CRISPR-Cas Systems: Bridging Bacterial Immunity and Host Interactions
Abstract
1. Introduction
1.1. Overview of CRISPR-Cas Systems
1.2. Historical Context
1.2.1. Evolutionary Development in Bacteria and Archaea
1.2.2. From Microbial Immunity to Genome Editing
2. CRISPR Role in Host-Pathogen Interaction
2.1. Adaptive Immunity Against Foreign Genetic Elements
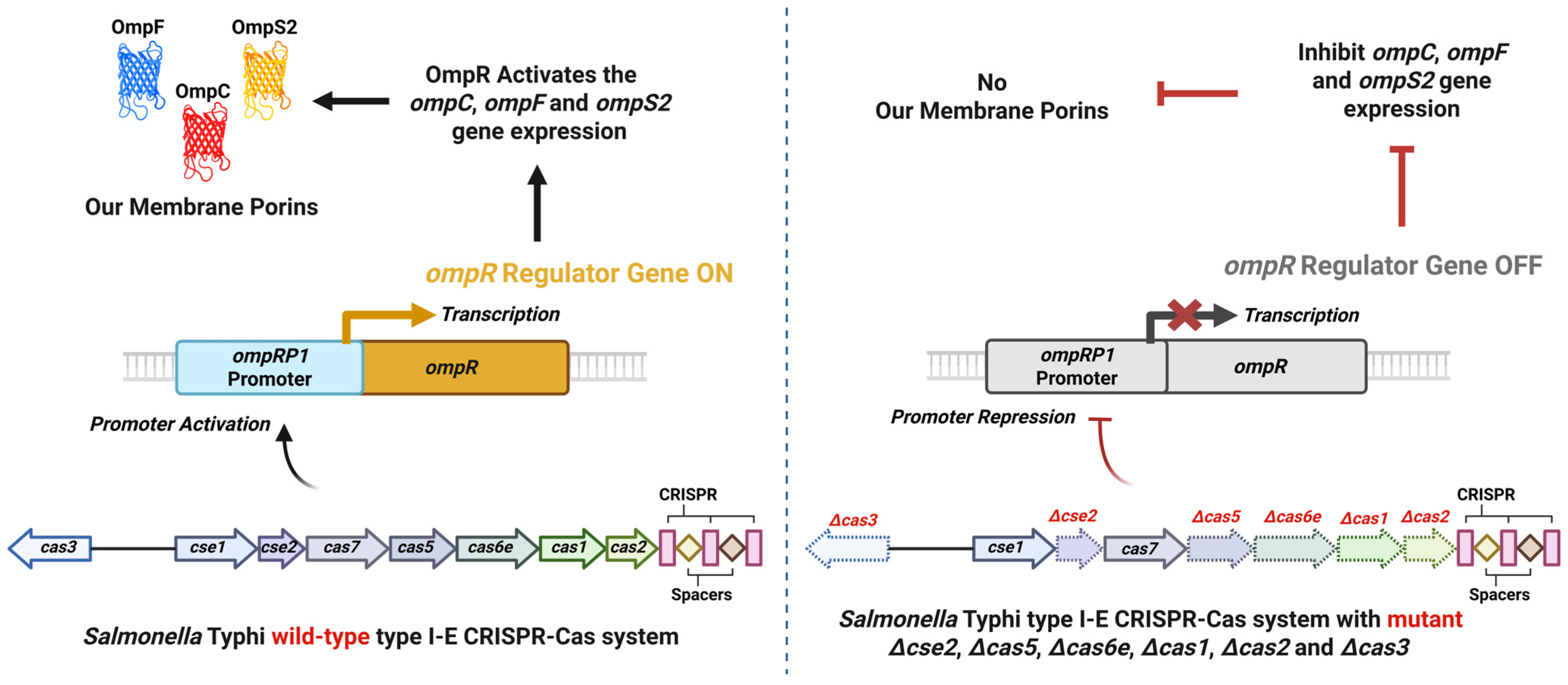
2.2. Regulation of Endogenous Gene Expression
2.3. Influence on Biofilm Formation
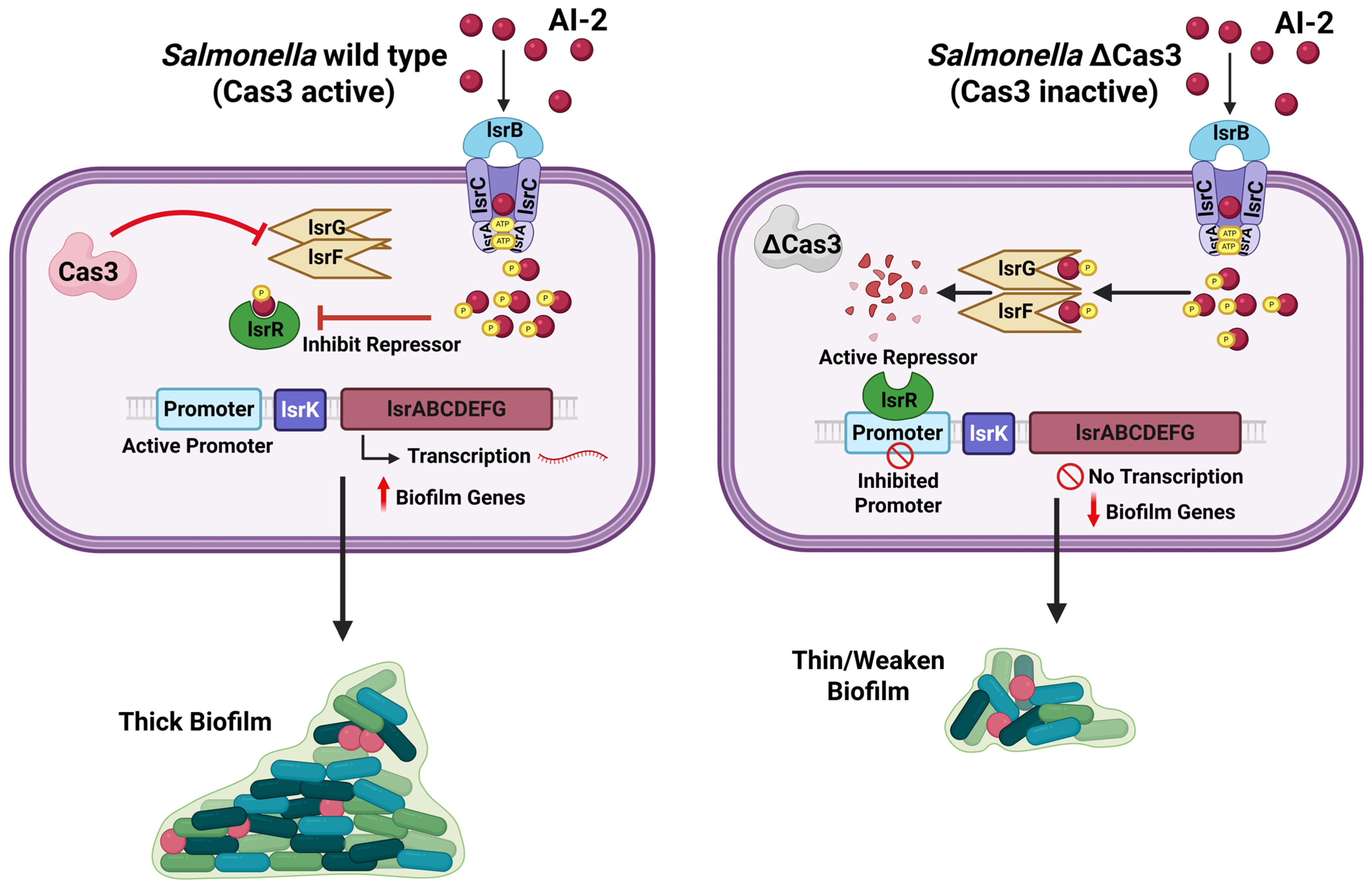
2.4. Interaction with Quorum-Sensing Mechanisms
2.5. Modulation of Virulence Factors
2.6. Evasion of Host Immune Responses
3. Host Defense Against Horizontal Gene Transfer (HGT)
3.1. CRISPR-Cas Systems as Barriers to HGT
3.2. Prevention of Lysogenic Conversion
3.3. Influence on Antibiotic Resistance Spread
3.4. Balancing Genetic Diversity and Stability
3.5. Acquisition of CRISPR-Cas Systems via HGT
4. Future Directions in CRISPR-Cas Research and Host Interaction
4.1. Expanding CRISPR Applications Beyond Immunity
4.2. Development of CRISPR-Based Antimicrobials
4.3. Understanding CRISPR–Host Co-Evolution
4.4. CRISPR-Cas in Host–Microbiome Engineering
4.5. Synthetic Biology and CRISPR Innovations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| Cas | CRISPR-associated protein |
| AI-2 | Autoinducer-2 (universal quorum-sensing molecule) |
| AHL | Acyl-homoserine lactone |
| BLP | Bacterial lipoprotein |
| EPS | Extracellular polymeric substances (biofilm matrix) |
| MGE | Mobile genetic element |
| QS | Quorum sensing |
| ROS | Reactive oxygen species |
| SPI-1 | Salmonella pathogenicity island 1 |
| TLR2 | Toll-like receptor 2 |
| TLR4 | Toll-like receptor 4 |
| WT | Wild type |
References
- Barrangou, R. Diversity of CRISPR-Cas Immune Systems and Molecular Machines. Genome Biol. 2015, 16, 247. [Google Scholar] [CrossRef]
- Yuan, B.; Yuan, C.; Li, L.; Long, M.; Chen, Z. Application of the CRISPR/Cas System in Pathogen Detection: A Review. Molecules 2022, 27, 6999. [Google Scholar] [CrossRef]
- Mengstie, M.A.; Wondimu, B.Z. Mechanism and Applications of Crispr/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Kumar, P.; Malik, Y.S.; Ganesh, B.; Rahangdale, S.; Saurabh, S.; Natesan, S.; Srivastava, A.; Sharun, K.; Yatoo, M.I.; Tiwari, R.; et al. CRISPR-Cas System: An Approach With Potentials for COVID-19 Diagnosis and Therapeutics. Front. Cell. Infect. Microbiol. 2020, 10, 576875. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Li, R.; Qiu, X.; Fan, T.; Wang, B.; Zhang, B.; Zhang, Y. Advances in Application of CRISPR-Cas13a System. Front. Cell. Infect. Microbiol. 2024, 14, 1291557. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Chen, H.; Li, N.; Liang, W. The Application of the CRISPR-Cas System in Antibiotic Resistance. Infect. Drug Resist. 2022, 15, 4155–4168. [Google Scholar] [CrossRef] [PubMed]
- Sinkunas, T.; Gasiunas, G.; Waghmare, S.P.; Dickman, M.J.; Barrangou, R.; Horvath, P.; Siksnys, V. In Vitro Reconstitution of Cascade-Mediated CRISPR Immunity in Streptococcus Thermophilus. EMBO J. 2013, 32, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Amlinger, L.; Rath, A.; Lundgren, M. The CRISPR-Cas Immune System: Biology, Mechanisms and Applications. Biochimie 2015, 117, 119–128. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary Classification of CRISPR–Cas Systems: A Burst of Class 2 and Derived Variants. Nat. Rev. Microbiol. 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Murugan, K.; Babu, K.; Sundaresan, R.; Rajan, R.; Sashital, D.G. The Revolution Continues: Newly Discovered Systems Expand the CRISPR-Cas Toolkit. Mol. Cell 2017, 68, 15–25. [Google Scholar] [CrossRef]
