Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review
Abstract
1. Introduction
2. Substances of Very High Concern (SVHC)
3. Solvent Guides
4. Replacement Solvents in Synthetic Chemistry
5. Solubility Parameters
6. Empirical Polarity Scales
7. Opportunities with Mixed Solvents
7.1. Chromatography Solvents
| Mixed Solvent a | Analyte b | System | Ref. |
|---|---|---|---|
| EtAc:EtOH (3:1) in heptanes | Neutral | LC | [43] |
| EtAc:EtOH in heptanes | Neutral | LC | [43] |
| iPrOH in heptanes | Neutral | LC | [43] |
| EtAc:EtOH (3:1) in MTBE | Neutral | LC | [43] |
| MeOH in MTBE | Neutral | LC | [43] |
| EtAc:EtOH (3:1) (2% NH4OH) in heptanes | Basic | LC | [43] |
| MeOH: NH4OH (10:1) in EtAc | Basic | LC | [43] |
| MeOH: NH4OH (10:1) in MTBE | Basic | LC | [43] |
| EtAc:EtOH (3:1) (2% AcOH) in heptanes | Acidic | LC | [43] |
| MeOH:AcOH (10:1) in EtAc | Acidic | LC | [43] |
| MeOH:AcOH (10:1) in MTBE | Acidic | LC | [43] |
| EtAc:EtOH (3:1) in cyclohexane | n.s. | LC | [46] |
| acetonitrile:water | Polar | LC | [46] |
| tert-butyl acetate | All | LC | [50] |
| sec-butyl acetate | All | LC | [50] |
| ethyl isobutyrate | All | LC | [50] |
| methyl pivalate | All | LC | [50] |
| CO2:EtAc | n.s. | TLC | [51] |
| EtAc in heptanes | n.s. | TLC | [51] |
| iPrOH in heptanes | n.s. | TLC | [51] |
| Ace in heptanes | n.s. | TLC | [51] |
| CO2:MeOH | Neutral | FC | [45] |
| CO2:EtAc | n.s. | FC | [51] |
| CO2:Ace | n.s. | FC | [51] |
| CO2:iPrOH | n.s. | FC | [51] |
7.2. Expanded Liquids and Supercritical Fluids
7.3. Low Transition Temperature Mixtures
7.4. Switchable Solvents
7.5. HBD—HBA Mixtures of Molecular Solvents
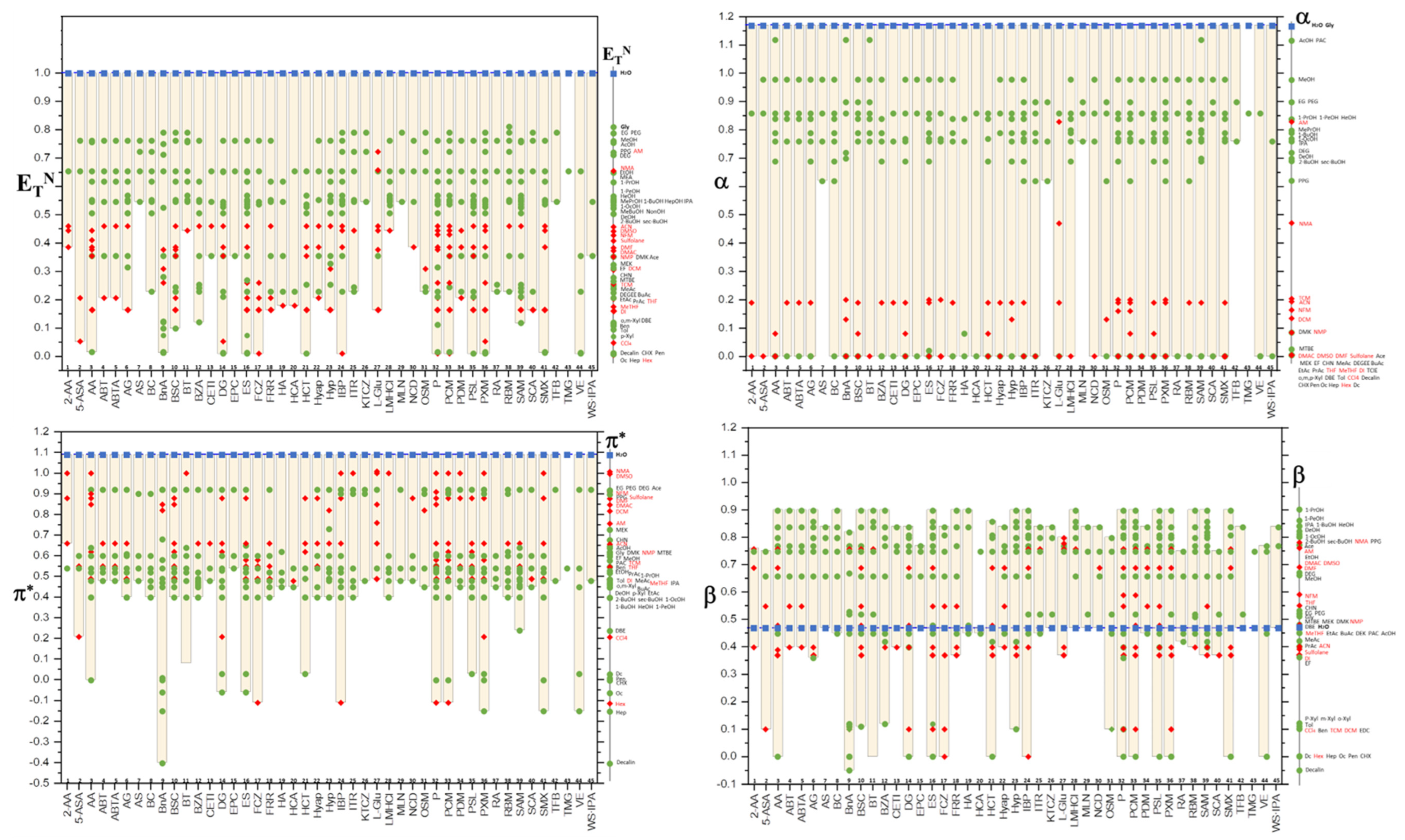
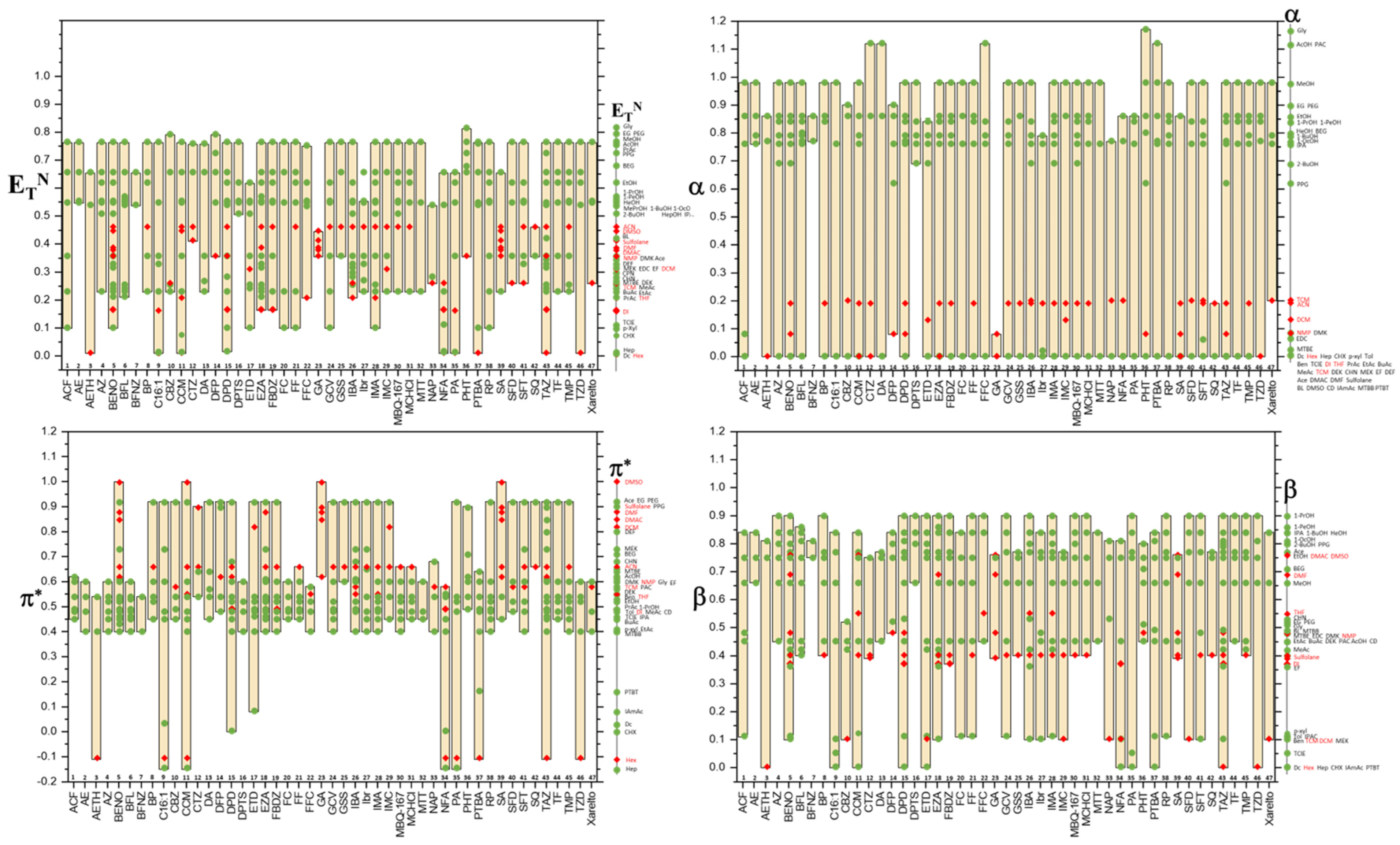
8. Kamlet—Taft Parameter Windows for APIs
9. Linear Solvation Energy Relationships (LSER)
10. Conclusions
11. Future Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reichardt, C. Empirical Parameters of Solvent Polarity as Linear Free-Energy Relationships. Angew. Chem. Int. Ed. Engl. 1979, 18, 98–110. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Wrona, P.K.; Zielkowska, U.; Reichardt, C. Empirical parameters of lewis acidity and basicity for aqueous binary solvent mixtures. Tetrahedron 1985, 41, 4519–4527. [Google Scholar] [CrossRef]
- Reichardt, C. Solvation Effects in Organic Chemistry: A Short Historical Overview. J. Org. Chem. 2022, 87, 1616–1629. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, M.J.; Taft, R.W. The Solvatochromic Comparison Method. I. The β-Scale Of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Catalán, J. On the empirical scales of organic solvents established using probe/homomorph pairs. J. Phys. Org. Chem. 2021, 34, e4206. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- ACS. ACS GCI Pharmaceutical Roundtable. Collaboration to Deliver a Solvent Selection Guide for the Pharmaceutical Industry. 2018. Available online: https://www.acs.org/content/dam/acsorg/greenchemistry/industriainnovation/roundtable/solvent-selection-guide.pdf (accessed on 16 December 2023).
- ACS. Tools for Innovation in Chemistry. 2023. Available online: https://www.acsgcipr.org/tools-for-innovation-in-chemistry/ (accessed on 16 December 2023).
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Richardson, P.; Sneddon, H.F.; Moores, A.; Briddell, C.; Martinez, I. Making Sustainability Assessment Accessible: Tools Developed by the ACS Green Chemistry Institute Pharmaceutical Roundtable. ACS Sustain. Chem. Eng. 2021, 9, 16862–16864. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.C.K.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol–water solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef]
- Ono, T.; Horikawa, K.; Ota, M.; Sato, Y.; Inomata, H. Insight into the local composition of the Wilson equation at high temperatures and pressures through molecular simulations of methanol-water mixtures. J. Chem. Eng. Data 2014, 59, 1024–1030. [Google Scholar] [CrossRef]
- Ono, T.; Ito, Y.; Ota, M.; Takebayashi, Y.; Furuya, T.; Inomata, H. Difference in aqueous solution structure at 293.2 and 473.2 K between ethanol and ethylene glycol via molecular dynamics. J. Mol. Liq. 2022, 368, 120764. [Google Scholar] [CrossRef]
- Jordan, A.; Hall, C.G.J.; Thorp, L.R.; Sneddon, H.F. Replacement of Less-Preferred Dipolar Aprotic and Ethereal Solvents in Synthetic Organic Chemistry with More Sustainable Alternatives. Chem. Rev. 2022, 122, 6749–6794. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Candidate List of Substances of Very High Concern for Authorisation. 2023. Available online: https://echa.europa.eu/candidate-list-table (accessed on 24 December 2023).
