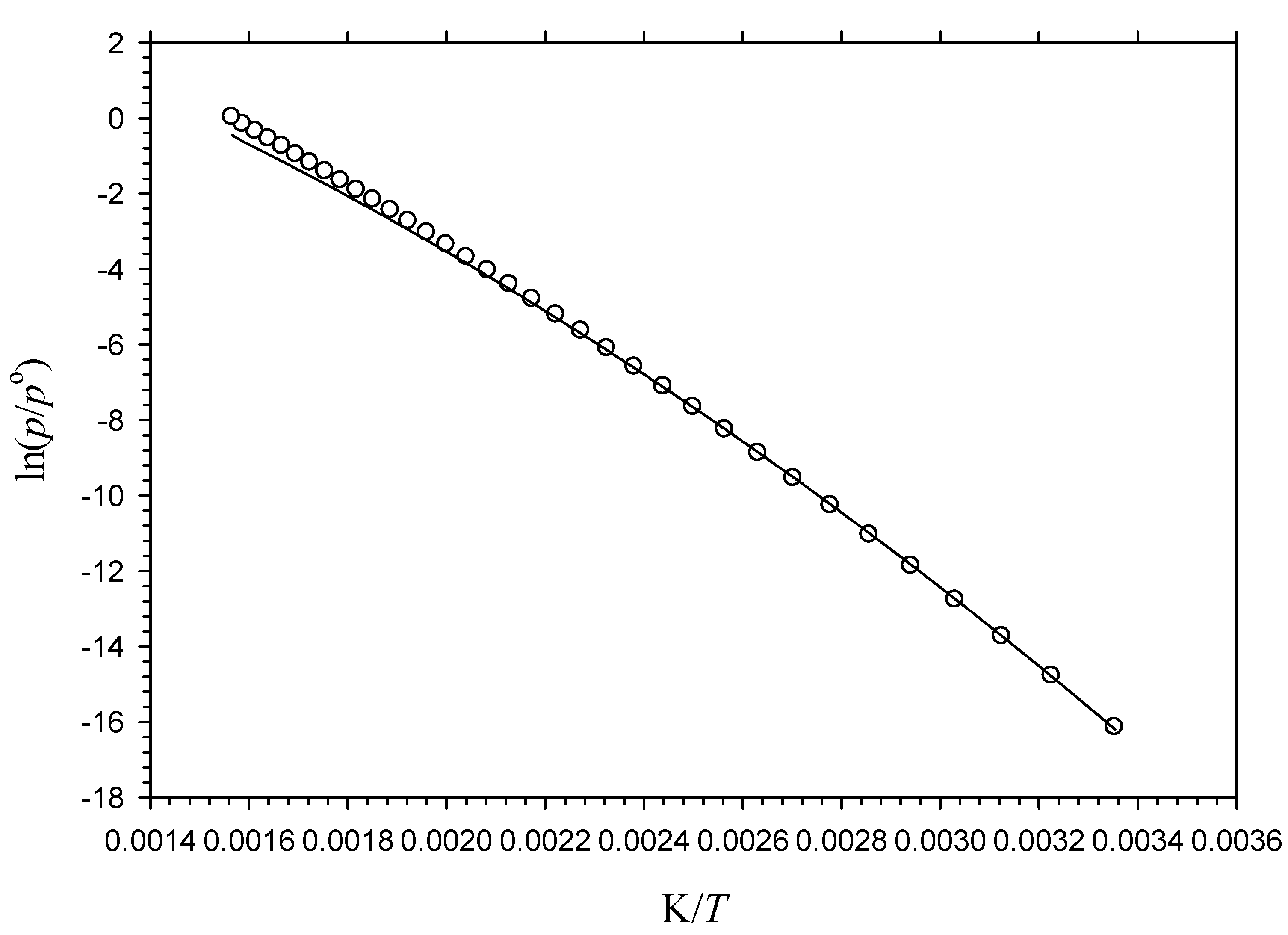
3.2. Vapor Pressures
4. Discussion
4.1. Vaporization Enthalpy of 5α-Cholestane Using Literature Data
4.2. Liquid Vapor Pressures of 5α-Cholestane
4.3. Sublimation Enthalpy and Vapor Pressure of Solid 5α-Cholestane
4.4. Vaporization Enthalpy of 5α-Androstane
4.5. Liquid Vapor Pressures of 5α-Androstane
4.6. Retention Times of 5α-Cholestane and 5α-Androstane
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bobrovskiy, I.; Hope, J.M.; Ivantsov, A.; Andrey, I.; Nettersheim, B.J.; Nettersheim, B.J.; Hallmann, C.; Brocks, J.J. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 2018, 361, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Segaloff, A.; Gabbard, R.B.A. 5α-Androstane-An Androgenic Hydrocarbon. Endocrinology 1960, 67, 887–889. [Google Scholar] [PubMed]
- Analytical Standards and Certified Reference Materials, 2012–2015, Fluka Analytical; Sigma Aldrich Schweiz, Industriestrasse 25 CH-9470 Buchs SG Schweiz.