- Pinilla-Redondo, R.; Russel, J.; Mayo-Muñoz, D.; Shah, S.A.; Garrett, R.A.; Nesme, J.; Madsen, J.S.; Fineran, P.C.; Sørensen, S.J. CRISPR-Cas Systems Are Widespread Accessory Elements across Bacterial and Archaeal Plasmids. Nucleic Acids Res. 2022, 50, 4315–4328. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.J.; Charpentier, E.; Haft, D.H.; et al. An Updated Evolutionary Classification of CRISPR-Cas Systems. Nat. Rev. Microbiol. 2015, 13, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Kolesnik, M.V.; Fedorova, I.; Karneyeva, K.A.; Artamonova, D.N.; Severinov, K.V. Type III CRISPR-Cas Systems: Deciphering the Most Complex Prokaryotic Immune System. Biochemistry 2021, 86, 1301–1314. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S. Origins and Evolution of CRISPR-Cas Systems. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180087. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Weissman, J.L.; Johnson, P.L.F. Ecological Drivers of CRISPR Immune Systems. mSystems 2024, 9, e0056824. [Google Scholar] [CrossRef]
- Antequera-Zambrano, L.; Parra-Sánchez, Á.; González-Paz, L.; Fernandez, E.; Martinez-Navarrete, G. Distribution of Genetic Determinants Associated with CRISPR-Cas Systems and Resistance to Antibiotics in the Genomes of Archaea and Bacteria. Microorganisms 2025, 13, 1321. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.; Liu, Y.; Koonin, E.V.; Severinov, K.; Prangishvili, D.; Krupovic, M. Virus-Borne Mini-CRISPR Arrays Are Involved in Interviral Conflicts. Nat. Commun. 2019, 10, 5204. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity One-Sentence Summary. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-Programmed Genome Editing in Human Cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef]
- Jia, F.; Li, X.; Zhang, C.; Tang, X. The Expanded Development and Application of CRISPR System for Sensitive Nucleotide Detection. Protein Cell 2020, 11, 624–629. [Google Scholar] [CrossRef]
- Saeed, K.; Ayub, F.; Durrani, M.A.; Mujahid, M. CRISPR Cas Systems: From Bacterial Defense Mechanisms to Revolutionary Tools Reshaping Genetic Research and Translation Therapeutics. Microbe 2025, 7, 100344. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR Technology: A Decade of Genome Editing Is Only the Beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef]
- Loureiro, A.; Da Silva, G.J. Crispr-Cas: Converting a Bacterial Defence Mechanism into a State-of-the-Art Genetic Manipulation Tool. Antibiotics 2019, 8, 18. [Google Scholar] [CrossRef]
- Li, Y.; Peng, N. Endogenous CRISPR-Cas System-Based Genome Editing and Antimicrobials: Review and Prospects. Front. Microbiol. 2019, 10, 2471. [Google Scholar] [CrossRef]
- Crawley, A.B.; Henriksen, E.D.; Stout, E.; Brandt, K.; Barrangou, R. Characterizing the Activity of Abundant, Diverse and Active CRISPR-Cas Systems in Lactobacilli. Sci. Rep. 2018, 8, 11544. [Google Scholar] [CrossRef] [PubMed]
- Pyenson, N.C.; Marraffini, L.A. Co-Evolution within Structured Bacterial Communities Results in Multiple Expansion of CRISPR Loci and Enhanced Immunity. eLife 2020, 9, e53078. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.; Parameshwaran, H.P.; Martin, L.; Rajan, R. The CRISPR-Cas Mechanism for Adaptive Immunity and Alternate Bacterial Functions Fuels Diverse Biotechnologies. Front. Cell. Infect. Microbiol. 2021, 10, 619763. [Google Scholar] [CrossRef] [PubMed]
- Haider, D.; Bauer, R.; Grempels, A.; Roscher, R.; Aslan, C.C.; Mauerer, S.; Spellerberg, B. The Stress of Carrying CRISPR-Cas. Virulence 2025, 16, 2541701. [Google Scholar] [CrossRef]
- Louwen, R.; Staals, R.H.J.; Endtz, H.P.; van Baarlen, P.; van der Oost, J. The Role of CRISPR-Cas Systems in Virulence of Pathogenic Bacteria. Microbiol. Mol. Biol. Rev. 2014, 78, 74–88. [Google Scholar] [CrossRef]
- Medina-Aparicio, L.; Rodriguez-Gutierrez, S.; Rebollar-Flores, J.E.; Martínez-Batallar, Á.G.; Mendoza-Mejía, B.D.; Aguirre-Partida, E.D.; Vázquez, A.; Encarnación, S.; Calva, E.; Hernández-Lucas, I. The CRISPR-Cas System Is Involved in OmpR Genetic Regulation for Outer Membrane Protein Synthesis in Salmonella Typhi. Front. Microbiol. 2021, 12, 657404. [Google Scholar] [CrossRef]
- Ratner, H.K.; Weiss, D.S. Francisella novicida CRISPR-Cas Systems Can Functionally Complement Each Other in DNA Defense While Providing Target Flexibility. J. Bacteriol. 2020, 202, e00670-19. [Google Scholar] [CrossRef] [PubMed]
- Nyerges, Á.; Bálint, B.; Cseklye, J.; Nagy, I.; Pál, C.; Feher, T. CRISPR-Interference-Based Modulation of Mobile Genetic Elements in Bacteria. Synth. Biol. 2019, 4, ysz008. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Sun, X.; Li, M.; Zhang, P.; Zhu, Z.; Jiao, H.; Guo, T.; Li, G. CRISPR-Cas in Acinetobacter baumannii Contributes to Antibiotic Susceptibility by Targeting Endogenous AbaI. Microbiol. Spectr. 2022, 10, e0082922. [Google Scholar] [CrossRef]