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide—Embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.C.; Cunningham, V.L. Expanding GSK’s Solvent Selection Guide—Application of life cycle assessment to enhance solvent selections. Clean Technol. Environ. Policy 2004, 7, 42–50. [Google Scholar] [CrossRef]
- Diorazio, L.J.; Hose, D.R.J.; Adlington, N.K. Toward a More Holistic Framework for Solvent Selection. Org. Process Res. Dev. 2016, 20, 760–773. [Google Scholar] [CrossRef]
- Gao, F.; Bai, R.; Ferlin, F.; Vaccaro, L.; Li, M.; Gu, Y. Replacement strategies for non-green dipolar aprotic solvents. Green Chem. 2020, 22, 6240–6257. [Google Scholar] [CrossRef]
- Gabriel, C.M.; Keener, M.; Gallou, F.; Lipshutz, B.H. Amide and Peptide Bond Formation in Water at Room Temperature. Org. Lett. 2015, 17, 3968–3971. [Google Scholar] [CrossRef]
- Dalla Torre, D.; Annatelli, M.; Aricò, F. Acid catalyzed synthesis of dimethyl isosorbide via dimethyl carbonate chemistry. Catal. Today 2023, 423, 113892. [Google Scholar] [CrossRef]
- Sherwood, J.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef]
- Campos, J.F.; Scherrmann, M.-C.; Berteina-Raboin, S. Eucalyptol: A new solvent for the synthesis of heterocycles containing oxygen, sulfur and nitrogen. Green Chem. 2019, 21, 1531–1539. [Google Scholar] [CrossRef]
- Domokos, A.; Nagy, B.; Szilágyi, B.; Marosi, G.; Nagy, Z.K. Integrated Continuous Pharmaceutical Technologies—A Review. Org. Process Res. Dev. 2021, 25, 721–739. [Google Scholar] [CrossRef]
- Lavayssiere, M.; Lamaty, F. Amidation by reactive extrusion for the synthesis of active pharmaceutical ingredients teriflunomide and moclobemide. Chem. Commun. 2023, 59, 3439–3442. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J.H. solubility. xii. Regular solutions1. J. Am. Chem. Soc. 1929, 51, 66–80. [Google Scholar] [CrossRef]
- Prausnitz, J.M.; Lichtenthaler, R.N.; Azevedo, E.G.D. Molecular Thermodynamics of Fluid-Phase Equilibria, 3rd ed.; Prentice Hall PTR: London, UK, 1999. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters. In A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Sue, K.; Furuya, T.; Yoda, S. Solubilities of Organic Semiconductors and Nonsteroidal Anti-inflammatory Drugs in Pure and Mixed Organic Solvents: Measurement and Modeling with Hansen Solubility Parameter. J. Chem. Eng. Data 2018, 63, 3889–3901. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Vovers, J.; Thuan Lu, H.; Stevens, G.W.; Mumford, K.A. Investigation of green solvents for the extraction of phenol and natural alkaloids: Solvent and extractant selection. Chem. Eng. J. 2022, 442, 136054. [Google Scholar] [CrossRef]
- Kumar, A.; Nanda, A. In-silico methods of cocrystal screening: A review on tools for rational design of pharmaceutical cocrystals. J. Drug Deliv. Sci. Technol. 2021, 63, 102527. [Google Scholar] [CrossRef]
- Abbott, S. Solubility, similarity, and compatibility: A general-purpose theory for the formulator. Curr. Opin. Colloid Interface Sci. 2020, 48, 65–76. [Google Scholar] [CrossRef]
- Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, C.; Zhou, Y.; Hu, D.; Chen, J.; Zhang, X. A review on the calculation and application of lignin Hansen solubility parameters. Int. J. Biol. Macromol. 2024, 256, 128506. [Google Scholar] [CrossRef]
- Novaes, F.J.M.; de Faria, D.C.; Ferraz, F.Z.; de Aquino Neto, F.R. Hansen Solubility Parameters Applied to the Extraction of Phytochemicals. Plants 2023, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Otárola-Sepúlveda, J.; Cea-Klapp, E.; Aravena, P.; Ormazábal-Latorre, S.; Canales, R.I.; Garrido, J.M.; Valerio, O. Assessment of Hansen solubility parameters in deep eutectic solvents for solubility predictions. J. Mol. Liq. 2023, 388, 122669. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The .pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N.; Schmidt, C.H.; Schreiter, K. Reappraisal of Empirical Solvent Polarity Scales for Organic Solvents. Chem. Methods 2021, 1, 42–60. [Google Scholar] [CrossRef]
- Spange, S.; Weiß, N. Empirical Hydrogen Bonding Donor (HBD) Parameters of Organic Solvents Using Solvatochromic Probes—A Critical Evaluation. ChemPhysChem 2023, 24, e202200780. [Google Scholar] [CrossRef] [PubMed]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H.; Pichierri, F. Does Synergism in Microscopic Polarity Correlate with Extrema in Macroscopic Properties for Aqueous Mixtures of Dipolar Aprotic Solvents? J. Phys. Chem. B 2017, 121, 6033–6041. [Google Scholar] [CrossRef]
- Taygerly, J.P.; Miller, L.M.; Yee, A.; Peterson, E.A. A convenient guide to help select replacement solvents for dichloromethane in chromatography. Green Chem. 2012, 14, 3020–3025. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Greener Chromatography Solvents. 2015. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/greener-chromatography-solvents-82207.pdf (accessed on 27 December 2023).
- McClain, R.; Rada, V.; Nomland, A.; Przybyciel, M.; Kohler, D.; Schlake, R.; Nantermet, P.; Welch, C.J. Greening Flash Chromatography. ACS Sustain. Chem. Eng. 2016, 4, 4905–4912. [Google Scholar] [CrossRef]
- Dutta, P.; McGranaghan, A.; Keller, I.; Patil, Y.; Mulholland, N.; Murudi, V.; Prescher, H.; Smith, A.; Carson, N.; Martin, C.; et al. A case study in green chemistry: The reduction of hazardous solvents in an industrial R&D environment. Green Chem. 2022, 24, 3943–3956. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H. Replacement of Hazardous Chemicals Used in Engineering Plastics with Safe and Renewable Hydrogen-Bond Donor and Acceptor Solvent-Pair Mixtures. ACS Sustain. Chem. Eng. 2015, 3, 1881–1889. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the move towards modernized greener separations: Introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Petřík, I.; Pěnčík, A.; Stýskala, J.; Tranová, L.; Amakorová, P.; Strnad, M.; Novák, O. Rapid profiling of cytokinins using supercritical fluid chromatography coupled with tandem mass spectrometry. Anal. Chim. Acta 2024, 1285, 342010. [Google Scholar] [CrossRef]
- Lynch, J.; Sherwood, J.; McElroy, C.R.; Murray, J.; Shimizu, S. Dichloromethane replacement: Towards greener chromatography via Kirkwood–Buff integrals. Anal. Methods 2023, 15, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Duereh, A.; Smith, R.L. Strategies for using hydrogen-bond donor/acceptor solvent pairs in developing green chemical processes with supercritical fluids. J. Supercrit. Fluids 2018, 141, 182–197. [Google Scholar] [CrossRef]
- Hoang, H.N.; Nagashima, Y.; Mori, S.; Kagechika, H.; Matsuda, T. CO2-expanded bio-based liquids as novel solvents for enantioselective biocatalysis. Tetrahedron 2017, 73, 2984–2989. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluids 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Temelli, F. Perspectives on the use of supercritical particle formation technologies for food ingredients. J. Supercrit. Fluids 2018, 134, 244–251. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Saravana, P.S.; Getachew, A.T.; Cho, Y.-J.; Choi, J.H.; Park, Y.B.; Woo, H.C.; Chun, B.S. Influence of co-solvents on fucoxanthin and phlorotannin recovery from brown seaweed using supercritical CO2. J. Supercrit. Fluids 2017, 120, 295–303. [Google Scholar] [CrossRef]
- Sun, M.; Temelli, F. Supercritical carbon dioxide extraction of carotenoids from carrot using canola oil as a continuous co-solvent. J. Supercrit. Fluids 2006, 37, 397–408. [Google Scholar] [CrossRef]
- Kok, S.L.; Lee, W.J.; Smith, R.L.; Suleiman, N.; Jom, K.N.; Vangnai, K.; Bin Sharaai, A.H.; Chong, G.H. Role of virgin coconut oil (VCO) as co-extractant for obtaining xanthones from mangosteen (Garcinia mangostana) pericarp with supercritical carbon dioxide extraction. J. Supercrit. Fluids 2021, 176, 105305. [Google Scholar] [CrossRef]
- Lee, W.J.; Ng, C.C.; Ng, J.S.; Smith, R.L.; Kok, S.L.; Hee, Y.Y.; Lee, S.Y.; Tan, W.K.; Zainal Abidin, N.H.; Halim Lim, S.A.; et al. Supercritical carbon dioxide extraction of α-mangostin from mangosteen pericarp with virgin coconut oil as co-extractant and in-vitro bio-accessibility measurement. Process Biochem. 2019, 87, 213–220. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, X.; Xu, H.; Zhao, J.; Wang, Q.; Liu, G.; Hao, Q. Optimization of supercritical carbon dioxide extraction of lutein esters from marigold (Tagetes erecta L.) with vegetable oils as continuous co-solvents. Sep. Purif. Technol. 2010, 71, 214–219. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, X.; Gao, Y.; Wang, Q.; Zhao, J. Optimisation of supercritical carbon dioxide extraction of lutein esters from marigold (Tagetes erect L.) with soybean oil as a co-solvent. Int. J. Food Sci. Technol. 2008, 43, 1763–1769. [Google Scholar] [CrossRef]
- Pattiram, P.D.; Abas, F.; Suleiman, N.; Mohamad Azman, E.; Chong, G.H. Edible oils as a co-extractant for the supercritical carbon dioxide extraction of flavonoids from propolis. PLoS ONE 2022, 17, e0266673. [Google Scholar] [CrossRef]
- Shi, X.; Wu, H.; Shi, J.; Xue, S.J.; Wang, D.; Wang, W.; Cheng, A.; Gong, Z.; Chen, X.; Wang, C. Effect of modifier on the composition and antioxidant activity of carotenoid extracts from pumpkin (Cucurbita maxima) by supercritical CO2. LWT-Food Sci. Technol. 2013, 51, 433–440. [Google Scholar] [CrossRef]
- Fikri, I.; Yulianah, Y.; Lin, T.-C.; Lin, R.-W.; Chen, U.-C.; Lay, H.-L. Optimization of supercritical fluid extraction of dihydrotanshinone, cryptotanshinone, tanshinone I, and tanshinone IIA from Salvia miltiorrhiza with a peanut oil modifier. Chem. Eng. Res. Des. 2022, 180, 220–231. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Temelli, F.; Guigard, S.E.; Tomberli, B.; Gray, C.G. Apparent solubility of lycopene and β-carotene in supercritical CO2, CO2+ethanol and CO2+canola oil using dynamic extraction of tomatoes. J. Food Eng. 2010, 99, 1–8. [Google Scholar] [CrossRef]
- Vasapollo, G.; Longo, L.; Rescio, L.; Ciurlia, L. Innovative supercritical CO2 extraction of lycopene from tomato in the presence of vegetable oil as co-solvent. J. Supercrit. Fluids 2004, 29, 87–96. [Google Scholar] [CrossRef]
- Shi, J.; Yi, C.; Xue, S.J.; Jiang, Y.; Ma, Y.; Li, D. Effects of modifiers on the profile of lycopene extracted from tomato skins by supercritical CO2. J. Food Eng. 2009, 93, 431–436. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Hashimoto, Y.; Sato, M.; Sato, Y.; Smith, R.L.; Inomata, H. Solubility of flavone, 6-methoxyflavone and anthracene in supercritical CO2 with/without a co-solvent of ethanol correlated by using a newly proposed entropy-based solubility parameter. Fluid Phase Equilibria 2016, 425, 65–71. [Google Scholar] [CrossRef]
- Ota, M.; Sugahara, S.; Sato, Y.; Smith, R.L.; Inomata, H. Vapor-liquid distribution coefficients of hops extract in high pressure CO2 and ethanol mixtures and data correlation with entropy-based solubility parameters. Fluid Phase Equilibria 2017, 434, 44–48. [Google Scholar] [CrossRef]
- Duereh, A.; Sugimoto, Y.; Ota, M.; Sato, Y.; Inomata, H. Kamlet-Taft Dipolarity/Polarizability of Binary Mixtures of Supercritical Carbon Dioxide with Cosolvents: Measurement, Prediction, and Applications in Separation Processes. Ind. Eng. Chem. Res. 2020, 59, 12319–12330. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-Transition-Temperature Mixtures (LTTMs): A New Generation of Designer Solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Goto, M. Ionic Liquids as Active Pharmaceutical Ingredients (APIs). In Application of Ionic Liquids in Drug Delivery; Goto, M., Moniruzzaman, M., Eds.; Springer Singapore: Singapore, 2021; pp. 13–33. [Google Scholar] [CrossRef]
- Md Moshikur, R.; Goto, M. Pharmaceutical Applications of Ionic Liquids: A Personal Account. Chem. Rec. 2023, 23, e202300026. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic liquids as a useful tool for tailoring active pharmaceutical ingredients. J. Control. Release 2021, 338, 268–283. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Carrier, R.L.; Moniruzzaman, M.; Goto, M. Recent Advances in Biocompatible Ionic Liquids in Drug Formulation and Delivery. Pharmaceutics 2023, 15, 1179. [Google Scholar] [CrossRef] [PubMed]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Cunha, I.T.; McKeeman, M.; Ramezani, M.; Hayashi-Mehedy, K.; Lloyd-Smith, A.; Bravi, M.; Jessop, P.G. Amine-free CO2-switchable hydrophilicity solvents and their application in extractions and polymer recycling. Green Chem. 2022, 24, 3704–3716. [Google Scholar] [CrossRef]
- Mercer, S.M.; Jessop, P.G. “Switchable water”: Aqueous solutions of switchable ionic strength. ChemSusChem 2010, 3, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Liberato, V.S.; Ferreira, T.F.; MacDonald, A.R.; Dias Ribeiro, B.; Zarur Coelho, M.A.; Jessop, P.G. A CO2-responsive method for separating hydrophilic organic molecules from aqueous solutions: Solvent-assisted switchable water. Green Chem. 2023, 25, 4705–4712. [Google Scholar] [CrossRef]
- Cunha, I.T.; Yang, H.; Jessop, P.G. High pressure switchable water: An alternative method for separating organic products from water. Green Chem. 2021, 23, 3996–4007. [Google Scholar] [CrossRef]
- Phan, L.; Jessop, P.G. Switching the hydrophilicity of a solute. Green Chem. 2009, 11, 307–330. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H. Methodology for replacing dipolar aprotic solvents used in API processing with safe hydrogen-bond donor and acceptor solvent-pair mixtures. Org. Process Res. Dev. 2017, 21, 114–124. [Google Scholar] [CrossRef]
- Jouyban, A.; Acree, W.E. A single model to represent physico-chemical properties of liquid mixtures at various temperatures. J. Mol. Liq. 2021, 323, 115054. [Google Scholar] [CrossRef]
- Nazemieh, A.; Acree, W.E.; Jouyban, A. Further computations on physico-chemical properties of binary mixtures of p-cymene with α-pinene, limonene and citral. J. Mol. Liq. 2022, 350, 118211. [Google Scholar] [CrossRef]