- Zafar, A.; Chickos, J.S. The vapor pressure and vaporization enthalpy of squalene and squalene by correlation gas chromatography. J. Chem. Thermodyn. 2019, 135, 192–197. [Google Scholar] [CrossRef]
- Thorton, M.; Gobble, C.; Chickos, J. The vaporization enthalpy and vapor pressure of S (+) methamphetamine at T = 298.15 K by correlation gas chromatography. J. Chem. Thermodyn. 2014, 73, 51–56. [Google Scholar]
- Umnahanant, P.; Zafar, A.; Kankala, V.; Chickos, J. Vapor pressure and vaporization enthalpy studies of (+) longifolene, (-) isolongifolene and β-myrcene by correlation gas chromatography. J. Chem. Thermodyn. 2019, 131, 583–591. [Google Scholar] [CrossRef]
- Orf, M.; Kurian, M.; Nelson, C.; Simmons, D.; Espinosa, L.; Chickos, J. Correlation gas chromatographic study of the vaporization enthalpies and vapor pressures of the major sesquiterpene hydrocarbons in patchouli oil. J. Chem. Eng. Data 2021, 66, 2538–2549. [Google Scholar] [CrossRef]
- Mokbel, I.; Ruzicka, K.; Majer, V.; Ruzicka, V.; Ribeiro, M.; Jose, J.; Zabransky, M. Vapor pressures and thermal data for three high-boiling compounds of petroleum interest: 1-phenyldodecane, 5α-cholestane, adamantane. Fluid Phase Equil. 2000, 169, 191–207. [Google Scholar] [CrossRef]
- American Petroleum Institute. Properties of Hydrocarbons of High Molar Mass; Research Project 42; Pennsylvania State University: State College, PA, USA, 1967. [Google Scholar]
- Barton, D.; Chickos, J.S. The vapor pressure and vaporization enthalpy of (-) β-Elemene and (-) β-Bisabolene by correlation gas chromatography. J. Chem. Thermodyn. 2020, 148, 106139. [Google Scholar] [CrossRef]
- 5alpha-Cholestane. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5alpha-Cholestane (accessed on 4 May 2024).
- Melting Point Standards. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/258/468/melting-point-standards-fl8678en-ms.pdf (accessed on 4 May 2024).
- Spectral Database for Organic Compounds. Available online: https://sdbs.db.aist.go.jp (accessed on 19 December 2020).
- Peacock, L.A.; Fuchs, R. Enthalpy of vaporization measurements by gas chromatography. J. Am. Chem. Soc. 1977, 99, 5524–5525. [Google Scholar] [CrossRef]
- Lipkind, D.; Chickos, J.S. An Examination of Factors Influencing the Thermodynamics of Correlation-Gas Chromatography as Applied to Large Molecules and Chiral Separations. J. Chem. Eng. Data 2010, 55, 698–707. [Google Scholar] [CrossRef]
- Shoemaker, D.P.; Garland, C.W.; Steinfield, J.I.; Niebler, J.W. Experiments in Physical Chemistry, 4th ed.; McGraw Hill Co.: New York, NY, USA, 1981. [Google Scholar]
- Chickos, J.S. Computational Thermochemistry, Prediction and Estimation of Molecular Thermodynamics; ACS Symposium Series 677; Irikura, K.K., Frurip, D.J., Eds.; ACS: Washington, DC, USA, 1996; Chapter 4; pp. 63–91. [Google Scholar]
- Ruzicka, K.; Majer, V. Simultaneous Treatment of Vapor Pressures and Related Thermal Data Between the Triple and Normal Boiling Temperatures for n-Alkanes C5–C20. J. Phys. Chem. Ref. Data 1994, 23, 1–39. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor pressures and vaporization enthalpies of the n-alkanes from C21-C30 at T = 298.15 by correlation-gas chromatography. J. Chem. Eng. Data 2004, 49, 77–85. [Google Scholar] [CrossRef]
- Chickos, J.S.; Hanshaw, W. Vapor pressures and vaporization enthalpies of the n-alkanes from C31-C38 at T = 298.15 by correlation-gas chromatography. J. Chem. Eng. Data 2004, 49, 620–630. [Google Scholar] [CrossRef]
- Stephenson, R.M.; Malanowski, S. Handbook of the Thermodynamics of Organic Compounds; Elsevier: New York, NY, USA, 1987. [Google Scholar]
- EPI Suite version 4.11 (Estimation Programs Interface). Available online: http://www.epa.gov/oppt/exposure/pubs/episuitedl.htm (accessed on 18 March 2020).