- Sampson, T.R.; Weiss, D.S. CRISPR-Cas Systems: New Players in Gene Regulation and Bacterial Physiology. Front. Cell. Infect. Microbiol. 2014, 4, 37. [Google Scholar] [CrossRef]
- Guo, T.; Yang, J.; Zhou, N.; Sun, X.; Huan, C.; Lin, T.; Bao, G.; Hu, J.; Li, G. Cas3 of Type I-Fa CRISPR-Cas System Upregulates Bacterial Biofilm Formation and Virulence in Acinetobacter baumannii. Commun. Biol. 2025, 8, 750. [Google Scholar] [CrossRef]
- Sampson, T.R.; Saroj, S.D.; Llewellyn, A.C.; Tzeng, Y.L.; Weiss, D.S. A CRISPR/Cas System Mediates Bacterial Innate Immune Evasion and Virulence. Nature 2013, 497, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Weiss, D.S. Alternative Roles for CRISPR/Cas Systems in Bacterial Pathogenesis. PLoS Pathog. 2013, 9, e1003621. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fang, L.; Tan, S.; Yu, M.; Li, X.; He, S.; Wei, Y.; Li, G.; Jiang, J.; Wu, M. Type I CRISPR-Cas Targets Endogenous Genes and Regulates Virulence to Evade Mammalian Host Immunity. Cell Res. 2016, 26, 1273–1287. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Huang, D.; Zhao, Y.; Feng, J.; Lu, Q.; Pu, Q.; Wang, Y.; Cheng, G.; Wu, M.; et al. CRISPR-Cas3 of Salmonella Upregulates Bacterial Biofilm Formation and Virulence to Host Cells by Targeting Quorum-Sensing Systems. Pathogens 2020, 9, 53. [Google Scholar] [CrossRef]
- Gao, N.J.; Al-Bassam, M.M.; Poudel, S.; Wozniak, J.M.; Gonzalez, D.J.; Olson, J.; Zengler, K.; Nizet, V.; Valderrama, J.A. Functional and Proteomic Analysis of Streptococcus pyogenes Virulence upon Loss of Its Native Cas9 Nuclease. Front. Microbiol. 2019, 10, 1967. [Google Scholar] [CrossRef]
- Dugar, G.; Leenay, R.T.; Eisenbart, S.K.; Bischler, T.; Aul, B.U.; Beisel, C.L.; Sharma, C.M. CRISPR RNA-Dependent Binding and Cleavage of Endogenous RNAs by the Campylobacter jejuni Cas9. Mol. Cell 2018, 69, 893–905.e7. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Tang, Y.; Xu, Z.; Lin, M.; Cheng, G.; Dai, M.; Wang, X.; Liu, Z.; Yuan, Z.; Hao, H. The Involvement of the Cas9 Gene in Virulence of Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2018, 8, 285. [Google Scholar] [CrossRef]
- Tang, B.; Gong, T.; Zhou, X.; Lu, M.; Zeng, J.; Peng, X.; Wang, S.; Li, Y. Deletion of Cas3 Gene in Streptococcus mutans Affects Biofilm Formation and Increases Fluoride Sensitivity. Arch. Oral Biol. 2019, 99, 190–197. [Google Scholar] [CrossRef]
- Spencer, B.L.; Deng, L.; Patras, K.A.; Burcham, Z.M.; Sanches, G.F.; Nagao, P.E.; Doran, K.S. Cas9 Contributes to Group b Streptococcal Colonization and Disease. Front. Microbiol. 2019, 10, 1930. [Google Scholar] [CrossRef] [PubMed]
- Hickok, N.J. What Are Biofilms? Spine 2018, 43, S7–S8. [Google Scholar]
- Afrasiabi, S.; Partoazar, A. Targeting Bacterial Biofilm-Related Genes with Nanoparticle-Based Strategies. Front. Microbiol. 2024, 15, 1387114. [Google Scholar] [CrossRef]
- Sharma, N.; Das, A.; Raja, P.; Marathe, S.A. The CRISPR-Cas System Differentially Regulates Surface-Attached and Pellicle Biofilm in Salmonella enterica Serovar Typhimurium. Microbiol. Spectr. 2022, 10, e0020222. [Google Scholar] [CrossRef] [PubMed]
- Saffari Natanzi, A.; Poudineh, M.; Karimi, E.; Khaledi, A.; Haddad Kashani, H. Innovative Approaches to Combat Antibiotic Resistance: Integrating CRISPR/Cas9 and Nanoparticles against Biofilm-Driven Infections. BMC Med. 2025, 23, 486. [Google Scholar] [CrossRef]
- Bano, S.; Hassan, N.; Rafiq, M.; Hassan, F.; Rehman, M.; Iqbal, N.; Ali, H.; Hasan, F.; Kang, Y.Q. Biofilms as Battlefield Armor for Bacteria against Antibiotics: Challenges and Combating Strategies. Microorganisms 2023, 11, 2595. [Google Scholar] [CrossRef]
- Hooton, S.P.T.; Brathwaite, K.J.; Connerton, I.F. The Bacteriophage Carrier State of Campylobacter jejuni Features Changes in Host Non-Coding RNAs and the Acquisition of New Host-Derived CRISPR Spacer Sequences. Front. Microbiol. 2016, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Hooton, S.P.T.; Connerton, I.F. Campylobacter jejuni Acquire New Host-Derived CRISPR Spacers When in Association with Bacteriophages Harboring a CRISPR-like Cas4 Protein. Front. Microbiol. 2015, 6, 744. [Google Scholar] [CrossRef]