- Lee, J.L.; Chong, G.H.; Kanno, A.; Ota, M.; Guo, H.; Smith, R.L. Local composition-regular solution theory for analysis of pharmaceutical solubility in mixed-solvents. J. Mol. Liq. 2024, 397, 124012. [Google Scholar] [CrossRef]
- Duereh, A.; Guo, H.; Honma, T.; Hiraga, Y.; Sato, Y.; Lee Smith, R.; Inomata, H. Solvent Polarity of Cyclic Ketone (Cyclopentanone, Cyclohexanone): Alcohol (Methanol, Ethanol) Renewable Mixed-Solvent Systems for Applications in Pharmaceutical and Chemical Processing. Ind. Eng. Chem. Res. 2018, 57, 7331–7344. [Google Scholar] [CrossRef]
- Duereh, A.; Sato, Y.; Smith, R.L.; Inomata, H. Analysis of the Cybotactic Region of Two Renewable Lactone-Water Mixed-Solvent Systems that Exhibit Synergistic Kamlet-Taft Basicity. J. Phys. Chem. B 2016, 120, 4467–4481. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Vaez-Gharamaleki, J.; Fazeli-Bakhtiyari, R.; Martinez, F.; Jouyban, A. Prediction of deferiprone solubility in some non-aqueous binary solvent mixtures at various temperatures. J. Mol. Liq. 2015, 203, 16–19. [Google Scholar] [CrossRef]
- Acree, W.E. IUPAC-NIST Solubility Data Series. 102. Solubility of Nonsteroidal Anti-inflammatory Drugs (NSAIDs) in Neat Organic Solvents and Organic Solvent Mixtures. J. Phys. Chem. Ref. Data 2014, 43, 023102. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Martínez, F. Solubility of fluconazole in (ethanol + water) mixtures: Determination, correlation, dissolution thermodynamics and preferential solvation. J. Mol. Liq. 2021, 333, 115987. [Google Scholar] [CrossRef]
- Akay, S.; Kayan, B.; Peña, M.Á.; Jouyban, A.; Martínez, F. Solubility of Salicylic Acid in Some (Ethanol + Water) Mixtures at Different Temperatures: Determination, Correlation, Thermodynamics and Preferential Solvation. Int. J. Thermophys. 2023, 44, 121. [Google Scholar] [CrossRef]
- Ali, H.S.M.; York, P.; Blagden, N.; Soltanpour, S.; Acree, W.E.; Jouyban, A. Solubility of Budesonide, Hydrocortisone, and Prednisolone in Ethanol + Water Mixtures at 298.2 K. J. Chem. Eng. Data 2009, 55, 578–582. [Google Scholar] [CrossRef]
- Almandoz, M.C.; Sancho, M.I.; Blanco, S.E. Spectroscopic and DFT study of solvent effects on the electronic absorption spectra of sulfamethoxazole in neat and binary solvent mixtures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 112–119. [Google Scholar] [CrossRef]
- Alsubaie, M.; Aljohani, M.; Erxleben, A.; McArdle, P. Cocrystal Forms of the BCS Class IV Drug Sulfamethoxazole. Cryst. Growth Des. 2018, 18, 3902–3912. [Google Scholar] [CrossRef]
- Alvani-Alamdari, S.; Rezaei, H.; Rahimpour, E.; Hemmati, S.; Martinez, F.; Barzegar-Jalali, M.; Jouyban, A. Mesalazine solubility in the binary mixtures of ethanol and water at various temperatures. Phys. Chem. Liq. 2019, 59, 12–25. [Google Scholar] [CrossRef]
- Aniya, V.; De, D.; Mohammed, A.M.; Thella, P.K.; Satyavathi, B. Measurement and modeling of solubility of para-tert-butylbenzoic acid in pure and mixed organic solvents at different temperatures. J. Chem. Eng. Data 2017, 62, 1411–1421. [Google Scholar] [CrossRef]
- Anwer, M.K.; Mohammad, M.; Fatima, F.; Alshahrani, S.M.; Aldawsari, M.F.; Alalaiwe, A.; Al-Shdefat, R.; Shakeel, F. Solubility, solution thermodynamics and molecular interactions of osimertinib in some pharmaceutically useful solvents. J. Mol. Liq. 2019, 284, 53–58. [Google Scholar] [CrossRef]
- Assis, G.P.; Garcia, R.H.L.; Derenzo, S.; Bernardo, A. Solid-liquid equilibrium of paracetamol in water-ethanol and water-propylene glycol mixtures. J. Mol. Liq. 2021, 323, 114617. [Google Scholar] [CrossRef]
- Aydi, A.; Claumann, C.A.; Wüst Zibetti, A.; Abderrabba, M. Differential Scanning Calorimetry Data and Solubility of Rosmarinic Acid in Different Pure Solvents and in Binary Mixtures (Methyl Acetate + Water) and (Ethyl Acetate + Water) from 293.2 to 313.2 K. J. Chem. Eng. Data 2016, 61, 3718–3723. [Google Scholar] [CrossRef]
- Banerjee, D.; Laha, A.K.; Bagchi, S. Preferential solvation in mixed binary solvent. J. Chem. Soc. Faraday Trans. 1995, 91, 631. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Mirheydari, S.N.; Rahimpour, E.; Shekaari, H.; Martinez, F.; Jouyban, A. Experimental determination and correlation of bosentan solubility in (PEG 200+ water) mixtures at T = (293.15–313.15) K. Phys. Chem. Liq. 2018, 57, 504–515. [Google Scholar] [CrossRef]
- Bernal-García, J.M.; Guzmán-López, A.; Cabrales-Torres, A.; Estrada-Baltazar, A.; Iglesias-Silva, G.A. Densities and viscosities of (N,N-dimethylformamide+ water) at atmospheric pressure from (283.15 to 353.15) K. J. Chem. Eng. Data 2008, 53, 1024–1027. [Google Scholar] [CrossRef]
- Bhesaniya, K.; Nandha, K.; Baluja, S. Thermodynamics of Fluconazole Solubility in Various Solvents at Different Temperatures. J. Chem. Eng. Data 2014, 59, 649–652. [Google Scholar] [CrossRef]
- Blanco-Márquez, J.H.; Ortiz, C.P.; Cerquera, N.E.; Martínez, F.; Jouyban, A.; Delgado, D.R. Thermodynamic analysis of the solubility and preferential solvation of sulfamerazine in (acetonitrile + water) cosolvent mixtures at different temperatures. J. Mol. Liq. 2019, 293, 111507. [Google Scholar] [CrossRef]
- Blokhina, S.V.; Sharapova, A.V.; Ol’khovich, M.V.; Levshin, I.B.; Perlovich, G.L. Solid–liquid phase equilibrium and thermodynamic analysis of novel thiazolidine-2,4-dione derivative in different solvents. J. Mol. Liq. 2021, 326, 115273. [Google Scholar] [CrossRef]
- Bosch, E.; Rived, F.; Rosés, M. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 4. Preferential solvation of solvatochromic indicators in mixtures of 2-methylpropan-2-ol with hexane, benzene, propan-2-ol, ethanol and methanol. J. Chem. Soc. Perkin Trans. 1996, 2, 2177–2184. [Google Scholar] [CrossRef]
- Bosch, E.; Rosés, M.; Herodes, K.; Koppel, I.; Leito, I.; Koppel, I.; Taal, V. Solute-solvent and solvent-solvent interactions in binary solvent mixtures. 2. Effect of temperature on the ET(30) polarity parameter of dipolar hydrogen bond acceptor-hydrogen bond donor mixtures. J. Phys. Org. Chem. 1996, 9, 403–410. [Google Scholar] [CrossRef]
- Calvo, B.; Cepeda, E.A. Solubilities of Stearic Acid in Organic Solvents and in Azeotropic Solvent Mixtures. J. Chem. Eng. Data 2008, 53, 628–633. [Google Scholar] [CrossRef]
- Calvo, B.; Collado, I.; Cepeda, E.A. Solubilities of Palmitic Acid in Pure Solvents and Its Mixtures. J. Chem. Eng. Data 2008, 54, 64–68. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; Díaz, I.; Rodríguez, M. Evaluation of bio-based solvents for phenolic acids extraction from aqueous matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Carmen Grande, M.d.; Juliá, J.A.; García, M.; Marschoff, C.M. On the density and viscosity of (water+dimethylsulphoxide) binary mixtures. J. Chem. Thermodyn. 2007, 39, 1049–1056. [Google Scholar] [CrossRef]
- Castro, G.T.; Filippa, M.A.; Peralta, C.M.; Davin, M.V.; Almandoz, M.C.; Gasull, E.I. Solubility and Preferential Solvation of Piroxicam in Neat Solvents and Binary Systems. Z. Für Phys. Chem. 2017, 232, 257–280. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, M.; Feng, L.; Ren, B. Measurement and Correlation for Solubility of Diosgenin in Some Mixed Solvents. Chin. J. Chem. Eng. 2014, 22, 170–176. [Google Scholar] [CrossRef]
- Chen, F.-X.; Zhao, M.-R.; Ren, B.-Z.; Zhou, C.-R.; Peng, F.-F. Solubility of diosgenin in different solvents. J. Chem. Thermodyn. 2012, 47, 341–346. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Q.; Dou, H.; Zhang, L.; Pei, L.; Huang, R.; Shu, G.; Yuan, Z.; Lin, J.; Zhang, W.; et al. Solubility and dissolution thermodynamic properties of Mequindox in binary solvent mixtures. J. Mol. Liq. 2020, 303, 112619. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Xie, L. Thermodynamic parameters on corresponding solid-liquid equilibrium of hydroxyapatite in pure and mixture organic solvents. J. Mol. Liq. 2017, 229, 189–197. [Google Scholar] [CrossRef]
- Chen, Z.; Zhai, J.; Liu, X.; Mao, S.; Zhang, L.; Rohani, S.; Lu, J. Solubility measurement and correlation of the form A of ibrutinib in organic solvents from 278.15 to 323.15 K. J. Chem. Thermodyn. 2016, 103, 342–348. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, L.; Ye, J.; Wang, Z.; Hu, Y. Solubility measurement and thermodynamic modelling of curcumin in twelve pure solvents and three binary solvents at different temperature (T = 278.15–323.15 K). J. Mol. Liq. 2021, 338, 116795. [Google Scholar] [CrossRef]
- Cysewski, P.; Jeliński, T.; Przybyłek, M.; Nowak, W.; Olczak, M. Solubility Characteristics of Acetaminophen and Phenacetin in Binary Mixtures of Aqueous Organic Solvents: Experimental and Deep Machine Learning Screening of Green Dissolution Media. Pharmaceutics 2022, 14, 2828. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, M.V.G.; Santiago, R.; Romero, J.M.; Duconge, J.; Monbaliu, J.-C.; López-Mejías, V.; Stelzer, T. Solubility Determination and Correlation of Warfarin Sodium 2-Propanol Solvate in Pure, Binary, and Ternary Solvent Mixtures. J. Chem. Eng. Data 2019, 64, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- del Mar Muñoz, M.; Delgado, D.R.; Peña, M.Á.; Jouyban, A.; Martínez, F. Solubility and preferential solvation of sulfadiazine, sulfamerazine and sulfamethazine in propylene glycol + water mixtures at 298.15K. J. Mol. Liq. 2015, 204, 132–136. [Google Scholar] [CrossRef]
- Delgado, D.R.; Martínez, F. Solubility and solution thermodynamics of sulfamerazine and sulfamethazine in some ethanol+water mixtures. Fluid Phase Equilibria 2013, 360, 88–96. [Google Scholar] [CrossRef]
- Delgado, D.R.; Martínez, F. Preferential solvation of sulfadiazine, sulfamerazine and sulfamethazine in ethanol+water solvent mixtures according to the IKBI method. J. Mol. Liq. 2014, 193, 152–159. [Google Scholar] [CrossRef]
- Dizechi, M.; Marschall, E. Viscosity of some binary and ternary liquid mixtures. J. Chem. Eng. Data 1982, 27, 358–363. [Google Scholar] [CrossRef]
- Domańska, U.; Pobudkowska, A.; Pelczarska, A.; Winiarska-Tusznio, M.; Gierycz, P. Solubility and pKa of select pharmaceuticals in water, ethanol, and 1-octanol. J. Chem. Thermodyn. 2010, 42, 1465–1472. [Google Scholar] [CrossRef]
- Dong, Q.; Yu, S.; Wang, X.; Ding, S.; Li, E.; Cai, Y.; Xue, F. Solubility Measurement and Correlation of Itraconazole Hydroxy Isobutyltriazolone in Four Kinds of Binary Solvent Mixtures with Temperature from 283.15 to 323.15 K. ACS Omega 2023, 8, 39390–39400. [Google Scholar] [CrossRef] [PubMed]
- Elizalde-Solis, O.; Arenas-Quevedo, M.G.; Verónico-Sánchez, F.J.; García-Morales, R.; Zúñiga-Moreno, A. Solubilities of Binary Systems α-Tocopherol + Capsaicin and α-Tocopherol + Palmitic Acid in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2019, 64, 1948–1955. [Google Scholar] [CrossRef]
- Fakhree, M.A.A.; Ahmadian, S.; Panahi-Azar, V.; Acree, W.E.; Jouyban, A. Solubility of 2-Hydroxybenzoic Acid in Water, 1-Propanol, 2-Propanol, and 2-Propanone at (298.2 to 338.2) K and Their Aqueous Binary Mixtures at 298.2 K. J. Chem. Eng. Data 2012, 57, 3303–3307. [Google Scholar] [CrossRef]
- Filippa, M.A.; Gasull, E.I. Ibuprofen solubility in pure organic solvents and aqueous mixtures of cosolvents: Interactions and thermodynamic parameters relating to the solvation process. Fluid Phase Equilibria 2013, 354, 185–190. [Google Scholar] [CrossRef]
- Gheitasi, N.; Nazari, A.H.; Haghtalab, A. Thermodynamic Modeling and Solubility Measurement of Cetirizine Hydrochloride and Deferiprone in Pure Solvents of Acetonitrile, Ethanol, Acetic Acid, Sulfolane, and Ethyl Acetate and Their Mixtures. J. Chem. Eng. Data 2019, 64, 5486–5496. [Google Scholar] [CrossRef]
- Gonçalves Bonassoli, A.B.; Oliveira, G.; Bordón Sosa, F.H.; Rolemberg, M.P.; Mota, M.A.; Basso, R.C.; Igarashi-Mafra, L.; Mafra, M.R. Solubility measurement of lauric, palmitic, and stearic acids in ethanol, n-propanol, and 2-propanol using differential scanning calorimetry. J. Chem. Eng. Data 2019, 64, 2084–2092. [Google Scholar] [CrossRef]
- Guo, S.; Yang, W.; Hu, Y.; Wang, K.; Luan, Y. Measurement and Correlation of the Solubility of N-Acetylglycine in Different Solvents at Temperatures from 278.15 to 319.15 K. J. Solut. Chem. 2013, 42, 1879–1887. [Google Scholar] [CrossRef]
- Guo, Y.; He, H.; Huang, H.; Qiu, J.; Han, J.; Hu, S.; Liu, H.; Zhao, Y.; Wang, P. Solubility determination and thermodynamic modeling of n-acetylglycine in different solvent systems. J. Chem. Eng. Data 2021, 66, 1344–1355. [Google Scholar] [CrossRef]
- Gusain, K.; Garg, S.; Kumar, R. Solubility Prediction of Pharmaceutical Compounds in Pure Solvent by Different Correlations and Thermodynamic Models. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Ha, E.-S.; Lee, S.-K.; Jeong, J.-S.; Sim, W.-Y.; Yang, J.-I.; Kim, J.-S.; Kim, M.-S. Solvent effect and solubility modeling of rebamipide in twelve solvents at different temperatures. J. Mol. Liq. 2019, 288, 111041. [Google Scholar] [CrossRef]
- Hatefi, A.; Jouyban, A.; Mohammadian, E.; Acree, W.E.; Rahimpour, E. Prediction of paracetamol solubility in cosolvency systems at different temperatures. J. Mol. Liq. 2019, 273, 282–291. [Google Scholar] [CrossRef]
- He, Q.; Zheng, M.; Zhao, H. Baicalin solubility in aqueous co-solvent mixtures of methanol, ethanol, isopropanol and n-propanol revisited: Solvent–solvent and solvent–solute interactions and IKBI preferential solvation analysis. Phys. Chem. Liq. 2019, 58, 820–832. [Google Scholar] [CrossRef]
- Hellstén, S.; Qu, H.; Louhi-Kultanen, M. Screening of Binary Solvent Mixtures and Solvate Formation of Indomethacin. Chem. Eng. Technol. 2011, 34, 1667–1674. [Google Scholar] [CrossRef]
- Heryanto, R.; Hasan, M.; Abdullah, E.C.; Kumoro, A.C. Solubility of Stearic Acid in Various Organic Solvents and Its Prediction using Non-ideal Solution Models. ScienceAsia 2007, 33, 469–472. Available online: https://www.scienceasia.org/2007.33.n4/v33_469_472.pdf (accessed on 30 December 2023).
