Bioactive Metabolites from Yeasts Presumptively Qualified as Safe as Functional Agents in the Management of Type 2 Diabetes
Abstract
1. Introduction
2. Discovery of Probiotics and Characteristics of Probiotic Yeasts
3. Metabolites of QPS Yeasts
3.1. Metabolite Profile of QPS Yeasts
3.2. Antidiabetic Potential of Yeast-Secreted Metabolites
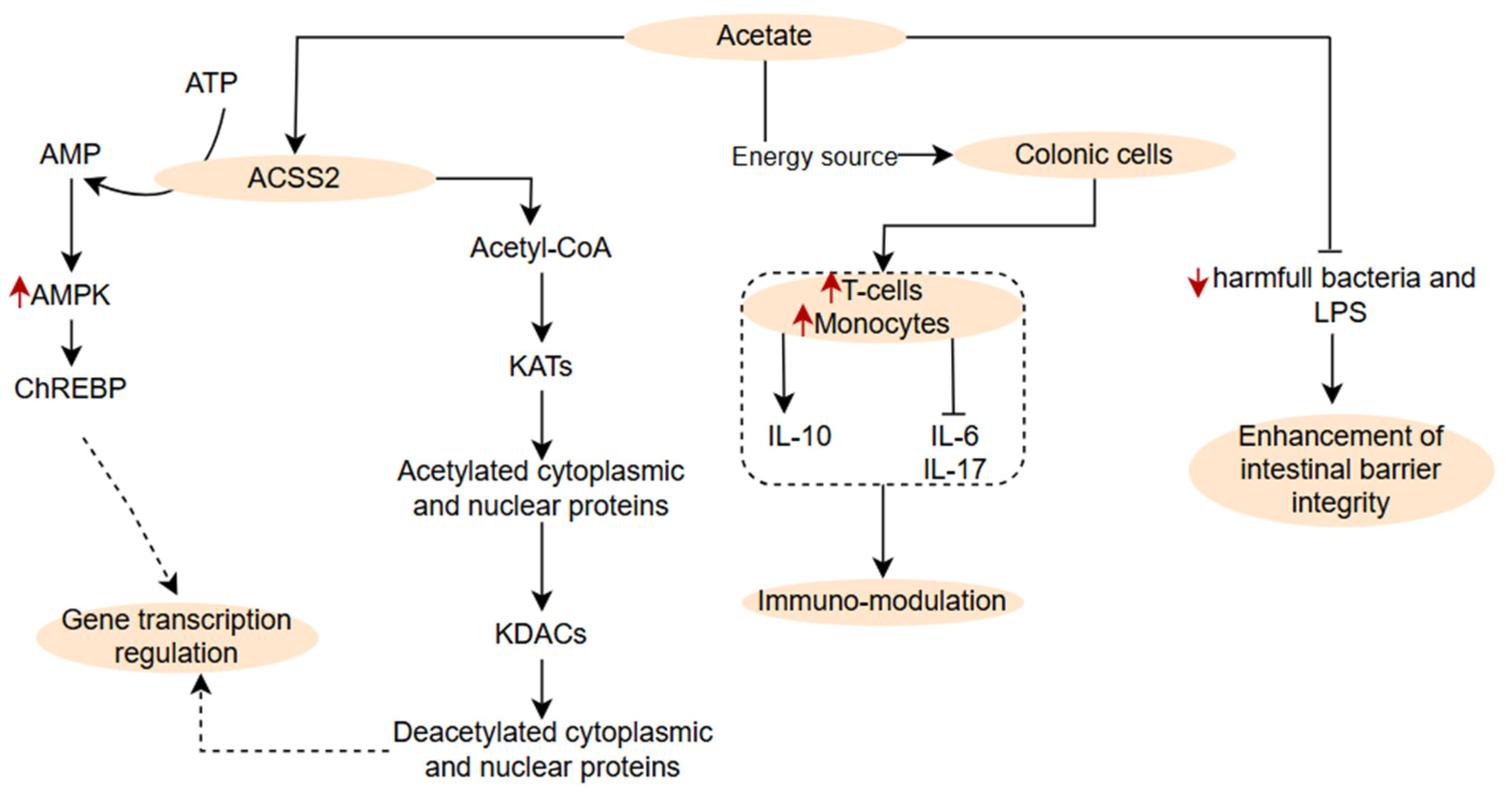
3.2.1. Branched and Non-Branched Short Chain Fatty Acids
3.2.2. Other Organic Acids
3.2.3. Bioactive Peptides
3.2.4. Carotenoids
| Carotenoid | Producing Yeast | Class | Antidiabetic Properties | Reference |
|---|---|---|---|---|
| β-Carotene | Rhodotorula glutinis, Sporobolomyces roseus | Carotene | ≈22% reduction in T2D risk, enhanced insulin sensitivity, antioxidant defence | [36] |
| γ-Carotene | Sporobolomyces spp., Sporidiobolus spp. | Carotene | Antioxidant properties, cardiovascular protection in diabetic individuals | [71] |
| Astaxanthin | Xanthophyllomyces dendrorhous | Xanthophyll | Improved glucose tolerance, reduced HbA1c, enhanced insulin responsiveness | [71] |
| Torularhodin | Sporidiobolus pararoseus, Rhodotorula spp. | Xanthophyll | Improved lipid metabolism, reduced hepatic inflammation, enhanced insulin sensitivity | [72] |
| Torulene | Sporidiobolus pararoseus, Rhodotorula glutinis | Carotene | Protection against hyperglycaemia-induced oxidative stress, superior antioxidant activity | [73] |
| Lycopene | Candida utilis (engineered), Saccharomyces cerevisiae (engineered) | Carotene | Reduced blood glucose, improved lipid profile, protection against diabetic complications | [74] |
| Canthaxanthin | Saccharomyces cerevisiae (engineered) | Xanthophyll | Oxidative stress tolerance, anti-inflammatory properties | [75] |
| Zeaxanthin | Saccharomyces cerevisiae (engineered) | Xanthophyll | Protection of diabetic retina, improved visual function | [75] |
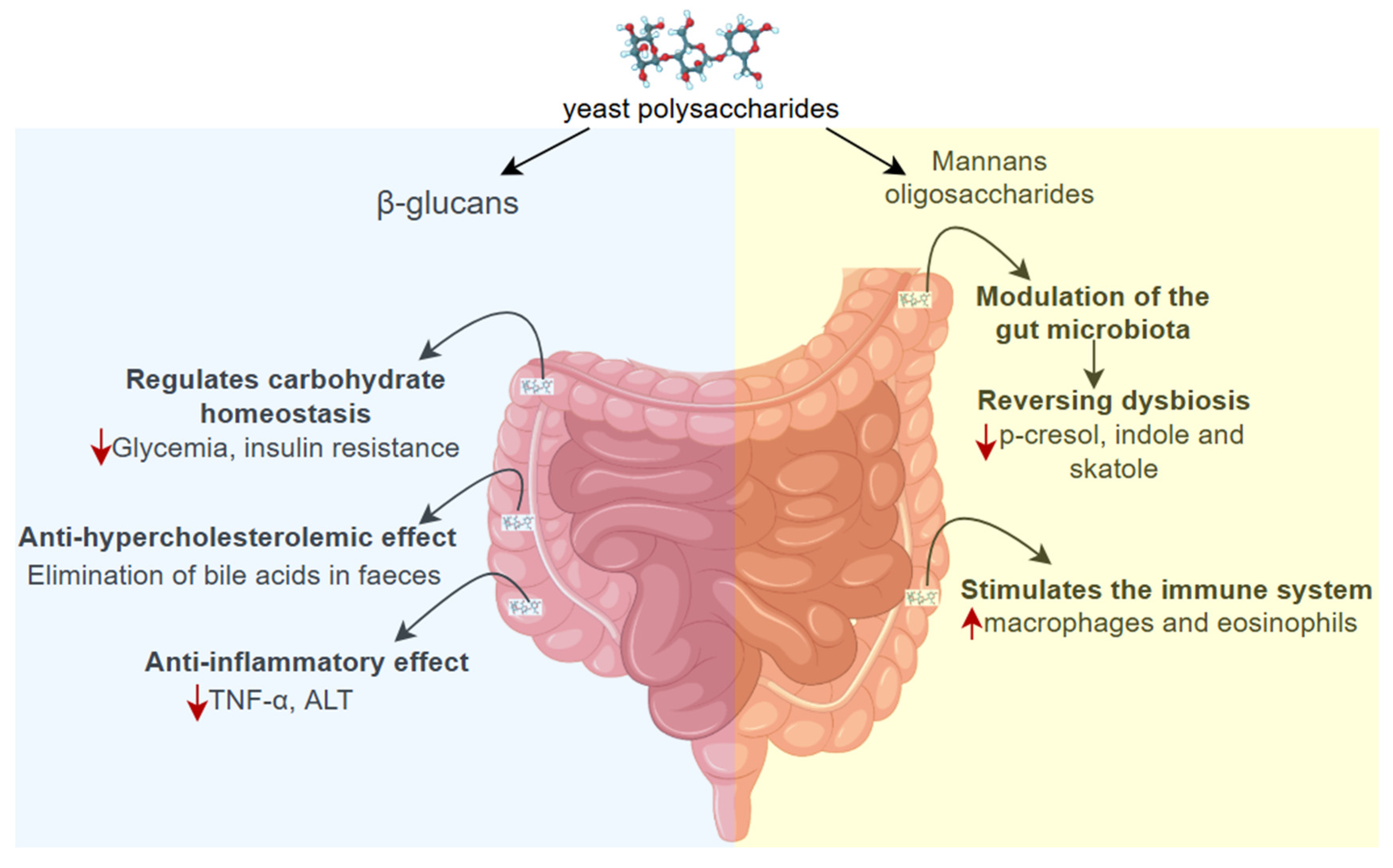
3.2.5. Polysaccharides
4. Potential of Postbiotic Formulations, Knowledge Gaps and Perspectives
4.1. Potential of Postbiotic Formulations from Yeast
4.2. Knowledge Gaps and Perspectives