- Ali, Q.; Wahl, L.M. Mathematical Modelling of CRISPR-Cas System Effects on Biofilm Formation. J. Biol. Dyn. 2017, 11, 264–284. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, C.; Li, S.; Liang, S.; Xu, X.; Zhao, Z. Quorum Sensing Positively Regulates CPS-Dependent Autographiviridae Phage Infection in Vibrio Alginolyticus. Appl. Environ. Microbiol. 2024, 90, e0221023. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.G.; Jackson, S.A.; Taylor, C.; Evans, G.B.; Salmond, G.P.C.; Przybilski, R.; Staals, R.H.J.; Fineran, P.C. Quorum Sensing Controls Adaptive Immunity through the Regulation of Multiple CRISPR-Cas Systems. Mol. Cell 2016, 64, 1102–1108. [Google Scholar] [CrossRef]
- Devi, V.; Harjai, K.; Chhibber, S. CRISPR-Cas Systems: Role in Cellular Processes beyond Adaptive Immunity. Folia Microbiol. 2022, 67, 837–850. [Google Scholar] [CrossRef]
- Maura, D.; Hazan, R.; Kitao, T.; Ballok, A.E.; Rahme, L.G. Evidence for Direct Control of Virulence and Defense Gene Circuits by the Pseudomonas aeruginosa Quorum Sensing Regulator, MvfR. Sci. Rep. 2016, 6, 34083. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm Dispersion and Quorum Sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas Systems Target Endogenous Genes to Impact Bacterial Physiology and Alter Mammalian Immune Responses. Mol. Biomed. 2022, 3, 22. [Google Scholar] [CrossRef]
- Bourgogne, A.; Garsin, D.A.; Qin, X.; Singh, K.V.; Sillanpaa, J.; Yerrapragada, S.; Ding, Y.; Dugan-Rocha, S.; Buhay, C.; Shen, H.; et al. Large Scale Variation in Enterococcus faecalis Illustrated by the Genome Analysis of Strain OG1RF. Genome Biol. 2008, 9, R110. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Cao, Y.; Chen, X.; Tang, Y.; Zhou, X.; Ingmer, H.; Jiao, X.; Li, Q. Oxidative Stress Elicited by Phage Infection Induces Staphylococcal Type III-A CRISPR–Cas System. Nucleic Acids Res. 2025, 53, gkaf541. [Google Scholar] [CrossRef]
- Serbanescu, M.A.; Cordova, M.; Krastel, K.; Flick, R.; Beloglazova, N.; Latos, A.; Yakunin, A.F.; Senadheera, D.B.; Cvitkovitch, D.G. Role of the Streptococcus mutans CRISPR-Cas Systems in Immunity and Cell Physiology. J. Bacteriol. 2015, 197, 749–761. [Google Scholar] [CrossRef]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Emamalipour, M.; Seidi, K.; Zununi Vahed, S.; Jahanban-Esfahlan, A.; Jaymand, M.; Majdi, H.; Amoozgar, Z.; Chitkushev, L.T.; Javaheri, T.; Jahanban-Esfahlan, R.; et al. Horizontal Gene Transfer: From Evolutionary Flexibility to Disease Progression. Front. Cell Dev. Biol. 2020, 8, 229. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Staals, R.H.J.; Fineran, P.C. CRISPR-Cas-Mediated Phage Resistance Enhances Horizontal Gene Transfer by Transduction. mBio 2018, 9, e02406-17. [Google Scholar] [CrossRef]
- Faure, G.; Shmakov, S.A.; Yan, W.X.; Cheng, D.R.; Scott, D.A.; Peters, J.E.; Makarova, K.S.; Koonin, E.V. CRISPR–Cas in Mobile Genetic Elements: Counter-Defence and Beyond. Nat. Rev. Microbiol. 2019, 17, 513–525. [Google Scholar] [CrossRef]
- Liu, T.Y.; Doudna, J.A. Chemistry of Class 1 CRISPR-Cas Effectors: Binding, Editing, and Regulation. J. Biol. Chem. 2020, 295, 14473–14487. [Google Scholar] [CrossRef]
- Pfeifer, E.; Bonnin, R.A.; Rocha, E.P.C. Phage-Plasmids Spread Antibiotic Resistance Genes through Infection and Lysogenic Conversion. mBio 2022, 13, e01851-22. [Google Scholar] [CrossRef]
- Westra, E.R.; Levin, B.R. It Is Unclear How Important CRISPR-Cas Systems Are for Protecting Natural Populations of Bacteria against Infections by Mobile Genetic Elements. Proc. Natl. Acad. Sci. USA 2020, 117, 27777–27785. [Google Scholar] [CrossRef]
- Price, V.J.; McBride, S.W.; Hullahalli, K.; Chatterjee, A.; Duerkop, B.A.; Palmer, K.L. Enterococcus faecalis CRISPR-Cas Is a Robust Barrier to Conjugative Antibiotic Resistance Dissemination in the Murine Intestine. mSphere 2019, 4, e00464-19. [Google Scholar] [CrossRef]
