Survey of Thirteen Novel Pseudomonas putida Bacteriophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Phage Isolation, Culturing, and Imaging
2.2. DNA Isolation
2.3. DNA Sequencing and Assembly
2.4. Genome Annotation and Other Analyses
2.5. Comparative Genomics
2.6. Lifestyle Prediction
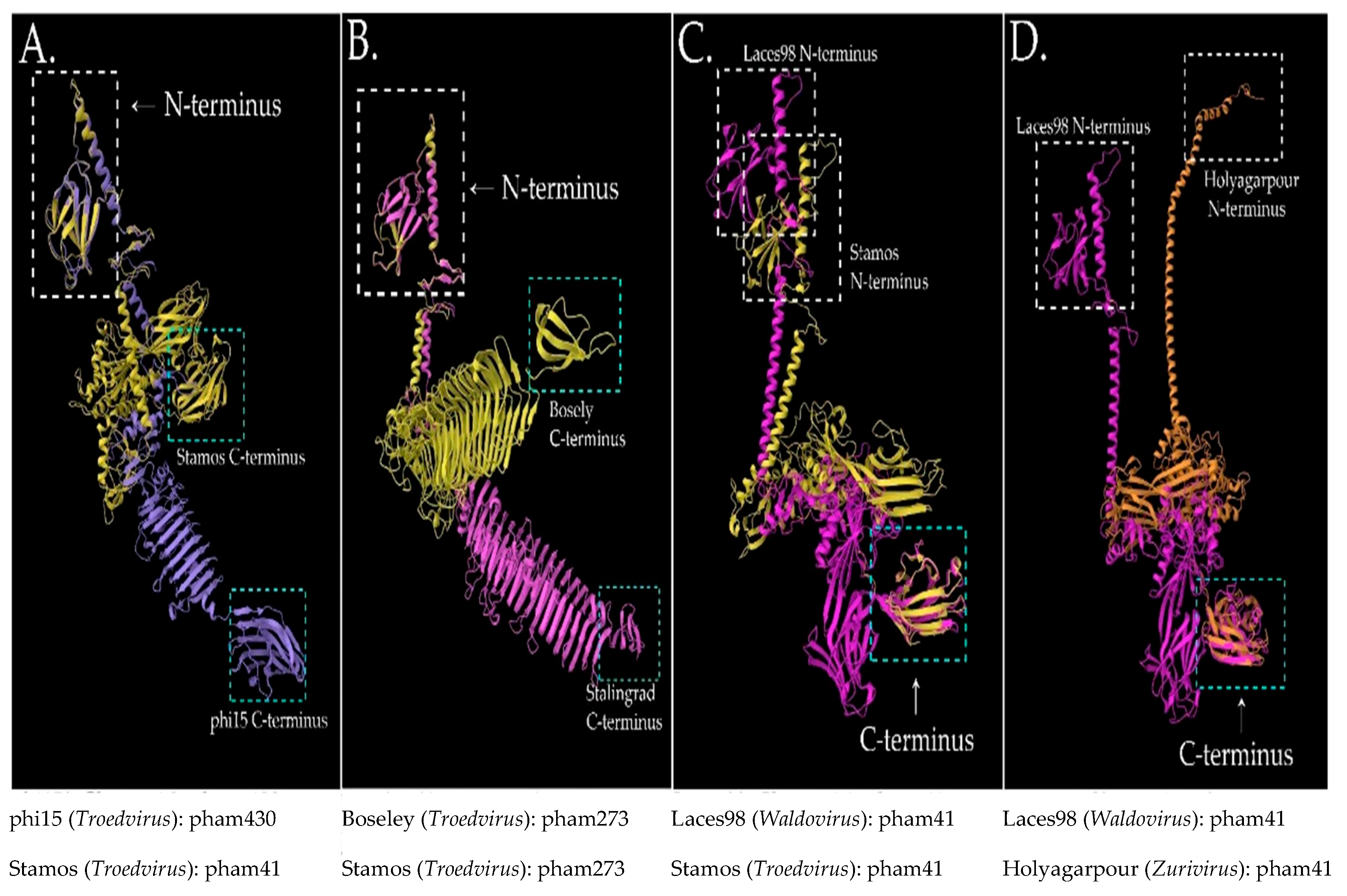
2.7. Protein Structure Predictions
2.8. Phages Added from GenBank