- Advanced Chemistry Development (ACD/Labs). ACD/Labs Software, V11.02; (© 1994–2023 ACD/Labs) through CAS SciFinder; ACD/Labs: Toronto, ON, Canada, 1994.
- Acree, W., Jr.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2015. Part 1. C1–C10. J. Phys. Chem. Ref. Data 2016, 45, 033101. [Google Scholar]
- Chickos, J. An update on liquid heat capacity estimations of cyclic organic compounds by group additivity and their application in estimations of complex molecules by synthetic and retrosynthetic analysis. J. Chem. Thermodyn. 2023, 182, 107039. [Google Scholar] [CrossRef]
- Chirico, R.D.; Nguyen, A.; Steele, W.V.; Strube, M.M. Vapor pressure data of n- alkanes revisited. New high precision vapor pressure data on n-decane, n-eicosane and n-octacosane. J. Chem. Eng. Data 1989, 34, 149–156. [Google Scholar] [CrossRef]

| Compound | CAS Registry No. | Supplier a | Mass Fraction b Supplier |
|---|---|---|---|
| n-Hexadecane | 544-76-3 | Sigma Aldrich | 0.99+ |
| n-Heptadecane | 62978-7 | Aldrich | 0.99 |
| n-Octadecane | 593-45-3 | Fischer | 0.99 |
| n-Nonadecane | 629-78-7 | Sigma Aldrich | 0.99 |
| n-Eicosane | 112-95-8 | Aldrich | 0.99 |
| n-Docosane | 629-97-0 | Aldrich | 0.99 |
| n-Tetracosane | 646-31-1 | Aldrich | 0.98 |
| n-Pentacosane | 629-99-2 | Aldrich | 0.99 |
| n-Octacosane | 630-02-4 | Aldrich | 0.99 |
| n-Nonacosane | 630-03-5 | Aldrich | 0.99 |
| n-Triacontane | 638-68-5 | Sigma | 0.99 |
| n-Hentriacontane | 630-04-6 | Fluka | 0.995 |
| 5α-Androstane/CH2Cl2 | 438-22-2 | Sigma Aldrich | 0.99+ c |
| 5α-Cholestane | 481-21-0 | Sigma Aldrich | 0.98 |
| (A) Standards a | (298.15 K) b kJ·mol−1 | A0 | A1 | A2 × 106 | To/K/po/kPa | |
|---|---|---|---|---|---|---|
| n-Heptadecane | 86.47 ± 1.7 | 3.21826 | −0.002036553 | 1.383899 | 575.375/101.325 | |
| n-Octadecane | 91.44 ± 1.8 | 3.24741 | −0.002048039 | 1.362445 | 590.023/101.325 | |
| n-Nonadecane | 96.44 ± 1.9 | 3.27626 | −0.002062714 | 1.346737 | 603.989/101.325 | |
| n-Eicosane | 101.80 ± 0.5 | 3.31181 | −0.002102218 | 1.348780 | 617.415/101.325 | |
| (B) x = l; cr | (351.8 K) | o | 1·(104) | 2·(107) | Tfus/K | ΔT/Krange |
| 5α-Cholestane (l) c | 108.4 | 3.948254 | −11.945709 | 6.8443691 | 352–478 | |
| 5α-Cholestane (cr) c | 133.8 | 3.936924 | −3.2336427 | 0 | 351.75 | 308–352 |
| (C) Standards d | (298.15 K) e kJ·mol−1 | At | Bt | Ct | Dt | |
| n-Heneicosane | 106.8 ± 2.5 | 6.6591 | −98.135 | −2,907,500 | 199,890,000 | |