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Guo, W.; Zhu, S.; Gong, G.; Chen, M.; Zhong, Z.; Guo, J.; Zhang, Y. Type 2 diabetes mellitus and the risk of abnormal spermatozoa: A Mendelian randomization study. Front. Endocrinol. 2022, 13, 1035338. [Google Scholar] [CrossRef] [PubMed]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S.; Ayesha, I.E. Probiotics and their role in the management of type 2 diabetes mellitus (Short-term versus long-term effect): A systematic review and meta-analysis. Cureus 2023, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ma, C.; Yang, Y.; Liu, X.; Wang, B.; Wang, Y.; Zhang, G.; Bian, X.; Zhang, N. The Role and Mechanism of Probiotics Supplementation in Blood Glucose Regulation: A Review. Foods 2024, 13, 2719. [Google Scholar] [CrossRef] [PubMed]
- Piame, L.T. Potential of Non-Dairy Probiotic Beverages to Modulate Gut Microbiota and Improve Management of Non-Communicable Diseases. Nov. Res. Microbiol. J. 2025, 9, 198–213. [Google Scholar] [CrossRef]
- Everard, A.; Matamoros, S.; Geurts, L.; Delzenne, N.M.; Cani, P.D. Saccharomyces boulardii administration changes gut microbiota and reduces hepatic steatosis, low-grade inflammation, and fat mass in obese and type 2 diabetic db/db mice. MBio 2014, 5, e01011-14. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Hurley, C.; Ryan, A.; Zamora-Úbeda, M.; Govindan, A.; Stanton, C.; Lane, G.P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Yeast β-glucan supplementation lowers insulin resistance without altering microbiota composition compared with placebo in subjects with type II diabetes: A phase I exploratory study. Br. J. Nutr. 2024, 132, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, R.C.M.F.; Brandão, A.B.P.; De Abreu, I.C.M.E.; Ferreira, F.G.; Santos, L.B.; Moreira, L.N.; Taddei, C.R.; Cunha, F.A.A.T.S. Saccharomyces boulardii Tht 500101 changes gut microbiota and ameliorates hyperglycaemia, dyslipidaemia, and liver inflammation in streptozotocin-diabetic mice. Benef. Microbes 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.; Albuquerque, R.; Brandão, A.B.P.; Barssotti, L.; de