2.9. Sequencing and Analysis of P. putida Host
3. Results
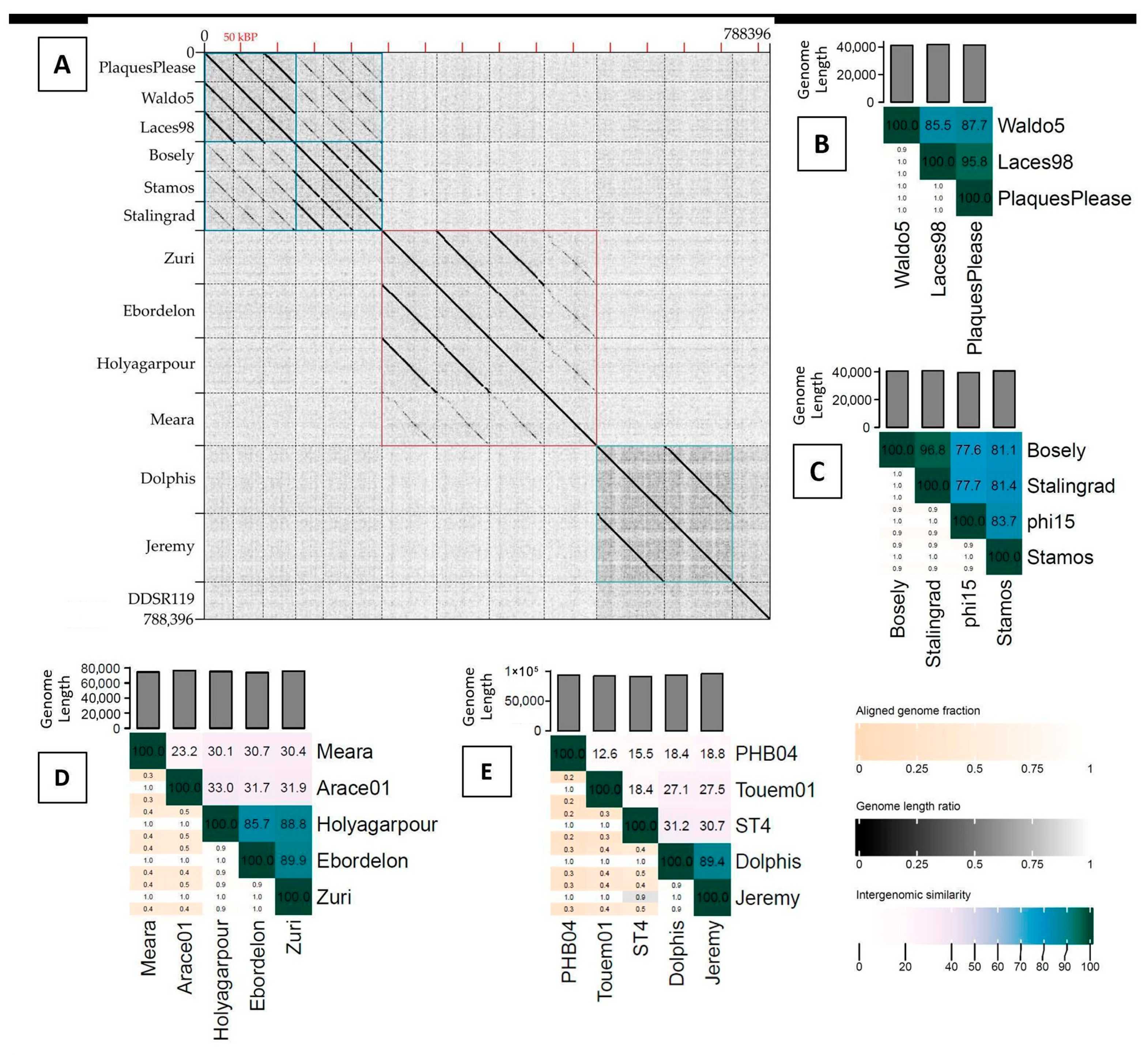
3.1. Genome Features and Relatedness to Other Phages
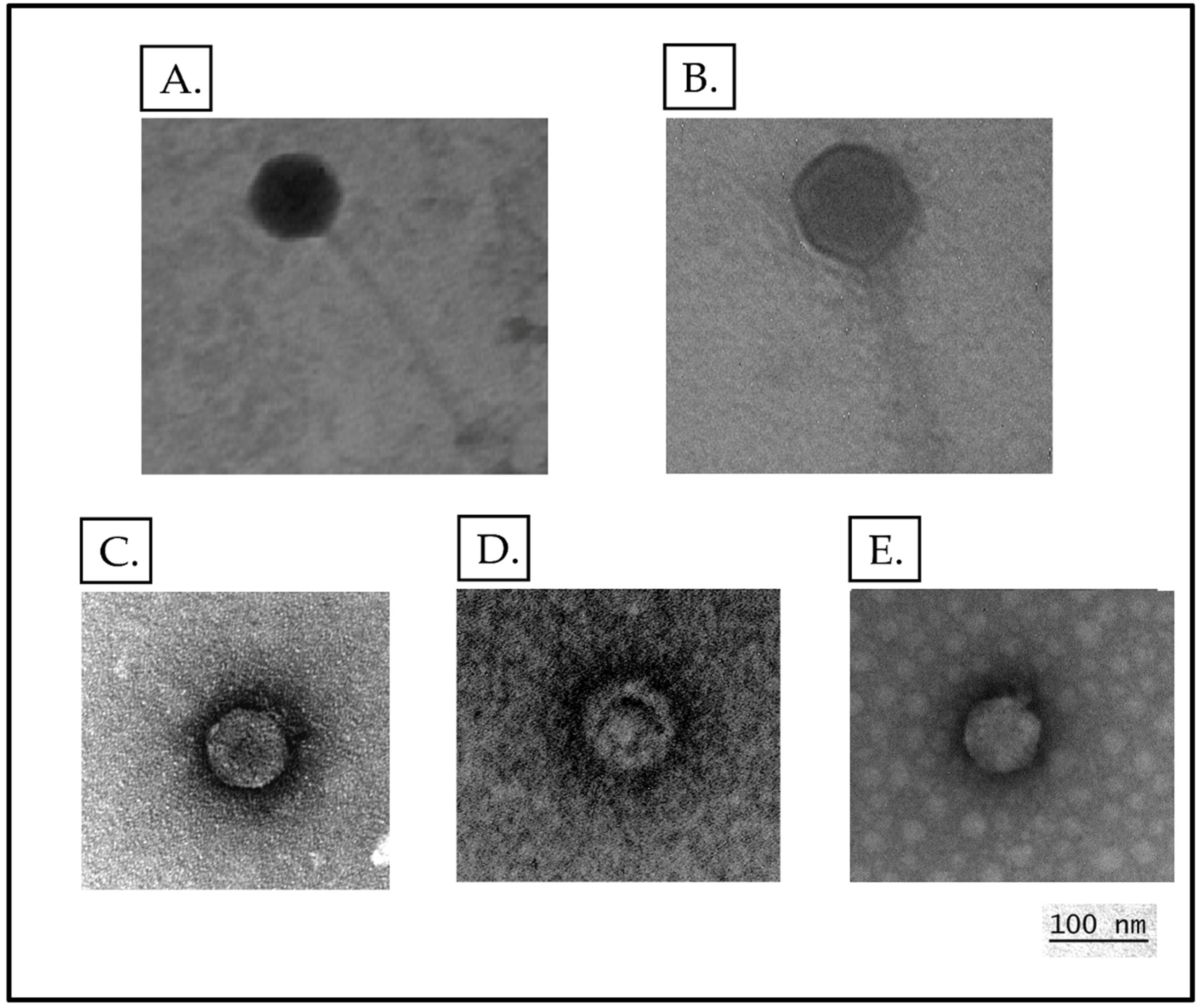
3.2. Phage Morphology
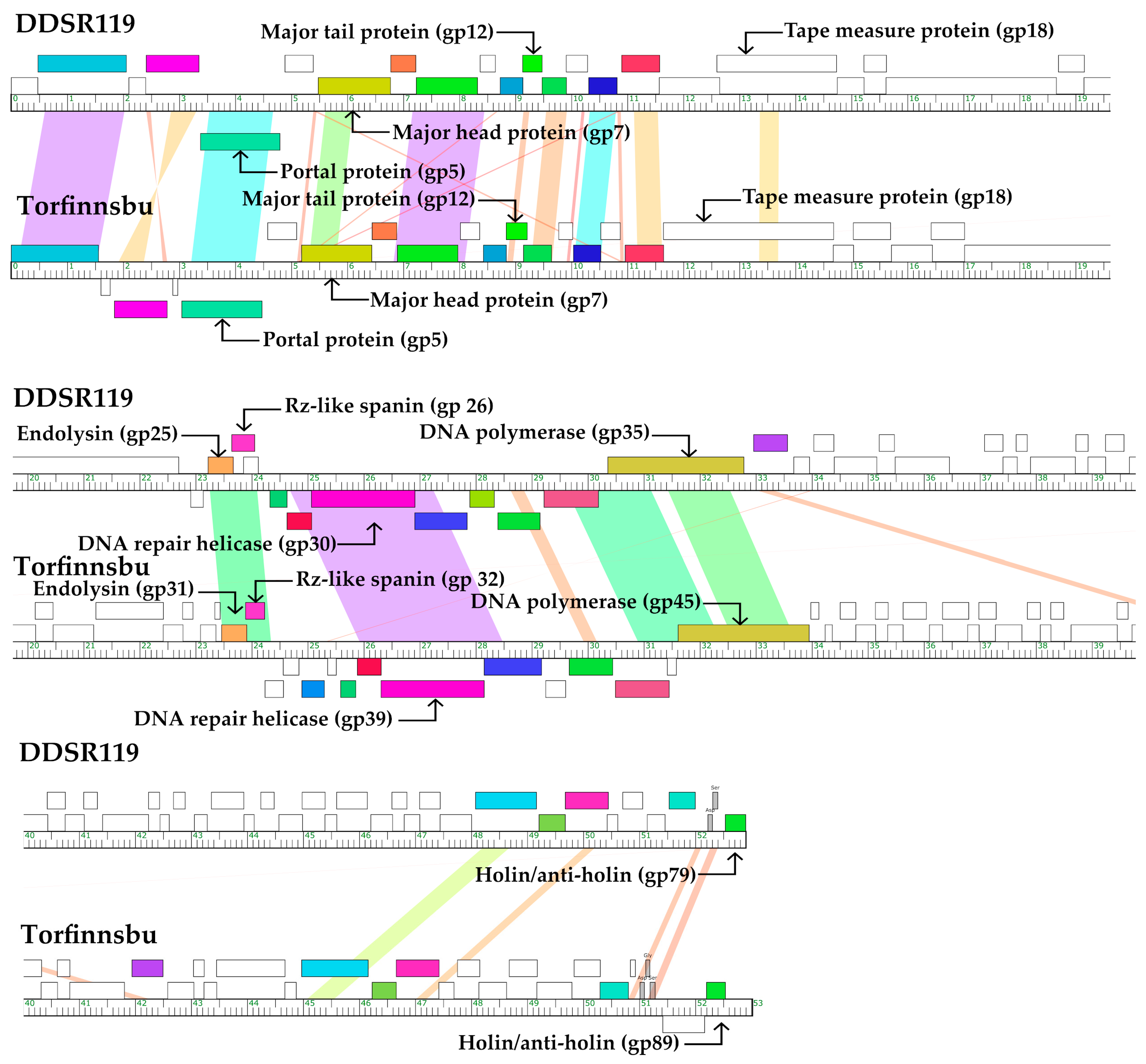
3.3. Protein Features of our Phages and Selected Relatives Using Phamerator
3.4. Phage Lifestyle
3.5. Lysis Systems
3.6. Auxiliary Metabolic Genes
3.7. Podovirus Tail Spike Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyng, M.; Kovács, Á.T. Frenemies of the soil: Bacillus and Pseudomonas interspecies interactions. Trends Microbiol. 2023, 31, 845–857. [Google Scholar] [CrossRef]
- Iglewski, B.H. Pseudomonas. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. Available online: http://www.ncbi.nlm.nih.gov/books/NBK8326/ (accessed on 2 April 2025).