- Sontheimer, E.J.; Davidson, A.R. Inhibition of CRISPR-Cas Systems by Mobile Genetic Elements. Curr. Opin. Microbiol. 2017, 37, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Zaayman, M.; Wheatley, R.M. Fitness Costs of CRISPR-Cas Systems in Bacteria. Microbiology 2022, 168, 001209. [Google Scholar] [CrossRef]
- Gummalla, V.S.; Zhang, Y.; Liao, Y.T.; Wu, V.C.H. The Role of Temperate Phages in Bacterial Pathogenicity. Microorganisms 2023, 11, 541. [Google Scholar] [CrossRef]
- Khambhati, K.; Bhattacharjee, G.; Gohil, N.; Dhanoa, G.K.; Sagona, A.P.; Mani, I.; Bui, N.L.; Chu, D.T.; Karapurkar, J.K.; Jang, S.H.; et al. Phage Engineering and Phage-Assisted CRISPR-Cas Delivery to Combat Multidrug-Resistant Pathogens. Bioeng. Transl. Med. 2023, 8, e10381. [Google Scholar] [CrossRef]
- Osuna, B.A.; Karambelkar, S.; Mahendra, C.; Christie, K.A.; Garcia, B.; Davidson, A.R.; Kleinstiver, B.P.; Kilcher, S.; Bondy-Denomy, J. Listeria Phages Induce Cas9 Degradation to Protect Lysogenic Genomes. Cell Host Microbe 2020, 28, 31–40.e9. [Google Scholar] [CrossRef] [PubMed]
- Pasechnek, A.; Rabinovich, L.; Stadnyuk, O.; Azulay, G.; Mioduser, J.; Argov, T.; Borovok, I.; Sigal, N.; Herskovits, A.A. Active Lysogeny in Listeria monocytogenes Is a Bacteria-Phage Adaptive Response in the Mammalian Environment. Cell Rep. 2020, 32, 107956. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Steens, J.A.; Staals, R.H.J.; Westra, E.R.; van Houte, S. Coevolution between Bacterial CRISPR-Cas Systems and Their Bacteriophages. Cell Host Microbe 2021, 29, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Landsberger, M.; Gandon, S.; Meaden, S.; Rollie, C.; Chevallereau, A.; Chabas, H.; Buckling, A.; Westra, E.R.; van Houte, S. Anti-CRISPR Phages Cooperate to Overcome CRISPR-Cas Immunity. Cell 2018, 174, 908–916.e12. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. CRISPR-Cas-Based Antimicrobials: Design, Challenges, and Bacterial Mechanisms of Resistance. ACS Infect. Dis. 2023, 9, 1283–1302. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Wang, T.; Liang, W. The Spread of Antibiotic Resistance Genes In Vivo Model. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3348695. [Google Scholar] [CrossRef]
- Tao, S.; Chen, H.; Li, N.; Fang, Y.; Zhang, H.; Xu, Y.; Chen, L.; Liang, W. Elimination of Bla KPC–2-Mediated Carbapenem Resistance in Escherichia coli by CRISPR-Cas9 System. BMC Microbiol. 2023, 23, 310. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Price, V.J.; Sharifi, A.; Zhang, M.Q.; Palmer, K.L. Enterococcus faecalis Strains with Compromised CRISPR-Cas Defense Emerge under Antibiotic Selection for a CRISPR-Targeted Plasmid. Appl. Environ. Microbiol. 2023, 89, e0012423. [Google Scholar] [CrossRef] [PubMed]
- Pursey, E.; Sünderhauf, D.; Gaze, W.H.; Westra, E.R.; van Houte, S. CRISPR-Cas Antimicrobials: Challenges and Future Prospects. PLoS Pathog. 2018, 14, e1006990. [Google Scholar] [CrossRef]
- Kadkhoda, H.; Gholizadeh, P.; Samadi Kafil, H.; Ghotaslou, R.; Pirzadeh, T.; Ahangarzadeh Rezaee, M.; Nabizadeh, E.; Feizi, H.; Aghazadeh, M. Role of CRISPR-Cas Systems and Anti-CRISPR Proteins in Bacterial Antibiotic Resistance. Heliyon 2024, 10, e34692. [Google Scholar] [CrossRef]
- Zakrzewska, M.; Burmistrz, M. Mechanisms Regulating the CRISPR-Cas Systems. Front. Microbiol. 2023, 14, 1060337. [Google Scholar] [CrossRef]
- Gunawardana, W.; Kalupahana, R.S.; Kottawatta, S.A.; Gamage, A.; Merah, O. A Review of the Dissemination of Antibiotic Resistance through Wastewater Treatment Plants: Current Situation in Sri Lanka and Future Perspectives. Life 2024, 14, 1065. [Google Scholar] [CrossRef]
- Duan, C.; Cao, H.; Zhang, L.H.; Xu, Z. Harnessing the CRISPR-Cas Systems to Combat Antimicrobial Resistance. Front. Microbiol. 2021, 12, 716064. [Google Scholar] [CrossRef]
- Lam, T.J.; Mortensen, K.; Ye, Y. Diversity and Dynamics of the CRISPR-Cas Systems Associated with Bacteroides Fragilis in Human Population. BMC Genom. 2022, 23, 573. [Google Scholar] [CrossRef]