- Hu, W.; Shang, Z.; Wei, N.; Hou, B.; Gong, J.; Wang, Y. Solubility of benorilate in twelve monosolvents: Determination, correlation and COSMO-RS analysis. J. Chem. Thermodyn. 2021, 152, 106272. [Google Scholar] [CrossRef]
- Hu, X.; Gong, Y.; Cao, Z.; Huang, Z.; Sha, J.; Li, Y.; Li, T.; Ren, B. Solubility, Hansen solubility parameter and thermodynamic properties of etodolac in twelve organic pure solvents at different temperatures. J. Mol. Liq. 2020, 316, 113779. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Y.; Cao, Z.; Sha, J.; Huang, Z.; Li, Y.; Li, T.; Ren, B. Solubility measurement, Hansen solubility parameter and thermodynamic modeling of etodolac in four binary solvents from 278.15 K to 323.15 K. J. Mol. Liq. 2020, 318, 114155. [Google Scholar] [CrossRef]
- Imran, S.; Hossain, A.; Mahali, K.; Guin, P.S.; Datta, A.; Roy, S. Solubility and peculiar thermodynamical behaviour of 2-aminobenzoic acid in aqueous binary solvent mixtures at 288.15 to 308.15 K. J. Mol. Liq. 2020, 302, 112566. [Google Scholar] [CrossRef]
- Ivanov, E.V.; Batov, D.V. Enthalpy-related parameters of interaction of simplest α-amino acids with the pharmaceutical mebicar (N-tetramethylglycoluril) in water at 298.15 K. J. Chem. Thermodyn. 2019, 128, 159–163. [Google Scholar] [CrossRef]
- Jia, L.; Yang, J.; Cui, P.; Wu, D.; Wang, S.; Hou, B.; Zhou, L.; Yin, Q. Uncovering solubility behavior of Prednisolone form II in eleven pure solvents by thermodynamic analysis and molecular simulation. J. Mol. Liq. 2021, 342, 117376. [Google Scholar] [CrossRef]
- Jiménez Cruz, J.M.; Vlaar, C.P.; López-Mejías, V.; Stelzer, T. Solubility Measurements and Correlation of MBQ-167 in Neat and Binary Solvent Mixtures. J. Chem. Eng. Data 2021, 66, 832–839. [Google Scholar] [CrossRef]
- Jiménez, D.M.; Cárdenas, Z.J.; Martínez, F. Solubility and solution thermodynamics of sulfadiazine in polyethylene glycol 400 + water mixtures. J. Mol. Liq. 2016, 216, 239–245. [Google Scholar] [CrossRef]
- Jouyban, A.; Acree, W.E.; Martínez, F. Dissolution thermodynamics and preferential solvation of ketoconazole in some {ethanol (1) + water (2)} mixtures. J. Mol. Liq. 2020, 313, 113579. [Google Scholar] [CrossRef]
- Jouyban, A.; Mazaher Haji Agha, E.; Rahimpour, E.; Acree, W.E., Jr. Further computation and some comments on “Stearic acid solubility in mixed solvents of (water + ethanol) and (ethanol + ethyl acetate): Experimental data and comparison among different thermodynamic models”. J. Mol. Liq. 2020, 310, 113228. [Google Scholar] [CrossRef]
- Jouyban, K.; Mazaher Haji Agha, E.; Hemmati, S.; Martinez, F.; Kuentz, M.; Jouyban, A. Solubility of 5-aminosalicylic acid in N-methyl-2-pyrrolidone + water mixtures at various temperatures. J. Mol. Liq. 2020, 310, 113143. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, V.; Jouyban, A.; Kuentz, M.; Hemmati, S.; Martinez, F.; Rahimpour, E. A laser monitoring technique for determination of mesalazine solubility in propylene glycol and ethanol mixtures at various temperatures. J. Mol. Liq. 2020, 304, 112714. [Google Scholar] [CrossRef]
- Jouyban-Gharamaleki, V.; Jouyban, A.; Martinez, F.; Zhao, H.; Rahimpour, E. A laser monitoring technique for solubility study of ketoconazole in propylene glycol and 2-propanol mixtures at various temperatures. J. Mol. Liq. 2020, 320, 114444. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshehri, S.; Alshamsan, A.; Haque, A.; Shakeel, F. Solid liquid equilibrium of an antifungal drug itraconazole in different neat solvents: Determination and correlation. J. Mol. Liq. 2017, 234, 81–87. [Google Scholar] [CrossRef]
- Kandi, S.; Charles, A.L. Measurement, correlation, and thermodynamic properties for solubilities of bioactive compound (−)-epicatechin in different pure solvents at 298.15 K to 338.15 K. J. Mol. Liq. 2018, 264, 269–274. [Google Scholar] [CrossRef]
- Karpiuk, I.; Wilczura-Wachnik, H.; Myśliński, A. α-Tocopherol/AOT/alkane/water system. J. Therm. Anal. Calorim. 2017, 131, 2885–2892. [Google Scholar] [CrossRef]
- Khajir, S.; Shayanfar, A.; Acree, W.E.; Jouyban, A. Effects of N-methylpyrrolidone and temperature on phenytoin solubility. J. Mol. Liq. 2019, 285, 58–61. [Google Scholar] [CrossRef]
- Kuhs, M.; Svärd, M.; Rasmuson, Å.C. Thermodynamics of fenoxycarb in solution. J. Chem. Thermodyn. 2013, 66, 50–58. [Google Scholar] [CrossRef]
- Kumari, A.; Kadakanchi, S.; Tangirala, R.; Thella, P.K.; Satyavathi, B. Measurement and modeling of solid–liquid equilibrium of para-tert-butylbenzoic acid in acetic acid/methanol+ water and acetic acid+ para-tert-butyltoluene binary systems at various temperatures. J. Chem. Eng. Data 2016, 62, 87–95. [Google Scholar] [CrossRef]
- Lange, L.; Heisel, S.; Sadowski, G. Predicting the Solubility of Pharmaceutical Cocrystals in Solvent/Anti-Solvent Mixtures. Molecules 2016, 21, 593. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Sim, W.-Y.; Ha, E.-S.; Park, H.; Kim, J.-S.; Jeong, J.-S.; Kim, M.-S. Solubility of bisacodyl in fourteen mono solvents and N-methyl-2-pyrrolidone + water mixed solvents at different temperatures, and its application for nanosuspension formation using liquid antisolvent precipitation. J. Mol. Liq. 2020, 310, 113264. [Google Scholar] [CrossRef]
- Li, A.; Si, Z.; Yan, Y.; Zhang, X. Solubility and thermodynamic properties of hydrate lenalidomide in phosphoric acid solution. J. Mol. Liq. 2021, 330, 115446. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Li, S.; Yang, Y.; Cui, Y.; Gong, J. Determination and Correlation of Dipyridamole p-Toluene Sulfonate Solubility in Seven Alcohol Solvents and Three Binary Solvents. J. Chem. Eng. Data 2017, 63, 208–216. [Google Scholar] [CrossRef]
- Li, R.; Fu, L.; Zhang, J.; Wang, W.; Chen, X.; Zhao, J.; Han, D. Solid-liquid equilibrium and thermodynamic properties of dipyridamole form II in pure solvents and mixture of (N-methyl pyrrolidone + isopropanol). J. Chem. Thermodyn. 2020, 142, 105981. [Google Scholar] [CrossRef]
- Li, R.; Jin, Y.; Yu, B.; Xu, Q.; Chen, X.; Han, D. Solubility determination and thermodynamic properties calculation of macitentan in mixtures of ethyl acetate and alcohols. J. Chem. Thermodyn. 2021, 156, 106344. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Khan, A.; Li, C.; He, Z.; Zhao, J.; Han, D. Effect of Cosolvents on the Solubility of Lenalidomide and Thermodynamic Model Correlation of Data. J. Chem. Eng. Data 2019, 64, 4272–4279. [Google Scholar] [CrossRef]
- Li, R.; Yan, H.; Wang, Z.; Gong, J. Correlation of Solubility and Prediction of the Mixing Properties of Ginsenoside Compound K in Various Solvents. Ind. Eng. Chem. Res. 2012, 51, 8141–8148. [Google Scholar] [CrossRef]
- Li, R.; Yin, X.; Jin, Y.; Chen, X.; Zhao, B.; Wang, W.; Zhong, S.; Han, D. The solubility profile and dissolution thermodynamic properties of minocycline hydrochloride in some pure and mixed solvents at several temperatures. J. Chem. Thermodyn. 2021, 157, 106399. [Google Scholar] [CrossRef]
- Li, R.; Zhao, B.; Chen, X.; Zhang, J.; Liu, Z.; Zhu, X.; Han, D. Solubility and apparent thermodynamic analysis of pomalidomide in (acetone + ethanol/isopropanol) and (ethyl acetate + ethanol/isopropanol) and its correlation with thermodynamic model. J. Chem. Thermodyn. 2021, 154, 106345. [Google Scholar] [CrossRef]
- Li, W.; Yuan, J.; Wang, X.; Shi, W.; Zhao, H.; Xing, R.; Jouyban, A.; Acree, W.E. Bifonazole dissolved in numerous aqueous alcohol mixtures: Solvent effect, enthalpy–entropy compensation, extended Hildebrand solubility parameter approach and preferential solvation. J. Mol. Liq. 2021, 338, 116671. [Google Scholar] [CrossRef]
- Li, X.; Du, C.; Cong, Y.; Zhao, H. Solubility determination and thermodynamic modelling of 3-amino-1,2,4-triazole in ten organic solvents from T = 283.15 K to T = 318.15 K and mixing properties of solutions. J. Chem. Thermodyn. 2017, 104, 189–200. [Google Scholar] [CrossRef]
- Li, X.; Ma, M.; Du, C.; Zhao, H. Solubility of cetilistat in neat solvents and preferential solvation in (acetone, isopropanol or acetonitrile) + water co-solvent mixtures. J. Mol. Liq. 2017, 242, 618–624. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Du, C.; Cong, Y.; Zhao, H. Preferential solvation of rosmarinic acid in binary solvent mixtures of ethanol + water and methanol + water according to the inverse Kirkwood–Buff integrals method. J. Mol. Liq. 2017, 240, 56–64. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ning, Z.; Cui, J.; Wu, Q.; Wang, X. Solubilities of Adipic Acid and Succinic Acid in a Glutaric Acid + Acetone or n-Butanol Mixture. J. Chem. Eng. Data 2014, 59, 4062–4069. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, K.; Yu, B.; Wang, H.; Chen, M.; Gong, J. Measurement and Correlation of Solubility of Cefathiamidine in Water + (Acetone, Ethanol, or 2-Propanol) from (278.15 to 308.15) K. J. Chem. Eng. Data 2015, 61, 412–419. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Wang, Y.; Tang, H.; Wu, S.; Li, Y.-Y.; Zhang, L.-Y.; Bai, Q.-Y.; Liu, X. Experimental Measurements and Modeling of the Solubility of Aceclofenac in Six Pure Solvents from (293.35 to 338.25) K. J. Chem. Eng. Data 2014, 59, 1588–1592. [Google Scholar] [CrossRef]
- Liu, W.; Bao, Z.; Shen, Y.; Yao, T.; Bai, H.; Jin, X. Solubility measurement and thermodynamic modeling of carbamazepine (form III) in five pure solvents at various temperatures. Chin. J. Chem. Eng. 2021, 33, 231–235. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Liu, Y.; Xu, S.; Chen, M.; Du, S.; Gong, J. Solubility of L-histidine in different aqueous binary solvent mixtures from 283.15 K to 318.15 K with experimental measurement and thermodynamic modelling. J. Chem. Thermodyn. 2017, 105, 1–14. [Google Scholar] [CrossRef]
- Lou, Y.; Wang, Y.; Li, Y.; He, M.; Su, N.; Xu, R.; Meng, X.; Hou, B.; Xie, C. Thermodynamic equilibrium and cosolvency of florfenicol in binary solvent system. J. Mol. Liq. 2018, 251, 83–91. [Google Scholar] [CrossRef]
- Mabhoot, A.; Jouyban, A. Solubility of Sodium Phenytoin in Propylene Glycol + Water Mixtures in the Presence of B-Cyclodextrin. Pharm. Sci. 2015, 21, 152–156. [Google Scholar] [CrossRef]
- Mahali, K.; Guin, P.S.; Roy, S.; Dolui, B.K. Solubility and solute–solvent interaction phenomenon of succinic acid in aqueous ethanol mixtures. J. Mol. Liq. 2017, 229, 172–177. [Google Scholar] [CrossRef]
- Marcus, Y. The use of chemical probes for the characterization of solvent mixtures. Part 2. Aqueous mixtures. J. Chem. Soc. Perkin Trans. 1994, 2, 1751. [Google Scholar] [CrossRef]
- Marcus, Y. Use of chemical probes for the characterization of solvent mixtures. Part 1. Completely non-aqueous mixtures. J. Chem. Soc. Perkin Trans. 1994, 2, 1015. [Google Scholar] [CrossRef]