| n-Docosane | 111.9 ± 2.7 | 6.5353 | 110.72 | −3,117,600 | 217,130,000 | |
| n-Tetracosane | 121.9 ± 2.8 | 6.2817 | 530.15 | −3,528,600 | 250,720,000 | |
| n-Pentacosane | 126.8 ± 2.9 | 6.1496 | 741.19 | −3,730,700 | 267,380,000 | |
| n-Hexacosane | 131.7 ± 3.2 | 6.0704 | 910.53 | −3,919,300 | 282,440,000 | |
| n-Octacosane | 141.9 ± 4.9 | 5.8835 | 1279.4 | −4,312,000 | 313,890,000 | |
| n-Nonacosane | 147.1 ± 5.1 | 5.8413 | 1431.2 | −4,504,300 | 328,710,000 | |
| n-Tricontane | 152.3 ± 5.3 | 5.7696 | 1601.6 | −4,699,800 | 344,040,000 | |
| n-Henitricontane f | 157.3 ± 0.7 | 5.679 | 1791.2 | −4,900,200 | 360,370,000 | |
| (D) Antoine Eq. g | AA | BA | CA | po/kPa | Trange/K | |
| 5α-Cholestane (l) | 11.35684 | 6038.938 | 0 | 1 | 481–538 | |
| (E) Heat capacity c | Trange/K | Acp | Bcp | Ccp | Cp(298 K)/J·K−1·mol−1 | |
| 5α-Cholestane (l) | 368–387 | 9.22607 | 0.256109 | −0.881339 | 646.4 h | |
| 5α-Cholestane (cr) c | 308–333 | −9.44908 | 0.257208 | 0 | 559 h | |
| Run 1 | -slope a/K | Intercept a | a/ kJ·mol−1 | / kJ·mol−1 | ||
|---|---|---|---|---|---|---|
| Lit. a | Calc. a | |||||
| Pentacosane | 9754 ± 97 | 16.95 ± 0.18 | 81.09 ± 0.81 | 126.8 ± 2.9 | 126.6 ± 3.9 | |
| Hexacosane | 10,123 ± 96 | 17.38 ± 0.18 | 84.15 ± 0.80 | 131.7 ± 3.2 | 131.8 ± 4.0 | |
| Octacosane | 10,846.0 ± 97 | 18.21 ± 0.18 | 90.17 ± 0.82 | 141.9 ± 4.9 | 142.0 ± 4.1 | |
| 5-α-Cholestane | 9720 ± 81 | 15.89 ± 0.15 | 80.81 ± 0.68 | 126.2 ± 3.9 | ||
| Nonacosane | 11,197 ± 88 | 18.61 ± 0.16 | 93.08 ± 0.73 | 147.1 ± 5.1 | 146.9 ± 4.2 | |
| Tricosane | 11,632 ± 95 | 19.16 ± 0.18 | 96.70 ± 0.79 | 152.3 ± 5.3 | 153.0 ± 4.3 | |
| Hentriacontane | 11,892 ± 95 | 19.40 ± 0.17 | 98.86 ± 0.78 | 157.3 ± 1.2 | 156.7 ± 4.3 | |
| /kJ·mol−1 = (1.692 ± 0.032) − (10.59 ± 2.9) | r2 = 0.9986 | (9) | ||||
| Run 3 | -slope a/K | Intercept a | a/ kJ·mol−1 | / kJ·mol−1 | ||
| Lit. a | Calc. a | |||||
| n-Tetracosane | 8596.9 ± 62 | 15.21 ± 0.12 | 71.47 ± 0.52 | 121.9 ± 2.8 | 121.7 ± 1.6 | |
| n-Pentacosane | 8961.0 ± 61 | 15.62 ± 0.12 | 74.50 ± 0.51 | 126.8 ± 2.9 | 126.8 ± 1.6 | |
| n-Hexacosane | 9327.2 ± 55 | 16.05 ± 0.11 | 77.54 ± 0.46 | 131.7 ± 3.2 | 131.9 ± 1.6 | |
| n-Octacosane | 10,047.5 ± 50 | 16.87 ± 0.10 | 83.53 ± 0.41 | 141.9 ± 4.9 | 142.0 ± 1.7 | |
| 5-α-Cholestane | 8870.8 ± 57 | 14.45 ± 0.09 | 73.75 ± 0.47 | 125.6 ± 1.6 | ||
| n-Nonacosane | 10,405.7 ± 45 | 17.29 ± 0.09 | 86.51 ± 0.38 | 147.1 ± 5.1 | 147.0 ± 1.7 | |
| /kJ·mol−1 = (1.677 ± 0.015) + (1.90 ± 1.2) | r2 = 0.9998 | (10) | ||||