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, L.; Hocquet, D.; Miller, S.I. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011, 19, 341–348. [Google Scholar] [CrossRef]
- Ahearn, D.G.; Borazjani, R.N.; Simmons, R.B.; Gabriel, M.M. Primary adhesion of Pseudomonas aeruginosa to inanimate surfaces including biomaterials. Methods Enzym. 1999, 310, 551–557. [Google Scholar] [CrossRef]
- Perkins, K.M.; Reddy, S.C.; Fagan, R.; Arduino, M.J.; Perz, J.F. Investigation of healthcare infection risks from water-related organisms: Summary of CDC consultations, 2014–2017. Infect. Control. Hosp. Epidemiol. 2019, 40, 621–626. [Google Scholar] [CrossRef]
- Sukhum, K.V.; Newcomer, E.P.; Cass, C.; Wallace, M.A.; Johnson, C.; Fine, J.; Sax, S.; Barlet, M.H.; Burnham, C.D.; Dantas, G.; et al. Antibiotic-resistant organisms establish reservoirs in new hospital built environments and are related to patient blood infection isolates. Commun. Med. 2022, 2, 62. [Google Scholar] [CrossRef]
- Xin, X.F.; Kvitko, B.; He, S.Y. Pseudomonas syringae: What it takes to be a pathogen. Nat. Rev. Microbiol. 2018, 16, 316–328. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; Stanton, B.A.; O’Toole, G.A. Pseudomonas aeruginosa biofilm formation in the cystic fibrosis airway. Pulm. Pharmacol. Ther. 2008, 21, 595–599. [Google Scholar] [CrossRef]
- Han, Q.; Feng, L.; Zhang, Y.; Zhang, R.; Wang, G.; Zhang, Y. Effect of Juglone against Pseudomonas syringae pv actinidiae Planktonic Growth and Biofilm Formation. Molecules 2021, 26, 7580. [Google Scholar] [CrossRef]
- Planchamp, C.; Glauser, G.; Mauch-Mani, B. Root inoculation with Pseudomonas putida KT2440 induces transcriptional and metabolic changes and systemic resistance in maize plants. Front. Plant Sci. 2015, 5, 719. [Google Scholar] [CrossRef]
- Timmis, K.N.; Steffan, R.J.; Unterman, R. Designing microorganisms for the treatment of toxic wastes. Annu. Rev. Microbiol. 1994, 48, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, P.R.; Zeyer, J.; Reineke, W.; Knackmuss, H.J.; Timmis, K.N. Enzyme recruitment in vitro: Use of cloned genes to extend the range of haloaromatics degraded by Pseudomonas sp. strain B13. J. Bacteriol. 1984, 158, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Dejonghe, W.; Boon, N.; Seghers, D.; Top, E.M.; Verstraete, W. Bioaugmentation of soils by increasing microbial richness: Missing links. Environ. Microbiol. 2001, 3, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Xu, X.; Dang, Y.; Kong, A.; Wu, Y.; Liang, P.; Wang, S.; Yu, H.; Xu, P.; Yang, C. An engineered Pseudomonas putida can simultaneously degrade organophosphates, pyrethroids and carbamates. Sci. Total Environ. 2018, 628–629, 1258–1265. [Google Scholar] [CrossRef]
- Qi, X.; Gao, X.; Wang, X.; Xu, P. Harnessing Pseudomonas putida in bioelectrochemical systems. Trends Biotechnol. 2024, 42, 877–894. [Google Scholar] [CrossRef]
- de Lorenzo, V.; Pérez-Pantoja, D.; Nikel, P.I. Pseudomonas putida KT2440: The long journey of a soil-dweller to become a synthetic biology chassis. J. Bacteriol. 2024, 206, e0013624. [Google Scholar] [CrossRef]
- Weimer, A.; Kohlstedt, M.; Volke, D.C.; Nikel, P.I.; Wittmann, C. Industrial biotechnology of Pseudomonas putida: Advances and prospects. Appl. Microbiol. Biotechnol. 2020, 104, 7745–7766. [Google Scholar] [CrossRef]
- Campbell, J.I.A.; Albrechtsen, M.; Sørensen, J. Large Pseudomonas phages isolated from barley rhizosphere. FEMS Microbiol. Ecol. 2006, 18, 63–74. [Google Scholar] [CrossRef]
- Il Kim, M.; Park, C.Y.; Seo, J.M.; Kang, K.S.; Park, K.S.; Kang, J.; Hong, K.S.; Choi, Y.; Lee, S.Y.; Park, J.P.; et al. In Situ Biosynthesis of a Metal Nanoparticle Encapsulated in Alginate Gel for Imageable Drug-Delivery System. ACS Appl. Mater. Interfaces 2021, 13, 36697–36708. [Google Scholar] [CrossRef]
- Avendaño, R.; Muñoz-Montero, S.; Rojas-Gätjens, D.; Fuentes-Schweizer, P.; Vieto, S.; Montenegro, R.; Salvador, M.; Frew, R.; Kim, J.; Chavarría, M.; et al. Production of selenium nanoparticles occurs through an interconnected pathway of sulphur metabolism and oxidative stress response in Pseudomonas putida KT2440. Microb. Biotechnol. 2023, 16, 931–946. [Google Scholar] [CrossRef]
- Shaburova, O.V.; Krylov, S.V.; Veĭko, V.P.; Pleteneva, E.A.; Burkal’tseva, M.V.; Miroshnokov, K.A.; Kornelissen, A.; Lavogne, R.; Sykilinda, N.N.; Kadykov, V.A.; et al. Search for destruction factors of bacterial biofilms: Comparison of phage properties in a group of Pseudomonas putida bacteriophages and specificity of their halo-formation products. Genetika 2009, 45, 185–195. [Google Scholar] [CrossRef]
- Fage, C.; Lemire, N.; Moineau, S. Delivery of CRISPR-Cas systems using phage-based vectors. Curr. Opin. Biotechnol. 2021, 68, 174–180. [Google Scholar] [CrossRef]
- Nikel, P.I.; de Lorenzo, V. Pseudomonas putida as a functional chassis for industrial biocatalysis: From native biochemistry to trans-metabolism. Metab. Eng. 2018, 50, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in Aquatic Ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Vandenheuvel, D.; Lavigne, R.; Brüssow, H. Bacteriophage Therapy: Advances in Formulation Strategies and Human Clinical Trials. Annu. Rev. Virol. 2015, 2, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Endersen, L.; O’Mahony, J.; Hill, C.; Ross, R.P.; McAuliffe, O.; Coffey, A. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for using bacteriophages for plant disease control. Bacteriophage 2012, 2, 208–214. [Google Scholar] [CrossRef]