- Matsuda, H.; Kaburagi, K.; Matsumoto, S.; Kurihara, K.; Tochigi, K.; Tomono, K. Solubilities of Salicylic Acid in Pure Solvents and Binary Mixtures Containing Cosolvent. J. Chem. Eng. Data 2008, 54, 480–484. [Google Scholar] [CrossRef]
- McHedlov-Petrosyan, N.O. Book review: Christian Reichardt and Thomas Welton, Solvents and Solvent Effects in Organic Chemistry (Fourth Edition, Updated and Enlarged; Wiley-VCH Verlag & Co. KGaA, Weinheim, 2011; 718 p. Hardcover). Russ. J. Phys. Chem. A 2011, 85, 1482. [Google Scholar] [CrossRef]
- Mealey, D.; Svärd, M.; Rasmuson, Å.C. Thermodynamics of risperidone and solubility in pure organic solvents. Fluid Phase Equilibria 2014, 375, 73–79. [Google Scholar] [CrossRef]
- Mirmehrabi, M.; Rohani, S. Measurement and Prediction of the Solubility of Stearic Acid Polymorphs by the UNIQUAC Equation. Can. J. Chem. Eng. 2004, 82, 335–342. [Google Scholar] [CrossRef]
- Mo, F.; Ma, J.; Zhang, P.; Zhang, D.; Fan, H.; Yang, X.; Zhi, L.; Zhang, J. Solubility and thermodynamic properties of baicalein in water and ethanol mixtures from 283.15 to 328.15 K. Chem. Eng. Commun. 2019, 208, 183–196. [Google Scholar] [CrossRef]
- Mohamadian, E.; Hamidi, S.; Martínez, F.; Jouyban, A. Solubility prediction of deferiprone in N-methyl-2-pyrrolidone+ ethanol mixtures at various temperatures using a minimum number of experimental data. Phys. Chem. Liq. 2017, 55, 805–816. [Google Scholar] [CrossRef]
- Mohammadian, E.; Jouyban, A.; Barzegar-Jalali, M.; Acree, W.E.; Rahimpour, E. Solubilization of naproxen: Experimental data and computational tools. J. Mol. Liq. 2019, 288, 110985. [Google Scholar] [CrossRef]
- Mohammadzade, M.; Barzegar-Jalali, M.; Jouyban, A. Solubility of naproxen in 2-propanol+water mixtures at various temperatures. J. Mol. Liq. 2015, 206, 110–113. [Google Scholar] [CrossRef]
- Moodley, K.; Rarey, J.; Ramjugernath, D. Experimental solubility of diosgenin and estriol in various solvents between T = (293.2–328.2)K. J. Chem. Thermodyn. 2017, 106, 199–207. [Google Scholar] [CrossRef]
- Moodley, K.; Rarey, J.; Ramjugernath, D. Experimental solubility data for prednisolone and hydrocortisone in various solvents between (293.2 and 328.2) K by employing combined DTA/TGA. J. Mol. Liq. 2017, 240, 303–312. [Google Scholar] [CrossRef]
- Mora, C.P.; Martínez, F. Solubility of naproxen in several organic solvents at different temperatures. Fluid Phase Equilibria 2007, 255, 70–77. [Google Scholar] [CrossRef]
- Moradi, M.; Mazaher Haji Agha, E.; Hemmati, S.; Martinez, F.; Kuentz, M.; Jouyban, A. Solubility of 5-aminosalicylic acid in {N-methyl-2-pyrrolidone + ethanol} mixtures at T = (293.2 to 313.2) K. J. Mol. Liq. 2020, 306, 112774. [Google Scholar] [CrossRef]
- Ning, L.; Gong, X.; Li, P.; Chen, X.; Wang, H.; Xu, J. Measurement and correlation of the solubility of estradiol and estradiol-urea co-crystal in fourteen pure solvents at temperatures from 273.15 K to 318.15 K. J. Mol. Liq. 2020, 304, 112599. [Google Scholar] [CrossRef]
- Noda, K.; Aono, Y.; Ishida, K. Viscosity and Density of Ethanol-Acetic Acid-Water Mixtures. Kagaku Kogaku Ronbunshu 1983, 9, 237–240. [Google Scholar] [CrossRef]
- Noda, K.; Ohashi, M.; Ishida, K. Viscosities and densities at 298.15 K for mixtures of methanol, acetone, and water. J. Chem. Eng. Data 1982, 27, 326–328. [Google Scholar] [CrossRef]
- Noubigh, A. Stearic acid solubility in mixed solvents of (water + ethanol) and (ethanol + ethyl acetate): Experimental data and comparison among different thermodynamic models. J. Mol. Liq. 2019, 296, 112101. [Google Scholar] [CrossRef]
- Oliveira, G.; Bonassoli, A.B.G.; Rolemberg, M.P.; Mota, M.A.; Basso, R.C.; Soares, R.d.P.; Igarashi-Mafra, L.; Mafra, M.R. Water Effect on Solubilities of Lauric and Palmitic Acids in Ethanol and 2-Propanol Determined by Differential Scanning Calorimetry. J. Chem. Eng. Data 2021, 66, 2366–2373. [Google Scholar] [CrossRef]
- Ortiz, C.P.; Cardenas-Torres, R.E.; Martínez, F.; Delgado, D.R. Solubility of Sulfamethazine in the Binary Mixture of Acetonitrile + Methanol from 278.15 to 318.15 K: Measurement, Dissolution Thermodynamics, Preferential Solvation, and Correlation. Molecules 2021, 26, 7588. [Google Scholar] [CrossRef]
- Osorio, I.P.; Martínez, F.; Peña, M.A.; Jouyban, A.; Acree, W.E. Solubility, dissolution thermodynamics and preferential solvation of sulfadiazine in (N-methyl-2-pyrrolidone + water) mixtures. J. Mol. Liq. 2021, 330, 115693. [Google Scholar] [CrossRef]
- Pabba, S.; Kumari, A.; Ravuri, M.G.; Thella, P.K.; Satyavathi, B.; Shah, K.; Kundu, S.; Bhargava, S.K. Experimental determination and modelling of the co-solvent and antisolvent behaviour of binary systems on the dissolution of pharma drug; L-aspartic acid and thermodynamic correlations. J. Mol. Liq. 2020, 314, 113657. [Google Scholar] [CrossRef]
- Pacheco, D.P.; Martínez, F. Thermodynamic analysis of the solubility of naproxen in ethanol + water cosolvent mixtures. Phys. Chem. Liq. 2007, 45, 581–595. [Google Scholar] [CrossRef]
- Padervand, M.; Naseri, S.; Boroujeni, H.C. Preferential solvation of pomalidomide, an anticancer compound, in some binary mixed solvents at 298.15 K. Chin. J. Chem. Eng. 2020, 28, 2626–2633. [Google Scholar] [CrossRef]
- Pasham, F.; Jabbari, M.; Farajtabar, A. Solvatochromic Measurement of KAT Parameters and Modeling Preferential Solvation in Green Potential Binary Mixtures of N-Formylmorpholine with Water, Alcohols, and Ethyl Acetate. J. Chem. Eng. Data 2020, 65, 5458–5466. [Google Scholar] [CrossRef]
- Patel, A.; Vaghasiya, A.; Gajera, R.; Baluja, S. Solubility of 5-Amino Salicylic Acid in Different Solvents at Various Temperatures. J. Chem. Eng. Data 2010, 55, 1453–1455. [Google Scholar] [CrossRef]
- Przybyłek, M.; Miernicka, A.; Nowak, M.; Cysewski, P. New Screening Protocol for Effective Green Solvents Selection of Benzamide, Salicylamide and Ethenzamide. Molecules 2022, 27, 3323. [Google Scholar] [CrossRef]
- Qiu, J.; Huang, H.; He, H.; Liu, H.; Hu, S.; Han, J.; Guo, Y.; Wang, P. Measurement and Correlation of trans-4-Hydroxyl-proline Solubility in Sixteen Individual Solvents and a Water + Acetonitrile Binary Solvent System. J. Chem. Eng. Data 2020, 66, 575–587. [Google Scholar] [CrossRef]
- Radmand, S.; Rezaei, H.; Zhao, H.; Rahimpour, E.; Jouyban, A. Solubility and thermodynamic study of deferiprone in propylene glycol and ethanol mixture. BMC Chem 2023, 17, 37. [Google Scholar] [CrossRef]
- Ràfols, C.; Rosés, M.; Bosch, E. Solute–solvent and solvent–solvent interactions in binary solvent mixtures. Part 5. Preferential solvation of solvatochromic indicators in mixtures of propan-2-ol with hexane, benzene, ethanol and methanol. J. Chem. Soc. Perkin Trans. 1997, 2, 243–248. [Google Scholar] [CrossRef]
- Rani, R.S.; Rao, G.N. Stability of binary complexes of L-aspartic acid in dioxan–water mixtures. Bull. Chem. Soc. Ethiop. 2013, 27, 367–376. [Google Scholar] [CrossRef][Green Version]
- Rashid, A.; White, E.T.; Howes, T.; Litster, J.D.; Marziano, I. Effect of Solvent Composition and Temperature on the Solubility of Ibuprofen in Aqueous Ethanol. J. Chem. Eng. Data 2014, 59, 2699–2703. [Google Scholar] [CrossRef]
- Rathi, P.B.; Kale, M.; Soleymani, J.; Jouyban, A. Solubility of Etoricoxib in Aqueous Solutions of Glycerin, Methanol, Polyethylene Glycols 200, 400, 600, and Propylene Glycol at 298.2 K. J. Chem. Eng. Data 2018, 63, 321–330. [Google Scholar] [CrossRef]
- Ren, J.; Chen, D.; Yu, Y.; Li, H. Solubility of dicarbohydrazide bis[3-(5-nitroimino-1,2,4-triazole)] in common pure solvents and binary solvents at different temperatures. R. Soc. Open Sci. 2019, 6, 190728. [Google Scholar] [CrossRef]
- Rezaei, H.; Rahimpour, E.; Martinez, F.; Jouyban, A. Measurement and correlation of solubility data for deferiprone in propylene glycol and 2-propanol at different temperatures. Heliyon 2023, 9, e17402. [Google Scholar] [CrossRef]
- Rezaei, H.; Rezaei, H.; Rahimpour, E.; Martinez, F.; Jouyban, A. Solubility profile of phenytoin in the mixture of 1-propanol and water at different temperatures. J. Mol. Liq. 2021, 334, 115936. [Google Scholar] [CrossRef]
- Rodríguez, A.; Trigo, M.; Aubourg, S.P.; Medina, I. Optimisation of Low-Toxicity Solvent Employment for Total Lipid and Tocopherol Compound Extraction from Patagonian Squid By-Products. Foods 2023, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Rosales-García, T.; Rosete-Barreto, J.M.; Pimentel-Rodas, A.; Davila-Ortiz, G.; Galicia-Luna, L.A. Solubility of Squalene and Fatty Acids in Carbon Dioxide at Supercritical Conditions: Binary and Ternary Systems. J. Chem. Eng. Data 2017, 63, 69–76. [Google Scholar] [CrossRef]
- Roses, M.; Ortega, J.; Bosch, E. Variation ofE T(30) polarity and the Kamlet-Taft solvatochromic parameters with composition in alcohol-alcohol mixtures. J. Solut. Chem. 1995, 24, 51–63. [Google Scholar] [CrossRef]
- Ruidiaz, M.A.; Delgado, D.R.; Martínez, F. Indomethacin solubility estimation in 1,4-dioxane + water mixtures by the extended hildebrand solubility approach. Química Nova 2011, 34, 1569–1574. [Google Scholar] [CrossRef]
- Sajedi-Amin, S.; Barzegar-Jalali, M.; Fathi-Azarbayjani, A.; Kebriaeezadeh, A.; Martínez, F.; Jouyban, A. Solubilization of bosentan using ethanol as a pharmaceutical cosolvent. J. Mol. Liq. 2017, 232, 152–158. [Google Scholar] [CrossRef]
- Serna-Carrizales, J.C.; Zárate-Guzmán, A.I.; Aguilar-Aguilar, A.; Forgionny, A.; Bailón-García, E.; Flórez, E.; Gómez-Durán, C.F.A.; Ocampo-Pérez, R. Optimization of Binary Adsorption of Metronidazole and Sulfamethoxazole in Aqueous Solution Supported with DFT Calculations. Processes 2023, 11, 1009. [Google Scholar] [CrossRef]
- Sha, J.; Ma, T.; Huang, Z.; Hu, X.; Zhang, R.; Cao, Z.; Wan, Y.; Sun, R.; He, H.; Jiang, G.; et al. Corrigendum to “Solubility determination, model evaluation, Hansen solubility parameter and thermodynamic properties of benorilate in six pure solvents and two binary solvent mixtures”. J. Chem. Thermodyn. 2021, 158, 106365. [Google Scholar] [CrossRef]
- Sha, J.; Yang, X.; Hu, X.; Huang, Z.; Cao, Z.; Wan, Y.; Sun, R.; Jiang, G.; He, H.; Li, Y.; et al. Solubility determination, model evaluation, Hansen solubility parameter and thermodynamic properties of benflumetol in pure alcohol and ester solvents. J. Chem. Thermodyn. 2021, 154, 106323. [Google Scholar] [CrossRef]
- Sha, J.; Yang, X.; Ji, L.; Cao, Z.; Niu, H.; Wan, Y.; Sun, R.; He, H.; Jiang, G.; Li, Y.; et al. Solubility determination, model evaluation, Hansen solubility parameter, molecular simulation and thermodynamic properties of benflumetol in four binary solvent mixtures from 278.15 K to 323.15 K. J. Mol. Liq. 2021, 333, 115867. [Google Scholar] [CrossRef]