- McDonald, N.D.; Regmi, A.; Morreale, D.P.; Borowski, J.D.; Fidelma Boyd, E. CRISPR-Cas Systems Are Present Predominantly on Mobile Genetic Elements in Vibrio Species. BMC Genom. 2019, 20, 105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas Systems for the Detection and Control of Antibiotic-Resistant Infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, H.; Su, Y.; Liu, S.; Xu, L.; Guo, Z.; Wu, J.; Cheng, C.; Feng, J. Horizontal Gene Transfer Contributes to Virulence and Antibiotic Resistance of Vibrio harveyi 345 Based on Complete Genome Sequence Analysis. BMC Genom. 2019, 20, 761. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, Y.; Liu, Z.; Dong, Z.; Xie, C.; Bravo, A.; Soberón, M.; Mahillon, J.; Sun, M.; Peng, D. The CRISPR-Cas Systems Were Selectively Inactivated during Evolution of Bacillus cereus Group for Adaptation to Diverse Environments. ISME J. 2020, 14, 1479–1493. [Google Scholar] [CrossRef]
- Wheatley, R.M.; MacLean, R.C. CRISPR-Cas Systems Restrict Horizontal Gene Transfer in Pseudomonas aeruginosa. ISME J. 2021, 15, 1420–1433. [Google Scholar] [CrossRef]
- Bonsma-Fisher, M.; Soutiere, D.; Goyal, S. How Adaptive Immunity Constrains the Composition and Fate of Large Bacterial Populations. Proc. Natl. Acad. Sci. USA 2018, 115, E7462–E7468. [Google Scholar] [CrossRef]
- López-Beltrán, A.; Botelho, J.; Iranzo, J. Dynamics of CRISPR-Mediated Virus-Host Interactions in the Human Gut Microbiome. ISME J. 2024, 18, wrae134. [Google Scholar] [CrossRef]
- Eghbalpoor, F.; Gorji, M.; Alavigeh, M.Z.; Moghadam, M.T. Genetically Engineered Phages and Engineered Phage-Derived Enzymes to Destroy Biofilms of Antibiotics Resistance Bacteria. Heliyon 2024, 10, e35666. [Google Scholar] [CrossRef] [PubMed]
- Bier, E.; Nizet, V. Driving to Safety: CRISPR-Based Genetic Approaches to Reducing Antibiotic Resistance. Trends Genet. 2021, 37, 745–757. [Google Scholar] [CrossRef]
- Deng, X.; Sun, W.; Li, X.; Wang, J.; Cheng, Z.; Sheng, G.; Wang, Y. An Anti-CRISPR That Represses Its Own Transcription While Blocking Cas9-Target DNA Binding. Nat. Commun. 2024, 15, 1806. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Han, S.R.; Lee, J.H.; Park, H.; Oh, T.J. A Computational Approach to Identify CRISPR-Cas Loci in the Complete Genomes of the Lichen-Associated Burkholderia sp. PAMC28687 and PAMC26561. Genomics 2021, 113, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Karginov, F.V.; Hannon, G.J. The CRISPR System: Small RNA-Guided Defense in Bacteria and Archaea. Mol. Cell 2010, 37, 7–19. [Google Scholar] [CrossRef]
- Medina-Aparicio, L.; Dávila, S.; Rebollar-Flores, J.E.; Calva, E.; Hernández-Lucas, I. The CRISPR-Cas System in Enterobacteriaceae. Pathog. Dis. 2018, 76, fty002. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Sashital, D.G. Mechanisms of Type I-E and I-F CRISPR-Cas Systems in Enterobacteriaceae. EcoSal Plus 2019, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Heidrich, N.; Ampattu, B.J.; Gunderson, C.W.; Seifert, H.S.; Schoen, C.; Vogel, J.; Sontheimer, E.J. Processing-Independent CRISPR RNAs Limit Natural Transformation in Neisseria Meningitidis. Mol. Cell 2013, 50, 488–503. [Google Scholar] [CrossRef]
- Varble, A.; Meaden, S.; Barrangou, R.; Westra, E.R.; Marraffini, L.A. Recombination between Phages and CRISPR–cas Loci Facilitates Horizontal Gene Transfer in Staphylococci. Nat. Microbiol. 2019, 4, 956–963. [Google Scholar] [CrossRef]
- Ramamurthy, T.; Ghosh, A.; Chowdhury, G.; Mukhopadhyay, A.K.; Dutta, S.; Miyoshi, S.I. Deciphering the Genetic Network and Programmed Regulation of Antimicrobial Resistance in Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2022, 12, 952491. [Google Scholar] [CrossRef] [PubMed]
- Ratner, H.K.; Sampson, T.R.; Weiss, D.S. I Can See CRISPR Now, Even When Phage Are Gone: A View on Alternative CRISPR-Cas Functions from the Prokaryotic Envelope. Curr. Opin. Infect. Dis. 2015, 28, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, A.; Ahmad, N.; Ahmad, H.; Saeed, M.; Ahmad, I. Beyond Antibiotics: CRISPR/Cas9 Triumph over Biofilm-Associated Antibiotic Resistance Infections. Front. Cell. Infect. Microbiol. 2024, 14, 1408569. [Google Scholar] [CrossRef]