- Di Lallo, G.; Evangelisti, M.; Mancuso, F.; Ferrante, P.; Marcelletti, S.; Tinari, A.; Superti, F.; Migliore, L.; D’Addabbo, P.; Frezza, D.; et al. Isolation and partial characterization of bacteriophages infecting Pseudomonas syringae pv. actinidiae, causal agent of kiwifruit bacterial canker. J. Basic Microbiol. 2014, 54, 1210–1221. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef]
- Slopek, S.; Kucharewicz-Krukowska, A.; Weber-Dabrowska, B.; Dabrowski, M. Results of bacteriophage treatment of suppurative bacterial infections. V. Evaluation of the results obtained in children. Arch. Immunol. Ther. Exp. 1985, 33, 241–259. [Google Scholar]
- Rabiey, M.; Roy, S.R.; Holtappels, D.; Franceschetti, L.; Quilty, B.J.; Creeth, R.; Sundin, G.W.; Wagemans, J.; Lavigne, R.; Jackson, R.W. Phage biocontrol to combat Pseudomonas syringae pathogens causing disease in cherry. Microb. Biotechnol. 2020, 13, 1428–1445. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, H.; Ryu, S. Bacteriophage and endolysin engineering for biocontrol of food pathogens/pathogens in the food: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2023, 63, 8919–8938. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Hu, Z.; Li, M.; Yang, Y.; Lu, S.; Rao, X. Therapeutic potential of bacteriophage endolysins for infections caused by Gram-positive bacteria. J. Biomed. Sci. 2023, 30, 29. [Google Scholar] [CrossRef] [PubMed]
- Brauer, A.; Rosendahl, S.; Kängsep, A.; Lewańczyk, A.C.; Rikberg, R.; Hõrak, R.; Tamman, H. Isolation and characterization of a phage collection against Pseudomonas putida. Environ. Microbiol. 2024, 26, e16671. [Google Scholar] [CrossRef]
- Lammens, E.M.; Nikel, P.I.; Lavigne, R. Exploring the synthetic biology potential of bacteriophages for engineering non-model bacteria. Nat. Commun. 2020, 11, 5294. [Google Scholar] [CrossRef]
- Lee, L.F.; Boezi, J.A. Characterization of bacteriophage gh-1 for Pseudomonas putida. J. Bacteriol. 1966, 92, 1821–1827. [Google Scholar] [CrossRef]
- Kovalyova, I.V.; Kropinski, A.M. The complete genomic sequence of lytic bacteriophage gh-1 infecting Pseudomonas putida—Evidence for close relationship to the T7 group. Virology 2003, 311, 305–315. [Google Scholar] [CrossRef]
- Ha, A.D.; Denver, D.R. Comparative Genomic Analysis of 130 Bacteriophages Infecting Bacteria in the Genus Pseudomonas. Front. Microbiol. 2018, 9, 1456. [Google Scholar] [CrossRef]
- Yehl, K.; Lemire, S.; Yang, A.C.; Ando, H.; Mimee, M.; Torres, M.T.; de la Fuente-Nunez, C.; Lu, T.K. Engineering Phage Host-Range and Suppressing Bacterial Resistance through Phage Tail Fiber Mutagenesis. Cell 2019, 179, 459–469.e9. [Google Scholar] [CrossRef]
- Mageeney, C.M.; Trubl, G.; Williams, K.P. Improved Mobilome Delineation in Fragmented Genomes. Front. Bioinform. 2022, 2, 866850. [Google Scholar] [CrossRef]
- Purtschert-Montenegro, G.; Cárcamo-Oyarce, G.; Pinto-Carbó, M.; Agnoli, K.; Bailly, A.; Eberl, L. Pseudomonas putida mediates bacterial killing, biofilm invasion and biocontrol with a type IVB secretion system. Nat. Microbiol. 2022, 7, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Poxleitner, M.; Pope, W.; Jacobs-Sera, D.; Sivanathan, V.; Hatfull, G. Phage Discovery Guide. Published Online 2018. Available online: https://seaphagesphagediscoveryguide.helpdocsonline.com/home (accessed on 27 March 2025).
- Promega. Wizard® DNA Clean-Up System Protocol. 2010. Available online: https://www.promega.com/resources/protocols/technical-bulletins/0/wizard-dna-cleanup-system-protocol/ (accessed on 7 July 2020).
- Fryslie, B. Newbler. 2012. Available online: https://swes.cals.arizona.edu/maier_lab/kartchner/documentation/index.php/home/docs/newbler (accessed on 26 March 2019).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.; Green, P. Consed: A graphical editor for next-generation sequencing. Bioinformatics 2013, 29, 2936–2937. [Google Scholar] [CrossRef] [PubMed]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Shaffer, M.; Borton, M.A.; McGivern, B.B.; Zayed, A.A.; La Rosa, S.L.; Solden, L.M.; Liu, P.; Narrowe, A.B.; Rodríguez-Ramos, J.; Bolduc, B.; et al. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 2020, 48, 8883–8900. [Google Scholar] [CrossRef]
- Arkin, A.P.; Cottingham, R.W.; Henry, C.S.; Harris, N.L.; Stevens, R.L.; Maslov, S.; Dehal, P.; Ware, D.; Perez, F.; Canon, S.; et al. KBase: The United States Department of Energy Systems Biology Knowledgebase. Nat. Biotechnol. 2018, 36, 566–569. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; et al. BLAST: A more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, W29. [Google Scholar] [CrossRef]
- Pope, W.H.; Jacobs-Sera, D. Annotation of Bacteriophage Genome Sequences Using DNA Master: An Overview. In Bacteriophages: Methods and Protocols; Clokie, M.R.J., Kropinski, A.M., Lavigne, R., Eds.; Springer: New York, NY, USA, 2018; pp. 217–229. [Google Scholar] [CrossRef]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Lukashin, A.V.; Borodovsky, M. GeneMark. hmm: New solutions for gene finding. Nucleic Acids Res. 1998, 26, 1107–1115. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005, 33 (Suppl. 2), W244–W248. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Hofmann, K.; TMbase—A Database of Membrane Spanning Protein Segments. TMpred Documentation. 1993. Available online: https://sbcb.bioch.ox.ac.uk/TM_noj/TM_noj.html (accessed on 19 September 2020).