| Run 5 | -slope a/K | Intercept a | a/kJ·mol−1 | / kJ·mol−1 | ||
| Lit. | Calc. a | |||||
| n-Heptadecane | 67,817 ± 16 | 13.61 ± 0.03 | 56.37 ± 0.13 | 86.47 ± 1.7 b | 86.3 ± 1.9 | |
| n-Octadecane | 7183 ± 19 | 14.09 ± 0.04 | 59.72 ± 0.16 | 91.44 ± 1.8 b | 91.5 ± 2.0 | |
| n-Nonadecane | 7582 ± 29 | 14.56 ± 0.06 | 63.03 ± 0.24 | 96.44 ± 2.0 b | 96.7 ± 2.1 | |
| n-Eicosane | 7987 ± 31 | 15.05 ± 0.07 | 66.40 ± 0.25 | 101.8 ± 0.5 b | 101.9 ± 2.2 | |
| 5-α-Androstane | 6891 ± 21 | 12.58 ± 0.04 | 57.29 ± 0.17 | 87.7 ± 1.9 | ||
| n-Heneicosane | 8344 ± 20 | 15.44 ± 0.04 | 69.37 ± 0.16 | 106.8 ± 2.5 a | 106.6 ± 2.1 | |
| n-Docosane | 87,956 ± 31 | 16.04 ± 0.07 | 73.12 ± 0.26 | 111.9 ± 2.7 a | 112.4 ± 2.2 | |
| /kJ·mol−1 = (1.560 ± 0.023) − (1.681 ± 1.44) | r2 = 0.9994 | (11) | ||||
| Run 1 | Run 2 | Run 3 | Run 4 | Avg. | Estimate b | Cox Eq. c | |
|---|---|---|---|---|---|---|---|
| 5α-Cholestane | 126.2 ± 3.9 | 124.1 ± 4.3 | 125.6 ± 1.6 | 126.6 ± 5.9 | 125.6 ± 3.9 | 122.9 ± 6.1 | 116.7 ± 3.3 |
| Run 5 | Run 6 | ||||||
| 5α-Androstane | 87.7 ± 1.9 | 87.8 ± 2.0 | 87.8 ± 2.0 | 85.3 ± 4.3 |
| A (Runs 1 and 2) | ln(to/tr)avg | ln(p/po) | ln(p/po) Calc | 106·p298.15 K/Pa Calc | 106·p298.15 K/Pa Lit. b |
|---|---|---|---|---|---|
| n-Pentacosane | −16.034 | −23.244 | −23.24 ± 0.45 | 8.3 ± 3.9 | 8.1 |
| n-Hexacosane | −16.807 | −24.309 | −24.31 ± 0.46 | 2.8 ± 1.3 | 2.8 |
| n-Octacosane | −18.370 | −26.490 | −26.51 ± 0.48 | 0.31 ± 0.16 | 0.32 |
| 5-α-Cholestane | −16.889 | −24.43 ± 0.47 | 2.5 ± 1.2 | 7.8 c | |
| n-Nonacosane | −19.149 | −27.627 | −27.61 ± 0.49 | 0.10 ± 0.05 | 0.10 |
| n-Tricontane | −20.035 | −28.748 | −28.86 ± 0.51 | 0.03 ± 0.02 | 0.033 |
| n-Hentriacontane | −20.665 | −29.841 | −29.75 ± 0.51 | 0.012 ± 0.007 | 0.011 d |
| (12) | r2 = 0.9997 | ||||
| B (Runs 3 and 4) | ln(to/tr)avg | ln(p/po) | ln(p/po) Calc | 106·p298.15 K/Pa Calc | 106·p298.15 K/Pa Lit. b |
| n-Tetracosane | −14.746 | −22.175 | −22.22 ± 0.44 | 24 ± 6 | 24 |
| n-Pentacosane | −15.447 | −23.244 | −23.17 ± 0.45 | 8.1 ± 2.1 | 8.1 |
| n-Hexacosane | −16.306 | −24.309 | −24.32 ± 0.46 | 2.7 ± 0.7 | 2.8 |
| n-Octacosane | −17.957 | −26.49 | −26.55 ± 0.48 | 0.31 ± 0.09 | 0.32 |
| 5-α-Cholestane | −16.408 | −24.46 ± 0.46 | 2.5 ± 0.7 | 7.8 c | |
| n-Nonacosane | −18.732 | −27.627 | −27.59 ± 0.49 | 0.11 ± 0.03 | 0.10 |
| (13) | r2 = 0.9998 | ||||
| C Runs (5 and 6) | ln(to/tr)avg | ln(p/po) | ln(p/po) Calc | 102·p298.15 K/Pa Calc | 102·p298.15 K/Pa Lit. e |