- Amen, R.A.; Hassan, Y.M.; Essmat, R.A.; Ahmed, R.H.; Azab, M.M.; Shehata, N.R.; Elgazzar, M.M.; El-Sayed, W.M. Harnessing the Microbiome: CRISPR-Based Gene Editing and Antimicrobial Peptides in Combating Antibiotic Resistance and Cancer. Probiotics Antimicrob. Proteins 2025, 17, 1938–1968. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-Phage Coevolution as a Driver of Ecological and Evolutionary Processes in Microbial Communities. FEMS Microbiol. Rev. 2014, 38, 916–931. [Google Scholar] [CrossRef]
- Ali, N.; Vora, C.; Mathuria, A.; Kataria, N.; Mani, I. Chapter Four—Advances in CRISPR-Cas Systems for Gut Microbiome. In CRISPR-Cas-Based Genome Editing for Treating Human Diseases-Part A; Singh, V., Ed.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2024; Volume 208, pp. 59–81. [Google Scholar]
- Echavarria Galindo, M.; Lai, Y. CRISPR-Based Genetic Tools for the Study of Host-Microbe Interactions. Infect. Immun. 2025, 93, e0051024. [Google Scholar] [CrossRef]
- Yaqub, M.O.; Jain, A.; Joseph, C.E.; Edison, L.K. Microbiome-Driven Therapeutics: From Gut Health to Precision Medicine. Gastrointest. Disord. 2025, 7, 7. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Yan, Z.; Xu, J.; Feng, L.; He, N.; Guo, M.; Zhao, J.; Chen, Z.; Chen, H.; et al. Recent Advances of CRISPR-Based Genome Editing for Enhancing Staple Crops. Front. Plant Sci. 2024, 15, 1478398. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Bauer, D.E.; Chiarle, R. Assessing and Advancing the Safety of CRISPR-Cas Tools: From DNA to RNA Editing. Nat. Commun. 2023, 14, 212. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef] [PubMed]
- Chuai, G.; Ma, H.; Yan, J.; Chen, M.; Hong, N.; Xue, D.; Zhou, C.; Zhu, C.; Chen, K.; Duan, B.; et al. DeepCRISPR: Optimized CRISPR Guide RNA Design by Deep Learning. Genome Biol. 2018, 19, 80. [Google Scholar] [CrossRef]
- Pandelakis, M.; Delgado, E.; Ebrahimkhani, M.R. CRISPR-Based Synthetic Transcription Factors In Vivo: The Future of Therapeutic Cellular Programming. Cell Syst. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Matsumoto, D.; Matsugi, E.; Kishi, K.; Inoue, Y.; Nigorikawa, K.; Nomura, W. SpCas9-HF1 Enhances Accuracy of Cell Cycle-Dependent Genome Editing by Increasing HDR Efficiency, and by Reducing off-Target Effects and Indel Rates. Mol. Ther. Nucleic Acids 2024, 35, 102124. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.W.; Gaidukov, L.; Lai, Y.; Wu, M.R.; Cao, J.; Gutbrod, M.J.; Choi, G.C.G.; Utomo, R.P.; Chen, Y.C.; Wroblewska, L.; et al. A Synthetic Transcription Platform for Programmable Gene Expression in Mammalian Cells. Nat. Commun. 2022, 13, 6167. [Google Scholar] [CrossRef]

| Class | Type and Subtypes | Signature Cas Nuclease | Target | Representative Organisms (Examples) |
|---|---|---|---|---|
| 1 | I-A | Cas3 (HD-nuclease helicase) | DNA | Archaeoglobus fulgidus (archaeon); Sulfolobus solfataricus (archaeon) |
| 1 | I-B | Cas3 | DNA | Clostridium kluyveri (anaerobic bacterium) |
| 1 | I-C | Cas3 | DNA | Bacillus halodurans (alkaliphilic bacterium) |
| 1 | I-D | Cas3 | DNA | Cyanothece sp. ATCC 51142 (cyanobacterium) |
| 1 | I-E | Cas3 | DNA | Escherichia coli K12 (enteric model bacterium) |
| 1 | I-F | Cas3 | DNA | Yersinia pseudotuberculosis (enteric pathogen); Shewanella putrefaciens (marine bacterium) |
| 1 | I-G | Cas3 | DNA | Geobacter sulfurreducens (metal-reducing bacterium) |
| 1 | III-A | Cas10 (large subunit with HD nuclease domain) | DNA & RNA † | Staphylococcus epidermidis (skin commensal bacterium) |
| 1 | III-B | Cas10 | DNA & RNA † | Pyrococcus furiosus (hyperthermophilic archaeon) |
| 1 | III-C | Cas10 | DNA & RNA † | Methanothermobacter thermautotrophicus (methanogenic archaeon) |
| 1 | III-D | Cas10 | RNA (primarily) | Synechocystis sp. PCC6803 (photosynthetic model cyanobacterium) |
| 1 | III-E | Cas10 | RNA (primarily) | Candidatus Scalindua brodae (anammox bacterium) |
| 1 | III-F | Cas10 | DNA (predicted) | Thermotoga lettingae (thermophilic bacterium) |
| 1 | IV-A | Csf1 (Cas8-like large subunit) | DNA (plasmid) ‡ | Thioalkalivibrio sp. K90mix (haloalkaliphilic bacterium) |
| 1 | IV-B | Csf1 | DNA (plasmid) ‡ | Rhodococcus jostii RHA1 (soil actinomycete) |
| 1 | IV-C | Csf1 | DNA (predicted) ‡ | Thermoflexile sp. (Anaerolineae bacterium) |
| 2 | II-A | Cas9 (RuvC + HNH nuclease domains) | DNA | Streptococcus pyogenes (Group A strep pathogen); Streptococcus thermophilus (dairy fermenter) |