- Shakeel, F.; Iqbal, M.; Ezzeldin, E.; Haq, N. Thermodynamics of solubility of ibrutinib in ethanol+water cosolvent mixtures at different temperatures. J. Mol. Liq. 2015, 209, 461–464. [Google Scholar] [CrossRef]
- Shao, D.; Yang, Z.; Zhou, G.; Chen, J.; Zheng, S.; Lv, X.; Li, R. Improving the solubility of acipimox by cosolvents and the study of thermodynamic properties on solvation process. J. Mol. Liq. 2018, 262, 389–395. [Google Scholar] [CrossRef]
- Sharapova, A.; Ol’khovich, M.; Blokhina, S.; Perlovich, G. Solubility and vapor pressure data of bioactive 6-(acetylamino)-N-(5-ethyl-1,3,4-thiadiazol-2-yl) hexanamide. J. Chem. Thermodyn. 2019, 135, 35–44. [Google Scholar] [CrossRef]
- Shen, B.; Wang, Q.; Wang, Y.; Ye, X.; Lei, F.; Gong, X. Solubilities of Adipic Acid in Acetic Acid + Water Mixtures and Acetic Acid + Cyclohexane Mixtures. J. Chem. Eng. Data 2013, 58, 938–942. [Google Scholar] [CrossRef]
- Sheng, X.; Luo, W.; Wang, Q. Determination and Correlation for the Solubilities of Succinic Acid in Cyclohexanol + Cyclohexanone + Cyclohexane Solvent Mixtures. J. Chem. Eng. Data 2018, 63, 801–811. [Google Scholar] [CrossRef]
- Shi, S.; Yan, M.; Tao, B.; Luo, W. Measurement and correlation for solubilities of succinic acid, glutaric acid and adipic acid in five organic solvents. J. Mol. Liq. 2020, 297, 111735. [Google Scholar] [CrossRef]
- Singh, S. Studies on the Interactions of Paracetamol in Water and Binary Solvent Mixtures at T = (298.15–313.15) K: Viscometric and Surface Tension Approach. Biointerface Res. Appl. Chem. 2021, 12, 2776–2786. [Google Scholar] [CrossRef]
- Smirnov, V.I. Thermochemical investigation of L-glutamine dissolution processes in aqueous co-solvent mixtures of acetonitrile, dioxane, acetone and dimethyl sulfoxide at T = 298.15 K. J. Chem. Thermodyn. 2020, 150, 106227. [Google Scholar] [CrossRef]
- Smirnov, V.I.; Badelin, V.G. Similarity and differences of the thermochemical characteristics of l-glutamine dissolution in aqueous solutions of some acetamides and formamides at T = 298.15 K. J. Mol. Liq. 2019, 285, 84–88. [Google Scholar] [CrossRef]
- Soltanpour, S.; Gharagozlu, A. Piroxicam Solubility in Binary and Ternary Solvents of Polyethylene Glycols 200 or 400 with Ethanol and Water at 298.2 K: Experimental Data Report and Modeling. J. Solut. Chem. 2015, 44, 1407–1423. [Google Scholar] [CrossRef]
- Soltanpour, S.; Jouyban, A. Solubility of Acetaminophen and Ibuprofen in Binary and Ternary Mixtures of Polyethylene Glycol 600, Ethanol and Water. Chem. Pharm. Bull. 2010, 58, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Soltanpour, S.; Nazemi, V. Solubility of Ketoconazole in Binary and Ternary Solvents of Polyethylene Glycols 200, 400 or 600 with Ethanol and Water at 298.2 K. Data Report and Analysis. J. Solut. Chem. 2018, 47, 65–79. [Google Scholar] [CrossRef]
- Soltanpour, S.; Shekarriz, A.-H. Naproxen solubility in binary and ternary solvents of polyethylene glycols 200, 400 or 600 with ethanol and water at 298.2 K—Experimental data report and modelling. Phys. Chem. Liq. 2015, 53, 748–762. [Google Scholar] [CrossRef]
- Solymosi, T.; Tóth, F.; Orosz, J.; Basa-Dénes, O.; Angi, R.; Jordán, T.; Ötvös, Z.; Glavinas, H. Solubility Measurements at 296 and 310 K and Physicochemical Characterization of Abiraterone and Abiraterone Acetate. J. Chem. Eng. Data 2018, 12, 4453–4458. [Google Scholar] [CrossRef]
- Sun, H.; Liu, B.; Liu, P.; Zhang, J.; Wang, Y. Solubility of Fenofibrate in Different Binary Solvents: Experimental Data and Results of Thermodynamic Modeling. J. Chem. Eng. Data 2016, 61, 3177–3183. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Fang, Z.; Mao, S.; Zhang, L.; Rohani, S.; Lu, J. Solubility Measurement and Simulation of Rivaroxaban (Form I) in Solvent Mixtures from 273.15 to 323.15 K. J. Chem. Eng. Data 2015, 61, 495–503. [Google Scholar] [CrossRef]
- Swinerd, G.G. Orbital Mechanics: Theory and Applications; Logsdon, T., Ed.; John Wiley and Sons Limited: Chichester, UK, 1998. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Z.; Feng, Y.; Xie, C.; Wang, J.; Yang, C.; Gong, J. Experimental Determination and Computational Prediction of Androstenedione Solubility in Alcohol + Water Mixtures. Ind. Eng. Chem. Res. 2014, 53, 11538–11549. [Google Scholar] [CrossRef]
- Tang, W.; Xie, C.; Wang, Z.; Wu, S.; Feng, Y.; Wang, X.; Wang, J.; Gong, J. Solubility of androstenedione in lower alcohols. Fluid Phase Equilibria 2014, 363, 86–96. [Google Scholar] [CrossRef]
- Teutenberg, T.; Wiese, S.; Wagner, P.; Gmehling, J. High-temperature liquid chromatography. Part II: Determination of the viscosities of binary solvent mixtures—Implications for liquid chromatographic separations. J. Chromatogr. A 2009, 1216, 8470–8479. [Google Scholar] [CrossRef]
- Thati, J.; Nordström, F.L.; Rasmuson, Å.C. Solubility of Benzoic Acid in Pure Solvents and Binary Mixtures. J. Chem. Eng. Data 2010, 55, 5124–5127. [Google Scholar] [CrossRef]
- Torres, N.; Escalera, B.; Martínez, F.; Peña, M.Á. Thermodynamic Analysis of Etoricoxib in Amphiprotic and Amphiprotic: Aprotic Solvent Mixtures at Several Temperatures. J. Solut. Chem. 2020, 49, 272–288. [Google Scholar] [CrossRef]
- Valavi, M.; Ukrainczyk, M.; Dehghani, M.R. Prediction of solubility of active pharmaceutical ingredients by semi- predictive Flory Huggins/Hansen model. J. Mol. Liq. 2017, 246, 166–172. [Google Scholar] [CrossRef]
- Vargas-Santana, M.S.; Cruz-González, A.M.; Ortiz, C.P.; Delgado, D.R.; Martínez, F.; Peña, M.Á.; Acree, W.E.; Jouyban, A. Solubility of sulfamerazine in (ethylene glycol + water) mixtures: Measurement, correlation, dissolution thermodynamics and preferential solvation. J. Mol. Liq. 2021, 337, 116330. [Google Scholar] [CrossRef]
- Vieira, A.W.; Molina, G.; Mageste, A.B.; Rodrigues, G.D.; de Lemos, L.R. Partitioning of salicylic and acetylsalicylic acids by aqueous two-phase systems: Mechanism aspects and optimization study. J. Mol. Liq. 2019, 296, 111775. [Google Scholar] [CrossRef]
- Volkova, T.V.; Levshin, I.B.; Perlovich, G.L. New antifungal compound: Solubility thermodynamics and partitioning processes in biologically relevant solvents. J. Mol. Liq. 2020, 310, 113148. [Google Scholar] [CrossRef]
- Wang, H.; Yao, G.; Zhang, H. Measurement and Correlation of the Solubility of Baicalin in Several Mixed Solvents. J. Chem. Eng. Data 2019, 64, 1281–1287. [Google Scholar] [CrossRef]
- Wang, S.; Chen, N.; Qu, Y. Solubility of Florfenicol in Different Solvents at Temperatures from (278 to 318) K. J. Chem. Eng. Data 2011, 56, 638–641. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Gong, T.; Dong, W.; Wang, G.; Li, H.; Wu, S. Solid-liquid equilibrium behavior and thermodynamic analysis of dipyridamole in pure and binary solvents from 293.15 K to 328.15 K. J. Mol. Liq. 2019, 275, 8–17. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.-S.; Su, M.-G. Solubility of 1H-1,2,4-Triazole in Ethanol, 1-Propanol, 2-Propanol, 1,2-Propanediol, Ethyl Formate, Methyl Acetate, Ethyl Acetate, and Butyl Acetate at (283 to 363) K. J. Chem. Eng. Data 2007, 52, 856–858. [Google Scholar] [CrossRef]
- Wang, S.; Qin, L.; Zhou, Z.; Wang, J. Solubility and Solution Thermodynamics of Betaine in Different Pure Solvents and Binary Mixtures. J. Chem. Eng. Data 2012, 57, 2128–2135. [Google Scholar] [CrossRef]
- Wang, S.; Song, Z.; Wang, J.; Dong, Y.; Wu, M. Solubilities of Ibuprofen in Different Pure Solvents. J. Chem. Eng. Data 2010, 55, 5283–5285. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Wang, J. Solubility Measurement and Modeling for Betaine in Different Pure Solvents. J. Chem. Eng. Data 2014, 59, 2511–2516. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Liu, S.; Chen, Y.; Jia, L.; Wu, S. Thermodynamic Study of Solubility for Imatinib Mesylate in Nine Monosolvents and Two Binary Solvent Mixtures from 278.15 to 318.15 K. J. Chem. Eng. Data 2018, 63, 4114–4127. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Z.; Xu, X.; Yang, A.; Luo, W.; Luo, Y. Solubility of benzoic acid in twelve organic solvents: Experimental measurement and thermodynamic modeling. J. Chem. Thermodyn. 2020, 150, 106234. [Google Scholar] [CrossRef]
- Watterson, S.; Hudson, S.; Svärd, M.; Rasmuson, Å.C. Thermodynamics of fenofibrate and solubility in pure organic solvents. Fluid Phase Equilibria 2014, 367, 143–150. [Google Scholar] [CrossRef]
- Wei, H.; Gao, N.; Dang, L. Solubility and Thermodynamic Properties of Sulfamethazine–Saccharin Cocrystal in Pure and Binary (Acetonitrile + 2-Propanol) Solvents. Trans. Tianjin Univ. 2020, 27, 460–472. [Google Scholar] [CrossRef]
- Wu, J.; Gu, L.; Wang, H.; Tao, L.; Wang, X. Solubility of Baicalein in Different Solvents from (287 to 323) K. Int. J. Thermophys. 2014, 35, 1465–1475. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y. Solubility and solution thermodynamics of isobutyramide in 15 pure solvents at temperatures from 273.15 to 324.75 K. J. Mol. Liq. 2020, 311, 113294. [Google Scholar] [CrossRef]
- Wu, S.; Shi, Y.; Zhang, H. Solubility Measurement and Correlation for Amrinone in Four Binary Solvent Systems at 278.15–323.15 K. J. Chem. Eng. Data 2020, 65, 4108–4115. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, M.; Zhang, X. Solubility Determination and Model Correlation of Benorilate between T = 278.18 and 318.15 K. J. Chem. Eng. Data 2020, 65, 3690–3695. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Yan, S.; Hu, B. Solubility of Bisacodyl in Pure Solvent at Various Temperatures: Data Correlation and Thermodynamic Property Analysis. J. Chem. Eng. Data 2019, 65, 43–48. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Wang, J.; Gao, J. Effect of Solvent Properties and Composition on the Solubility of Ganciclovir Form I. J. Chem. Eng. Data 2019, 64, 1501–1507. [Google Scholar] [CrossRef]
- Wüst Zibetti, A.; Aydi, A.; Claumann, C.A.; Eladeb, A.; Adberraba, M. Correlation of solubility and prediction of the mixing properties of rosmarinic acid in different pure solvents and in binary solvent mixtures of ethanol + water and methanol + water from (293.2 to 318.2) K. J. Mol. Liq. 2016, 216, 370–376. [Google Scholar] [CrossRef]
- Xia, Q.; Chen, S.-N.; Chen, Y.-S.; Zhang, M.-S.; Zhang, F.-B.; Zhang, G.-L. Solubility of decanedioic acid in binary solvent mixtures. Fluid Phase Equilibria 2011, 304, 105–109. [Google Scholar] [CrossRef]