| n-Heptadecane | −9.187 | −14.314 | −14.308 | 6.3 ± 1.3 | 6.2 |
| n-Octadecane | −10.059 | −15.435 | −15.439 | 2.0 ± 0.4 | 2.0 |
| n-Nonadecane | −10.924 | −16.551 | −16.562 | 0.64 ± 0.1 | 0.66 |
| n-Eicosane | −11.793 | −17.696 | −17.688 | 0.20 ± 0.05 | 0.21 |
| 5α-Androstane | −10.594 | −16.133 | 1.0 ± 0.2 | 0.23 f | |
| n-Heneicosane | −12.600 | −18.836 | −18.736 | 0.070 ± 0.02 | 0.067 d |
| n-Docosane | −13.520 | −19.972 | −19.928 | 0.021 ± 0.005 | 0.021 b |
| (14) | r2 = 0.9997 | ||||
| Runs | As | −Bs | −Cs | Trange/K | TB/K | TB/K (Lit) |
|---|---|---|---|---|---|---|
| 5α-Cholestane | ||||||
| 1&2 | 11.45 ± 0.20 | 6016.6 ± 140 | 1,395,542 ± 14,123 | 298.15 to 400 | 700 | 740.4 b |
| 1&2 | 8.42 ± 0.20 | 3863.5 ± 161 | 1,773,500 ± 31,755 | 298.15 to 550 | 743 | 665 c |
| 3&4 | 11.59 ± 0.21 | 6097.3 ± 144 | 1,387,138 ± 24,713 | 298.15 to 400 | 698 | |
| 3&4 | 8.42 ± 0.21 | 3843.0 ± 171 | 1,783,043 ± 33,662 | 298.15 to 550 | 743 | |
| 5α-Androstane | ||||||
| 5&6 | 9.045 ± 0.140 | 4261.0 ± 95 | 969,095 ± 16,268 | 298.15 to 400 | 638.8 | 587.6 c |
| 5&6 | 6.578 ± 0.180 | 2500.4 ± 145 | 1,279,139 ± 28,441 | 298.15 to 550 | 670.1 | 609 ± 9 d |
| Runs | As | −Bs | −Cs | Trange/K | TB/K | TB/K (Lit) |
|---|---|---|---|---|---|---|
| 5α-Cholestane | ||||||
| 1–4 | 11.518 ± 0.210 | 6057.3 ± 142 | 1,391,260 ± 24,410 | 298.15 to 400 | 699 | 665 b, 740.4 c |
| 1–4 | 8.419 ± 0.210 | 3853.7 ± 166 | 1,778,173 ± 32,710 | 298.15 to 550 | 743 | 714 d |
| 5α-Androstane | ||||||
| 5&6 | 9.045 ± 0.140 | 4261.0 ± 95 | 969,095 ± 16,268 | 298.15 to 400 | 638.8 | 588 b, 609 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer-Lodike, C.; Albinsaad, M.; Chickos, J.S. Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography. Liquids 2024, 4, 456-469. https://doi.org/10.3390/liquids4030025
Fischer-Lodike C, Albinsaad M, Chickos JS. Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography. Liquids. 2024; 4(3):456-469. https://doi.org/10.3390/liquids4030025
Chicago/Turabian StyleFischer-Lodike, Christian, Mohammad Albinsaad, and James S. Chickos. 2024. "Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography" Liquids 4, no. 3: 456-469. https://doi.org/10.3390/liquids4030025
APA StyleFischer-Lodike, C., Albinsaad, M., & Chickos, J. S. (2024). Vaporization Enthalpies and Vapor Pressures of 5α-Androstane and 5α-Cholestane by Correlation Gas Chromatography. Liquids, 4(3), 456-469. https://doi.org/10.3390/liquids4030025