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022, 2202–2204. [Google Scholar] [CrossRef]
- Kahsay, R.Y.; Gao, G.; Liao, L. An improved hidden Markov model for transmembrane protein detection and topology prediction and its applications to complete genomes. Bioinformatics 2005, 21, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Juncker, A.S.; Willenbrock, H.; Von Heijne, G.; Brunak, S.; Nielsen, H.; Krogh, A. Prediction of lipoprotein signal peptides in Gram-negative bacteria. Protein Sci. 2003, 12, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Moraru, C.; Varsani, A.; Kropinski, A.M. VIRIDIC-A Novel Tool to Calculate the Intergenomic Similarities of Prokaryote-Infecting Viruses. Viruses 2020, 12, 1268. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The viral proteomic tree server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Cresawn, S.G.; Bogel, M.; Day, N.; Jacobs-Sera, D.; Hendrix, R.W.; Hatfull, G.F. Phamerator: A bioinformatic tool for comparative bacteriophage genomics. BMC Bioinform. 2011, 12, 395. [Google Scholar] [CrossRef]
- Gauthier, C.H.; Hatfull, G.F. A Bioinformatic Ecosystem for Bacteriophage Genomics: PhaMMSeqs, Phamerator, pdm_utils, PhagesDB, DEPhT, and PhamClust. Viruses 2024, 16, 1278. [Google Scholar] [CrossRef]
- Hockenberry, A.J.; Wilke, C.O. BACPHLIP: Predicting bacteriophage lifestyle from conserved protein domains. PeerJ 2021, 9, e11396. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Cornelissen, A.; Ceyssens, P.J.; T’Syen, J.; Van Praet, H.; Noben, J.P.; Shaburova, O.V.; Krylov, V.N.; Volckaert, G.; Lavigne, R. The T7-related Pseudomonas putida phage φ15 displays virion-associated biofilm degradation properties. PLoS ONE 2011, 6, e18597. [Google Scholar] [CrossRef]
- Feltin, C.; Garneau, J.R.; Morris, C.E.; Bérard, A.; Torres-Barceló, C. Novel phages of Pseudomonas syringae unveil numerous potential auxiliary metabolic genes. J. Gen. Virol. 2024, 105, 001990. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Sun, E.; Song, J.; Wu, B. Characterisation of a newly detected bacteriophage infecting Bordetella bronchiseptica in swine. Arch. Virol. 2019, 164, 33–40. [Google Scholar] [CrossRef]
- Dougherty, P.E.; Pedersen, M.S.; Forero-Junco, L.M.; Carstens, A.B.; Raaijmakers, J.M.; Riber, L.; Hansen, L.H. Novel bacteriophages targeting wheat phyllosphere bacteria carry DNA modifications and single-strand breaks. Virus Res. 2025, 352, 199524. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Brister, J.R. How to Name and Classify Your Phage: An Informal Guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef]
- Wittmann, J.; Turner, D.; Millard, A.D.; Mahadevan, P.; Kropinski, A.M.; Adriaenssens, E.M. From orphan phage to a proposed new family–the diversity of N4-like viruses. Antibiotics 2020, 9, 663. [Google Scholar] [CrossRef]
- Belcaid, M.; Bergeron, A.; Poisson, G. The evolution of the tape measure protein: Units, duplications and losses. BMC Bioinform. 2011, 12 (Suppl. 9), S10. [Google Scholar] [CrossRef] [PubMed]
- Cumby, N.; Reimer, K.; Mengin-Lecreulx, D.; Davidson, A.R.; Maxwell, K.L. The phage tail tape measure protein, an inner membrane protein and a periplasmic chaperone play connected roles in the genome injection process of E. coli phage HK97. Mol. Microbiol. 2015, 96, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Saini, T.; Al Qaffas, A.; Erill, I.; Caruso, S.M.; Temple, L.; Johnson, A.A. Evidence of a Set of Core-Function Genes in 16 Bacillus Podoviral Genomes with Considerable Genomic Diversity. Viruses 2023, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Pedulla, M.L.; Ford, M.E.; Houtz, J.M.; Karthikeyan, T.; Wadsworth, C.; Lewis, J.A.; Jacobs-Sera, D.; Falbo, J.; Gross, J.; Pannunzio, N.R.; et al. Origins of highly mosaic mycobacteriophage genomes. Cell 2003, 113, 172–182. [Google Scholar] [CrossRef]
- Wetzel, K.S.; Aull, H.G.; Zack, K.M.; Garlena, R.A.; Hatfull, G.F. Protein-Mediated and RNA-Based Origins of Replication of Extrachromosomal Mycobacterial Prophages. mBio 2020, 11, e00385-20. [Google Scholar] [CrossRef]
- Deutsch, D.R.; Utter, B.; Verratti, K.J.; Sichtig, H.; Tallon, L.J.; Fischetti, V.A. Extra-Chromosomal DNA Sequencing Reveals Episomal Prophages Capable of Impacting Virulence Factor Expression in Staphylococcus aureus. Front. Microbiol. 2018, 9, 1406. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.01406 (accessed on 25 December 2023). [CrossRef]
- Landy, A. The λ Integrase Site-specific Recombination Pathway. Microbiol. Spectr. 2015, 3, MDNA3-0051-2014. [Google Scholar] [CrossRef]