- Xu, R.; Han, T.; Shen, L.; Zhao, J.; Lu, X.a. Solubility Determination and Modeling for Artesunate in Binary Solvent Mixtures of Methanol, Ethanol, Isopropanol, and Propylene Glycol + Water. J. Chem. Eng. Data 2019, 64, 755–762. [Google Scholar] [CrossRef]
- Marcus, Y. Preferential Solvation of Drugs in Binary Solvent Mixtures. Pharm. Anal. Acta 2017, 10, 4172. [Google Scholar] [CrossRef]
- Yan, M.; Li, X.; Tao, B.; Yang, L.; Luo, W. Solubility of succinic acid, glutaric acid and adipic acid in propionic acid + ε-caprolactone mixtures and propionic acid + cyclohexanone mixtures: Experimental measurement and thermodynamic modeling. J. Mol. Liq. 2018, 272, 106–119. [Google Scholar] [CrossRef]
- Yang, H.; Rasmuson, Å.C. Solubility of Butyl Paraben in Methanol, Ethanol, Propanol, Ethyl Acetate, Acetone, and Acetonitrile. J. Chem. Eng. Data 2010, 55, 5091–5093. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Xu, S.; Han, D.; Liu, S.; Yang, Y.; Du, S.; Li, M.; Gong, J. Measurement and Correlation of the Solubility of Azoxystrobin in Seven Monosolvents and Two Different Binary Mixed Solvents. J. Chem. Eng. Data 2017, 62, 3967–3980. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Cheng, J.; Yang, C. Solubility and thermodynamics of polymorphic indomethacin in binary solvent mixtures. J. Mol. Liq. 2019, 295, 111717. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, D.; Zhou, G. Analysis of solubility parameters of fenbendazole in pure and mixed solvents and evaluation of thermodynamic model. J. Chem. Thermodyn. 2020, 140, 105876. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, D.; Zhou, G. Solubility profile of imatinib in pure and mixed solvents and calculation of thermodynamic properties. J. Chem. Thermodyn. 2020, 144, 106031. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, D.; Zhou, G. Improvement of solubility and analysis thermodynamic properties of β tegafur in pure and mixed organic solvents. J. Chem. Thermodyn. 2020, 146, 106090. [Google Scholar] [CrossRef]
- Yaws, C.L. Physical Properties—Inorganic Compounds. In The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals; Elsevier: Amsterdam, The Netherlands, 2015; pp. 684–810. [Google Scholar]
- Yu, Q.; Ma, X.; Xu, L. Solubility, dissolution enthalpy and entropy of l-glutamine in mixed solvents of ethanol+water and acetone+water. Thermochim. Acta 2013, 558, 6–9. [Google Scholar] [CrossRef]
- Zadaliasghar, S.; Jouyban, A.; Martinez, F.; Barzegar-Jalali, M.; Rahimpour, E. Solubility of ketoconazole in the binary mixtures of 2-propanol and water at different temperatures. J. Mol. Liq. 2020, 300, 112259. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Li, B.-Y.; Wang, Y. Solubilities of Sulfadiazine in Methanol, Ethanol, 1-Propanol, 2-Propanol, Acetone, and Chloroform from (294.15 to 318.15) K. J. Chem. Eng. Data 2010, 55, 2338–2339. [Google Scholar] [CrossRef]
- Zhang, F. Commentary on the “Solubility of l-histidine in different aqueous binary solvent mixtures from 283.15 K to 318.15 K with experimental measurement and thermodynamic modelling”. J. Chem. Thermodyn. 2018, 124, 98–100. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, Q.; Liu, Z.; Gong, J.; Bao, Y.; Zhang, M.; Hao, H.; Hou, B.; Xie, C. Measurement and correlation of solubility of dodecanedioic acid in different pure solvents from T = (288.15 to 323.15)K. J. Chem. Thermodyn. 2014, 68, 270–274. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Chen, J.; Xu, R. Equilibrium Solubility Determination and Modeling of Fenbendazole in Cosolvent Mixtures at (283.15–328.15) K. J. Chem. Eng. Data 2019, 64, 4095–4102. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Xu, R. Solubility of Bifonazole in Four Binary Solvent Mixtures: Experimental Measurement and Thermodynamic Modeling. J. Chem. Eng. Data 2019, 64, 2641–2648. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, C.; Xu, R. Solubility Determination and Mathematical Modeling of Nicorandil in Several Aqueous Cosolvent Systems at Temperature Ranges of 278.15–323.15 K. J. Chem. Eng. Data 2020, 65, 4063–4070. [Google Scholar] [CrossRef]
- Zhang, J.; Song, X.; Xu, R. Solubility Determination and Modeling for Milrinone in Binary Solvent Mixtures of Ethanol, Isopropanol, Ethylene Glycol, and N,N-Dimethylformamide + Water. J. Chem. Eng. Data 2020, 65, 4100–4107. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Xu, R. Solubility Determination and Modeling for Tirofiban in Several Mixed Solvents at 278.15–323.15 K. J. Chem. Eng. Data 2020, 65, 4071–4078. [Google Scholar] [CrossRef]
- Zhang, N.; Li, S.; Yang, H.; Li, M.; Yang, Y.; Tang, W. Measurement and Correlation of the Solubility of Tetramethylpyrazine in Nine Monosolvents and Two Binary Solvent Systems. J. Chem. Eng. Data 2019, 64, 995–1006. [Google Scholar] [CrossRef]
- Zhang, P.; Sha, J.; Wan, Y.; Zhang, C.; Li, T.; Ren, B. Apparent thermodynamic analysis and the dissolution behavior of levamisole hydrochloride in three binary solvent mixtures. Thermochim. Acta 2019, 681, 178375. [Google Scholar] [CrossRef]
- Zhang, P.; Wan, Y.; Zhang, C.; Zhao, R.; Sha, J.; Li, Y.; Li, T.; Ren, B. Solubility and mixing thermodynamic properties of levamisole hydrochloride in twelve pure solvents at various temperatures. J. Chem. Thermodyn. 2019, 139, 105882. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Zhao, R.; Wan, Y.; Yang, Z.; He, R.; Chen, Q.; Li, T.; Ren, B. Measurement and Correlation of the Solubility of Florfenicol Form A in Several Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 2046–2055. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Hu, J.; Liu, M.; Cai, Z.; Xu, Y.; Sun, B. The solubilities of benzoic acid and its nitro-derivatives, 3-nitro and 3,5-dinitrobenzoic acids. J. Chem. Res. 2021, 45, 1100–1106. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, P.; Yin, Q.; Zhou, L. Measurement and Correlation of the Solubility of Florfenicol in Four Binary Solvent Mixtures from T = (278.15 to 318.15) K. Crystals 2022, 12, 1176. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, H.; Si, Z.; Zhang, X. Solubility and thermodynamics of l-hydroxyproline in water and (methanol, ethanol, n-propanol) binary solvent mixtures. J. Mol. Liq. 2020, 298, 112043. [Google Scholar] [CrossRef]
- Zorrilla-Veloz, R.I.; Stelzer, T.; López-Mejías, V. Measurement and Correlation of the Solubility of 5-Fluorouracil in Pure and Binary Solvents. J. Chem. Eng. Data 2018, 63, 3809–3817. [Google Scholar] [CrossRef]
- Milescu, R.A.; Zhenova, A.; Vastano, M.; Gammons, R.; Lin, S.; Lau, C.H.; Clark, J.H.; McElroy, C.R.; Pellis, A. Polymer Chemistry Applications of Cyrene and its Derivative Cygnet 0.0 as Safer Replacements for Polar Aprotic Solvents. ChemSusChem 2021, 14, 3367–3381. [Google Scholar] [CrossRef]
- Glass, M.; Aigner, M.; Viell, J.; Jupke, A.; Mitsos, A. Liquid-liquid equilibrium of 2-methyltetrahydrofuran/water over wide temperature range: Measurements and rigorous regression. Fluid Phase Equilibria 2017, 433, 212–225. [Google Scholar] [CrossRef]
- Dargo, G.; Kis, D.; Gede, M.; Kumar, S.; Kupai, J.; Szekely, G. MeSesamol, a bio-based and versatile polar aprotic solvent for organic synthesis and depolymerization. Chem. Eng. J. 2023, 471, 144365. [Google Scholar] [CrossRef]
- Komarova, A.O.; Dick, G.R.; Luterbacher, J.S. Diformylxylose as a new polar aprotic solvent produced from renewable biomass. Green Chem. 2021, 23, 4790–4799. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters,. pi.*,. alpha., and. beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of solute hydrogen-bonding: Their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1993, 22, 73–83. [Google Scholar] [CrossRef]
- Liu, X.; Acree, W.E.; Abraham, M.H. Descriptors for some compounds with pharmacological activity; calculation of properties. Int. J. Pharm. 2022, 617, 121597. [Google Scholar] [CrossRef]
- Vitha, M.; Carr, P.W. The chemical interpretation and practice of linear solvation energy relationships in chromatography. J. Chromatogr. A 2006, 1126, 143–194. [Google Scholar] [CrossRef]
- West, C.; Lesellier, E. Characterisation of stationary phases in subcritical fluid chromatography with the solvation parameter model: III. Polar stationary phases. J. Chromatogr. A 2006, 1110, 200–213. [Google Scholar] [CrossRef]
- Efimov, I.; Povarov, V.G.; Rudko, V.A. Comparison of UNIFAC and LSER Models for Calculating Partition Coefficients in the Hexane–Acetonitrile System Using Middle Distillate Petroleum Products as an Example. Ind. Eng. Chem. Res. 2022, 61, 9575–9585. [Google Scholar] [CrossRef]
- EPA. CompTox Chemicals Dashboard v2.3.0. 2023. Available online: https://comptox.epa.gov/dashboard/ (accessed on 31 December 2023).
- Hou, P.; Jolliet, O.; Zhu, J.; Xu, M. Estimate ecotoxicity characterization factors for chemicals in life cycle assessment using machine learning models. Environ. Int. 2020, 135, 105393. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Abranches, D.O.; Ferreira, A.M.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS to Predict Solvatochromic Parameters for Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 10240–10249. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Okura, T.; Pinheiro Rolemberg, M.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS to Predict Hansen Solubility Parameters. Ind. Eng. Chem. Res. 2022, 61, 15631–15638. [Google Scholar] [CrossRef]


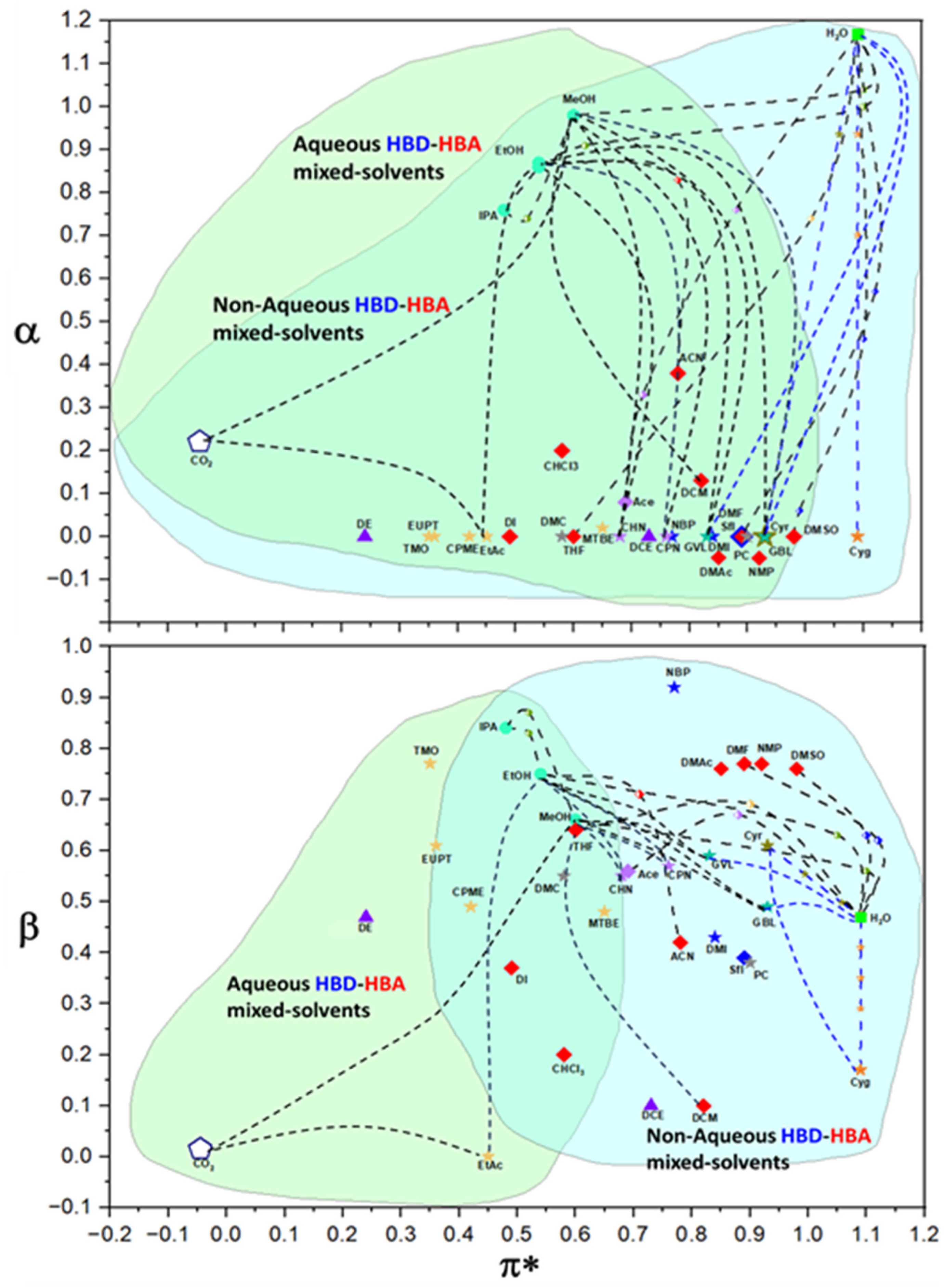
 ACN), γ-valerolactone (
ACN), γ-valerolactone ( GVL), γ-butyrolactone (
GVL), γ-butyrolactone ( GBL), tetrahydrofuran (
GBL), tetrahydrofuran ( THF), 1,4-dioxane (
THF), 1,4-dioxane ( DI), acetone (
DI), acetone ( Ace), pyridine (
Ace), pyridine ( PYR), N-methyl-2-pyrrolidone
(
PYR), N-methyl-2-pyrrolidone
( NMP), N,N-dimethylformamide
(
NMP), N,N-dimethylformamide
( DMF), N,N-dimethylacetamide
(
DMF), N,N-dimethylacetamide
( DMA), and dimethyl
sulfoxide (
DMA), and dimethyl
sulfoxide ( DMSO).
Reprinted with permission from [42]. Copyright American Chemical Society, 2017.
DMSO).
Reprinted with permission from [42]. Copyright American Chemical Society, 2017.
 ACN), γ-valerolactone (
ACN), γ-valerolactone ( GVL), γ-butyrolactone (
GVL), γ-butyrolactone ( GBL), tetrahydrofuran (
GBL), tetrahydrofuran ( THF), 1,4-dioxane (
THF), 1,4-dioxane ( DI), acetone (
DI), acetone ( Ace), pyridine (
Ace), pyridine ( PYR), N-methyl-2-pyrrolidone
(
PYR), N-methyl-2-pyrrolidone
( NMP), N,N-dimethylformamide
(
NMP), N,N-dimethylformamide
( DMF), N,N-dimethylacetamide
(
DMF), N,N-dimethylacetamide
( DMA), and dimethyl
sulfoxide (
DMA), and dimethyl
sulfoxide ( DMSO).
Reprinted with permission from [42]. Copyright American Chemical Society, 2017.
DMSO).