| 2 | II-B | Cas9 | DNA | Legionella pneumophila (intracellular pathogen) |
| 2 | II-C | Cas9 | DNA | Neisseria meningitidis (meningococcus pathogen); Campylobacter jejuni (enteric pathogen); Micrarchaeum acidiphilum (ARMAN-1 archaeon) |
| 2 | V-A | Cas12a (Cpf1 family) | DNA | Francisella novicida (tularemia-like bacterium) |
| 2 | V-B | Cas12b (C2c1) | DNA | Alicyclobacillus acidoterrestris (thermoacidophilic bacterium); Gluconacetobacter sp. (planctomycete bacterium) |
| 2 | V-C | Cas12c (C2c3) | DNA | Oleiphilus sp. SM1 (marine hydrocarbon-degrader) |
| 2 | V-D | Cas12d (CasY) | DNA | Uncultured bacterium (metagenomic assembly) |
| 2 | V-E | Cas12e (CasX) | DNA | “Candidatus” Deltaproteobacteria bacterium (metagenome) |
| 2 | V-F | Cas12f (Cas14a–c) | DNA | Uncultured archaeon (nanoarchaeote; hot spring); Bacillus thuringiensis (spore-forming bacterium) |
| 2 | V-G | Cas12g | RNA | Hot spring metagenome (unidentified thermophiles) |
| 2 | V-H | Cas12h | DNA | Hypersaline lake sediment metagenome (unidentified) |
| 2 | V-I | Cas12i | DNA | Freshwater pond metagenome (unidentified) |
| 2 | V-K | Cas12k (C2c5, Tn7-linked) | DNA | Cyanothece sp. PCC 8801 (cyanobacterium; CRISPR-associated transposon) |
| 2 | VI-A | Cas13a (C2c2 family; dual HEPN RNase domains) | RNA | Leptotrichia shahii (human oral bacterium) |
| 2 | VI-B | Cas13b (dual HEPN domains) | RNA | Prevotella buccae (human gut anaerobe); Bergeyella zoohelcum (oral bacterium) |
| 2 | VI-C | Cas13c (dual HEPN domains) | RNA | Fusobacterium perfoetens (oral/fusiform bacterium) |
| 2 | VI-D | Cas13d (dual HEPN domains) | RNA | Ruminococcus bicirculans (gut anaerobe) |
| Bacterial Strain | CRISPR Type | Regulated Gene/Pathway | Effect on Physiology (Metabolism, Stress, Virulence) | References |
|---|---|---|---|---|
| Francisella novicida | II-B (Cas9) | Bacterial lipoprotein (BLP) transcript; scaRNA-Cas9 complex | ↓ surface BLP → ↓ TLR2 recognition → ↑ immune evasion/virulence | [36,37] |
| Pseudomonas aeruginosa PA14 | I-F (Cas3) | lasR (QS master regulator) mRNA | Post-transcriptional control of QS → dampened host TLR4 response → ↑ immune evasion | [38] |
| Salmonella enterica (Enteritidis) | I-E (Cas3) | lsr operon/AI-2 uptake & processing (QS) | ↑ AI-2 signaling → ↑ biofilm & host-cell virulence | [39] |
| Streptococcus pyogenes (GAS) | II-A (Cas9) | Global virulence regulons (e.g., Mga/CovR-S; multiple factors proteomically affected) | Δcas9 → ↓ adherence, ↓ survival in blood, ↓ virulence in mouse skin model | [40] |
| Campylobacter jejuni NCTC11168 | II-C (Cas9) | Endogenous mRNAs (crRNA-dependent binding/cleavage) | Cas9 regulates virulence programs; Δcas9 → ↓ adhesion/invasion, ↓ biofilm | [41,42] |
| Streptococcus mutans UA159 | I-C (Cas3) | VicRK-linked biofilm genes; stress tolerance | Δcas3 → ↓ biofilm; ↑ fluoride sensitivity (metabolic/stress shift) | [27,43] |
| Acinetobacter baumannii ATCC19606 | I-Fb (Cas3) | abaI (AHL synthase; QS) mRNA; OmpA & biofilm genes | Cas3 activity → ↑ QS/biofilm/virulence; Δcas3 → ↓ biofilm & pathogenicity | [33] |
| Streptococcus agalactiae (GBS) | II-A (Cas9) | Endogenous regulation linked to colonization/immune evasion | Cas9 contributes to mucosal colonization & host interaction | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, C.E.; Jain, A.; Yaqub, M.O.; Edison, L.K. CRISPR-Cas Systems: Bridging Bacterial Immunity and Host Interactions. Appl. Microbiol. 2025, 5, 118. https://doi.org/10.3390/applmicrobiol5040118
Joseph CE, Jain A, Yaqub MO, Edison LK. CRISPR-Cas Systems: Bridging Bacterial Immunity and Host Interactions. Applied Microbiology. 2025; 5(4):118. https://doi.org/10.3390/applmicrobiol5040118
Chicago/Turabian StyleJoseph, Chinedu Eucharia, Aashika Jain, Muneer Oladipupo Yaqub, and Lekshmi K. Edison. 2025. "CRISPR-Cas Systems: Bridging Bacterial Immunity and Host Interactions" Applied Microbiology 5, no. 4: 118. https://doi.org/10.3390/applmicrobiol5040118
APA StyleJoseph, C. E., Jain, A., Yaqub, M. O., & Edison, L. K. (2025). CRISPR-Cas Systems: Bridging Bacterial Immunity and Host Interactions. Applied Microbiology, 5(4), 118. https://doi.org/10.3390/applmicrobiol5040118