- Cahill, J.; Young, R. Phage Lysis: Multiple Genes for Multiple Barriers. Adv. Virus Res. 2019, 103, 33–70. [Google Scholar] [CrossRef]
- Pratama, A.A.; Bolduc, B.; Zayed, A.A.; Zhong, Z.P.; Guo, J.; Vik, D.R.; Gazitúa, M.C.; Wainaina, J.M.; Roux, S.; Sullivan, M.B. Expanding standards in viromics: In silico evaluation of dsDNA viral genome identification, classification, and auxiliary metabolic gene curation. PeerJ 2021, 9, e11447. [Google Scholar] [CrossRef]
- Murphy, J.; Mahony, J.; Ainsworth, S.; Nauta, A.; van Sinderen, D. Bacteriophage orphan DNA methyltransferases: Insights from their bacterial origin, function, and occurrence. Appl. Environ. Microbiol. 2013, 79, 7547–7555. [Google Scholar] [CrossRef]
- Steven, A.C.; Trus, B.L.; Maizel, J.V.; Unser, M.; Parry, D.A.; Wall, J.S.; Hainfeld, J.F.; Studier, F.W. Molecular substructure of a viral receptor-recognition protein. The gp17 tail-fiber of bacteriophage T7. J. Mol. Biol. 1988, 200, 351–365. [Google Scholar] [CrossRef]
- Steinbacher, S.; Miller, S.; Baxa, U.; Budisa, N.; Weintraub, A.; Seckler, R.; Huber, R. Phage P22 tailspike protein: Crystal structure of the head-binding domain at 2.3 A, fully refined structure of the endorhamnosidase at 1.56 A resolution, and the molecular basis of O-antigen recognition and cleavage. J. Mol. Biol. 1997, 267, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Glukhov, A.S.; Krutilina, A.I.; Shlyapnikov, M.G.; Severinov, K.; Lavysh, D.; Kochetkov, V.V.; McGrath, J.W.; de Leeuwe, C.; Shaburova, O.V.; Krylov, V.N.; et al. Genomic Analysis of Pseudomonas putida Phage tf with Localized Single-Strand DNA Interruptions. PLoS ONE 2012, 7, e51163. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, A.; Ceyssens, P.J.; Krylov, V.N.; Noben, J.P.; Volckaert, G.; Lavigne, R. Identification of EPS-degrading activity within the tail spikes of the novel Pseudomonas putida phage AF. Virology 2012, 434, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, L.; Lins, B.; Barrett, J.; Montgomery, A.; Trapani, S.; Schindler, A.; Christie, G.E.; Cresawn, S.G.; Temple, L. Genomic characterization of six novel Bacillus pumilus bacteriophages. Virology 2013, 444, 374–383. [Google Scholar] [CrossRef]
- Sauder, A.B.; Quinn, M.R.; Brouillette, A.; Caruso, S.; Cresawn, S.; Erill, I.; Lewis, L.; Loesser-Casey, K.; Pate, M.; Scott, C.; et al. Genomic characterization and comparison of seven Myoviridae bacteriophage infecting Bacillus thuringiensis. Virology 2016, 489, 243–251. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, T.; Yu, M.; Chen, Y.L.; Jin, M. The Life Cycle Transitions of Temperate Phages: Regulating Factors and Potential Ecological Implications. Viruses 2022, 14, 1904. [Google Scholar] [CrossRef]
- Freifelder, D. A Comprehensive Introduction to Prokaryotes and Eukaryotes. In More DNA Molecular Biology; Biosicence; Jones and Bartlett Publishers, Inc.: Burlington, MA, USA, 1984; p. 660. [Google Scholar]
- Yu, S.L.; McClain, H.M.; Torrance, E.L.; Schoeniger, J.S.; Mageeney, C.M.; Williams, K.P. The TIGER/Islander resource for genomic islands. Microbiol. Resour. Announc. 2025, 14, e0049225. [Google Scholar] [CrossRef]
- Oliveira, H.; Melo, L.D.; Santos, S.B.; Nóbrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular Aspects and Comparative Genomics of Bacteriophage Endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef]
- Zhang, X.; Studier, F.W. Multiple roles of T7 RNA polymerase and T7 lysozyme during bacteriophage T7 infection. J. Mol. Biol. 2004, 340, 707–730. [Google Scholar] [CrossRef]
- Scholl, D. Phage Tail–Like Bacteriocins. Annu. Rev. Virol. 2017, 4, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Ghequire, M.G.K.; Dillen, Y.; Lambrichts, I.; Proost, P.; Wattiez, R.; De Mot, R. Different Ancestries of R Tailocins in Rhizospheric Pseudomonas Isolates. Genome Biol. Evol. 2015, 7, 2810–2828. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.M.; Dunstan, R.A.; Lithgow, T.; Coulibaly, F. Tall tails: Cryo-electron microscopy of phage tail DNA ejection conduits. Biochem. Soc. Trans. 2022, 50, 459-22W. [Google Scholar] [CrossRef]
- Johnson, J.E.; Chiu, W. DNA packaging and delivery machines in tailed bacteriophages. Curr. Opin. Struct. Biol. 2007, 17, 237–243. [Google Scholar] [CrossRef]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef]
- Ouyang, R.; Ongenae, V.; Muok, A.; Claessen, D.; Briegel, A. Phage fibers and spikes: A nanoscale Swiss army knife for host infection. Curr. Opin. Microbiol. 2024, 77, 102429. [Google Scholar] [CrossRef]