Reprinted with permission from [42]. Copyright American Chemical Society, 2017.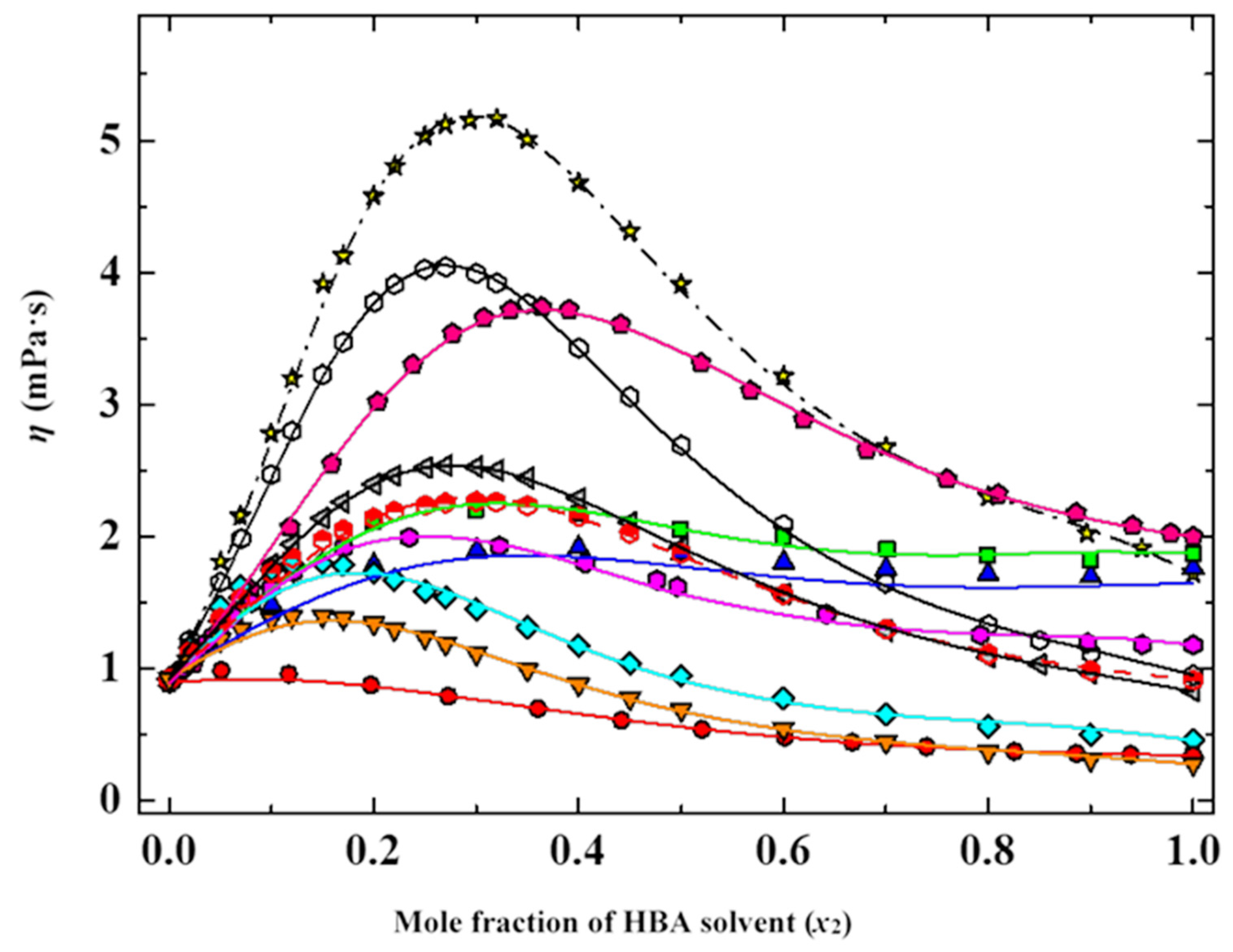
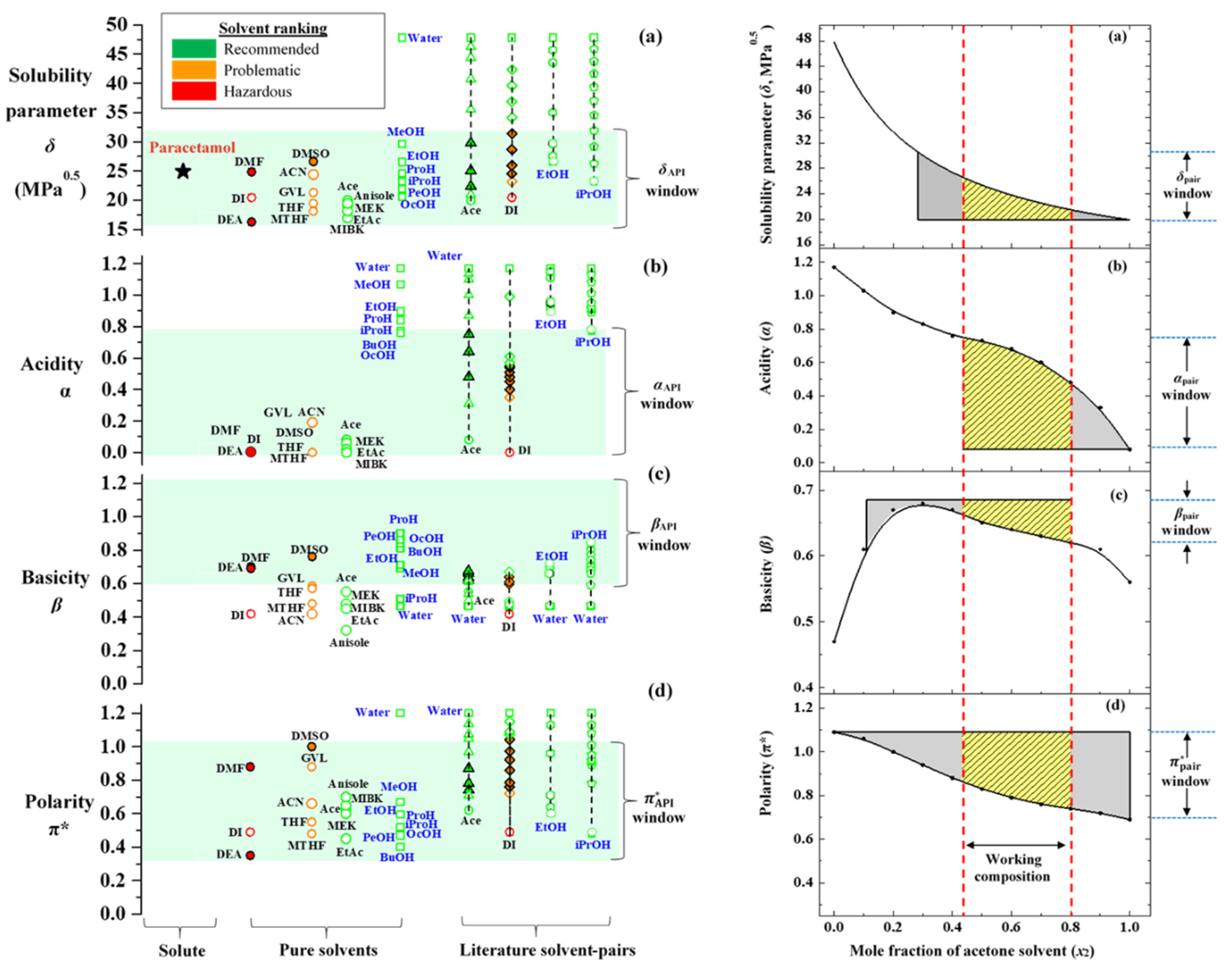

| Chemical (CAS No.) | LD50 (mg/kg) | Chemical (CAS No.) | LD50 (mg/kg) |
|---|---|---|---|
| Carcinogenic | Respiratory Sensitizing | ||
| 1,2,3-trichloropropane (96-18-4) | 120 | cis-cyclohexane-1,2-dicarboxylic anhydride (13149-00-3) | - |
| 1,2-dichloroethane (107-06-2) | 670 | Cyclohexane-1,2-dicarboxylic anhydride (85-42-7) | 958 |
| 1,4-dioxane (123-91-1) (DI) | 1550 | Glutaral (111-30-8) | 134 |
| 2,4-dinitrotoluene (121-14-2) | 268 | Toxic to Reproduction | |
| 4,4′-Diaminodiphenylmethane (101-77-9) | 120 | 1-Methyl-2-pyrrolidone (NMP) (872-50-4) | 3914 |
| 4-aminoazobenzene (60-09-3) | 200 | 1-vinylimidazole (1072-63-5) | 180 |
| Acrylamide (79-06-1) | 170 | 2-ethoxyethanol (110-80-5) | 2125 |
| Anthracene oil (90640-80-5) | 2000 d | 2-ethoxyethyl acetate (111-15-9) | 2700 |
| Biphenyl-4-ylamine (92-67-1) | 205 | 2-methoxyethanol (109-86-4) | 2370 |
| Chrysene (218-01-9) | 320 | 2-methoxyethyl acetate (110-49-6) | 2900 |
| Furan (110-00-9) | 5.2 | 2-methylimidazole (693-98-1) | 1400 |
| Propylene oxide (75-56-9) | 1245 d | 4,4′-sulphonyldiphenol (80-09-1) | 4556 |
| N-(hydroxymethyl)acrylamide (924-42-5) | 474 | Dibutyl phthalate (84-74-2) (DBP) | 7499 |
| o-aminoazotoluene (97-56-3) | 300 (dog) | Dicyclohexyl phthalate (84-61-7) | 30 |
| o-toluidine (95-53-4) | 670 | Dihexyl phthalate (84-75-3) | 29,600 |
| Phenolphthalein (77-09-8) | >1 | Diisobutyl phthalate (84-69-5) | 15 |
| Potassium dichromate (7778-50-9) | 25 | Diisohexyl phthalate (71850-09-4) | - |
| Trichloroethylene (79-01-6) | 1282 | Diisopentyl phthalate (605-50-5) | 2000 |
| Endocrine disruptor | Dioctyltin dilaurate (3648-18-8) | 6450 | |
| 2-(isononylphenoxy)ethanol (85005-55-6) | - | Formamide (75-12-7) | 5577 |
| 4-(1-ethyl-1-methylhexyl)phenol (52427-13-1) | - | Methoxyacetic acid (625-45-6) | 1000 |
| 4,4′-(1-methylpropylidene)bisphenol (77-40-7) | 500.1 | N,N-dimethylformamide (68-12-2) (DMF) | 2800 |
| 4-tert-butylphenol (98-54-4) | 2951 | Nitrobenzene (98-95-3) | 349 |
| Isobutyl 4-hydroxybenzoate (4247-02-3) | 2600 | N-methylacetamide (79-16-3) | 5 |
| Nonylphenol (25154-52-3) | 1200 | n-pentyl-isopentyl phthalate (776297-69-9) | - |
| Nonylphenol, ethoxylated (9016-45-9) | 1300 | Perfluoroheptanoic acid (375-85-9) | 500 |
| Human health effects | Phenol, 4-dodecyl, branched (210555-94-5) | 2000 | |
| Melamine (108-78-1) | 3161 | Phenol, tetrapropylene- (57427-55-1) | 2000 |
| Persistent, Bioaccumulative and Toxic (PBT) | Very Persistent, Very Bioaccumulative (vPvB) | ||
| Alkanes, C14-16, chloro (1372804-76-6) | 23 | Phenanthrene (85-01-8) | 700 |
| Anthracene (120-12-7) | >17 | Terphenyl, hydrogenated (61788-32-7) | 17,500 |
| Dodecamethylcyclohexasiloxane (540-97-6) | >50 | ||
| Octamethylcyclotetrasiloxane (556-67-2) | 1540 | ||
| Pyrene (129-00-0) | 2700 | ||
| Reaction | Unsafe Dipolar Aprotics | Replacement Solvents |
|---|---|---|
| Amide formation | DCM; DMF | Cyrene; surfactant-water |
| Boc deprotection | DI | HCl in CPME; TFA in PC |
| Borylation chemistry | DI | 2-MeTHF:MeOH (1:1); CPME; MTBE; CH |
| Buchwald—Hartwig amination | DI | 2-MeTHF; tBuOH |
| Carbonylation | THF; DE | DMC |
| Carboxylation | THF; DE | 2-MeTHF; DMI; DMC |
| C-H activation | THF; DMF; DI | 2-MeTHF; CH |
| Mizoroki—Heck cross-coupling | DI; THF; DMF | NBP; DMI; PC |
| Nucleophilic aromatic substitution | THF; DMF; DI | 2-MeTHF; PEG-400 |
| Organometallic reaction | R-MgX; R-Li; hydrides | 2-MeTHF; CPME |
| Solid-phase peptide synthesis | DMF; DMAc; NMP | NBP; GVL |
| Sonogashira cross-coupling | THF; DMF | Cyrene; NBP; DMI; Eucalyptol |
| Steglich Esterification | DMF | DMC |
| Suzuki-Miyaura cross-coupling | DI; THF; DMF | Cyrene; NBP; DMI; 2-MeTHF |
| Urea synthesis | DMF; THF | Cyrene |
| Natural Source | Co-Extractant | Bio-Product | T (°C) | P (MPa) | %Yield | Ref. |
|---|---|---|---|---|---|---|
| Algae | Soybean oil | astaxanthin | 70 | 40 | 36 | [57] |
| Brown seaweed | Sunflower oil | carotenoids | 50 | 30 | 99 | [58] |
| Carrots | Canola oil | carotenoids | 70 | 55 | 92 | [59] |
| Mangosteen | Virgin coconut oil | xanthonoids | 70 | 43 | 31 | [60] |
| Mangosteen | Virgin coconut oil | α-mangostin | 60 | 35 | 76 | [61] |
| Marigold | Medium-chain TAGs | lutein esters | 65 | 43 | 98 | [62] |
| Marigold | Soybean oil | lutein esters | 53 | 30 | 93 | [63] |
| Propolis | Virgin coconut oil | flavonoids | 50 | 15 | 25 | [64] |
| Pumpkin | Olive oil | carotenoid | 50 | 25 | 41 | [65] |
| Red sage | Peanut oil | diterpenoids | 50 | 38 | 90 | [66] |
| Tomato | Canola oil | lycopene | 40 | 40 | 86 | [67] |
| Tomato | Hazelnut oil | lycopene | 66 | 45 | 40 | [68] |
| Tomato skin | Olive oil | lycopene | 75 | 35 | 58 | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.L.; Chong, G.H.; Ota, M.; Guo, H.; Smith, R.L., Jr. Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review. Liquids 2024, 4, 352-381. https://doi.org/10.3390/liquids4020018
Lee JL, Chong GH, Ota M, Guo H, Smith RL Jr. Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review. Liquids. 2024; 4(2):352-381. https://doi.org/10.3390/liquids4020018
Chicago/Turabian StyleLee, Jia Lin, Gun Hean Chong, Masaki Ota, Haixin Guo, and Richard Lee Smith, Jr. 2024. "Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review" Liquids 4, no. 2: 352-381. https://doi.org/10.3390/liquids4020018
APA StyleLee, J. L., Chong, G. H., Ota, M., Guo, H., & Smith, R. L., Jr. (2024). Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review. Liquids, 4(2), 352-381. https://doi.org/10.3390/liquids4020018