- Hanauer, D.I.; Graham, M.J.; SEA-PHAGES; Betancur, L.; Bobrownicki, A.; Cresawn, S.G.; Garlena, R.A.; Jacobs-Sera, D.; Kaufmann, N.; Pope, W.H.; et al. An inclusive Research Education Community (iREC): Impact of the SEA-PHAGES program on research outcomes and student learning. Proc. Natl. Acad. Sci. USA 2017, 114, 13531–13536. [Google Scholar] [CrossRef]
- Hanauer, D.I.; Graham, M.J.; Jacobs-Sera, D.; Garlena, R.A.; Russell, D.A.; Sivanathan, V.; Asai, D.J.; Hatfull, G.F. Broadening Access to STEM through the Community College: Investigating the Role of Course-Based Research Experiences (CREs). CBE Life Sci. Educ. 2022, 21, ar38. [Google Scholar] [CrossRef]




| Phage Name | GenBank Accession | Year Isolated | Source |
|---|---|---|---|
| Ebordelon | PV565042.1 | 2017 | Soil |
| Holyagarpour | PV565043.1 | 2017 | Soil |
| Jeremy | PV565041.1 | 2017 | Soil |
| Laces98 | PV477980.1 | 2017 | Soil |
| Meara | PV565044.1 | 2017 | Soil |
| Stamos | PV565045.1 | 2017 | Soil |
| Waldo5 | MT711889.1 | 2017 | Soil |
| Bosely | PV329693.1 | 2018 | Soil |
| Zuri | MK863032.2 | 2018 | Soil |
| Dolphis | MT711888.1 | 2018 | Soil |
| Stalingrad | MT711887.2 | 2018 | Stream water |
| PlaquesPlease | MT711890.1 | 2018 | Streambed clay |
| DDSR119 | MT663720.1 | 2019 | Tree root soil |
| Phage Name | GenBank Accession | Bacterial Host | Publication PMID |
|---|---|---|---|
| phi15 | NC_015208.1 | P. putida | 21526174 [71] |
| Arace01 | PP179312.1 | P. putida | 38833289 [72] |
| gh-1 | NC_004665.1 | P. putida | 12842620 [38] |
| Henninger | NC_047922.1 | P. sp. | Unpublished |
| PHB04 | NC_047861.1 | Bordetella bronchiseptica | 30229303 [73] |
| ST4 | OR261032.1 | Aeromonas hydrophila | unpublished |
| Torfinnsbu | PQ464596.1 | P. poae | 39742975 [74] |
| Touem01 | PP179325.1 | P. syringae | 38833289 [72] |
| Phages * | GenBank Accession | Genome Length (bp) | GC Content | Protein- Coding Genes (tRNA Genes) | Proteins with Putative Functions | Packaged Genome Structure ** | Lifestyle |
|---|---|---|---|---|---|---|---|
| Autotranscriptaviridae | |||||||
| Waldovirus | |||||||
| PlaquesPlease | MT711890.1 | 41,456 | 58.07% | 51 (0) | 30 | 218 bp DTR | Virulent |
| Waldo5 | MT711889.1 | 41,195 | 57.73% | 53 (0) | 29 | 219 bp DTR | Virulent |
| Laces98 | PV477980.1 | 41,870 | 58.15% | 50 (0) | 32 | 219 bp DTR | Virulent |
| Troedvirus | |||||||
| Bosely | PV329693.1 | 40,504 | 57.82% | 50 (0) | 32 | 253 bp DTR | Virulent |
| Stamos | PV565045.1 | 40,667 | 58.38% | 48 (0) | 32 | 253 bp DTR | Virulent |
| Stalingrad | MT711887.2 | 40,723 | 57.85% | 49 (0) | 32 | 253 bp DTR | Virulent |
| Unclassified Caudoviricetes | |||||||
| Zurivirus | |||||||
| Zuri | NC_049456.2 | 75,873 | 53.53% | 104 (3) | 37 | 457 bp DTR | Virulent |
| Ebordelon | PV565042.1 | 74,043 | 53.70% | 93 (3) | 41 | 466 bp DTR | Virulent |
| Holyagarpour | PV565043.1 | 75,462 | 53.55% | 95 (3) | 43 | 467 bp DTR | Virulent |
| Novel genus related to Zurivirus | |||||||
| Meara | PV565044.1 | 74,705 | 53.68% | 97 (3) | 34 | Circularly permuted, TR | Virulent |
| Novel genus, no close relative | |||||||
| Dolphis | MT711888.1 | 93,507 | 63.71% | 125 (0) | 56 | Circularly permuted, TR | Temperate |
| Jeremy | PV565041.1 | 95,487 | 63.53% | 126 (0) | 54 | Circularly permuted, TR | Temperate |
| Novel genus, no close relative | |||||||
| DDSR119 | MT663720.1 | 52,905 | 60.39% | 79 (2) | 34 | Circularly permuted, TR | Virulent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, S.; Persinger, R.; Patel, A.; Rupe, E.; Osu, J.; Cooper, K.I.; Lehman, S.M.; Kongari, R.; Jaryenneh, J.D.; Mageeney, C.M.; et al. Survey of Thirteen Novel Pseudomonas putida Bacteriophages. Appl. Microbiol. 2025, 5, 108. https://doi.org/10.3390/applmicrobiol5040108
Anderson S, Persinger R, Patel A, Rupe E, Osu J, Cooper KI, Lehman SM, Kongari R, Jaryenneh JD, Mageeney CM, et al. Survey of Thirteen Novel Pseudomonas putida Bacteriophages. Applied Microbiology. 2025; 5(4):108. https://doi.org/10.3390/applmicrobiol5040108
Chicago/Turabian StyleAnderson, Simon, Rachel Persinger, Akaash Patel, Easton Rupe, Johnathan Osu, Katherine I. Cooper, Susan M. Lehman, Rohit Kongari, James D. Jaryenneh, Catherine M. Mageeney, and et al. 2025. "Survey of Thirteen Novel Pseudomonas putida Bacteriophages" Applied Microbiology 5, no. 4: 108. https://doi.org/10.3390/applmicrobiol5040108
APA StyleAnderson, S., Persinger, R., Patel, A., Rupe, E., Osu, J., Cooper, K. I., Lehman, S. M., Kongari, R., Jaryenneh, J. D., Mageeney, C. M., Cresawn, S. G., & Temple, L. (2025). Survey of Thirteen Novel Pseudomonas putida Bacteriophages. Applied Microbiology, 5(4), 108. https://doi.org/10.3390/applmicrobiol5040108