Souza, L.B.; Ferreira, F.G.; Oliveira, L.C.G.; Yokota, R.; Sparvoli, L.G.; Dias, D.D.S.; et al. Saccharomyces boulardii exerts renoprotection by modulating oxidative stress, renin angiotensin system and uropathogenic microbiota in a murine model of diabetes. Life Sci. 2022, 301, 120616. [Google Scholar] [CrossRef] [PubMed]
- Barssotti, L.; Abreu, I.C.M.E.; Brandão, A.B.P.; Albuquerque, R.C.M.F.; Ferreira, F.G.; Salgado, M.A.C.; Dias, D.D.S.; De Angelis, K.; Yokota, R.; Casarini, D.E.; et al. Saccharomyces boulardii modulates oxidative stress and renin angiotensin system attenuating diabetes-induced liver injury in mice. Sci. Rep. 2021, 11, 9189. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R. Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 19: Suitability of taxonomic units notified to EFSA until September 2023. EFSA J. 2024, 22, e8517. [Google Scholar] [CrossRef] [PubMed]
- Aquino, M.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O.; Cian, R.E. Anti-diabetic properties of brewer’s spent yeast peptides. In vitro, in silico and ex vivo study after simulated gastrointestinal digestion. Food Funct. 2024, 15, 3778–3790. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Zou, S.; Li, M.; Xu, X. Orally Administered Baker’s Yeast β-Glucan Promotes Glucose and Lipid Homeostasis in the Livers of Obesity and Diabetes Model Mice. J. Agric. Food Chem. 2017, 65, 9665–9674. [Google Scholar] [CrossRef] [PubMed]
- Poorniammal, R.; Sarathambal, C.; Sakthi, A.; Arun, S. Yeast carotenoids importance in food and feed industries and its health benefits–A review. Agric. Rev. 2013, 34, 307–312. [Google Scholar] [CrossRef]
- Noh, Y.-H.; Lee, D.-B.; Lee, Y.-W.; Pyo, Y.-H. In vitro inhibitory effects of organic acids identified in commercial vinegars on α-amylase and α-glucosidase. Prev. Nutr. Food Sci. 2020, 25, 319. [Google Scholar] [CrossRef] [PubMed]
- Baek, K.-R.; Lee, H.-J.; Jeong, K.-H.; Park, B.-R.; Kim, S.-J.; Seo, S.-O. Parabiotic immunomodulatory activity of yeast cell wall polysaccharides from Saccharomyces cerevisiae and S. boulardii. J. Funct. Foods 2024, 123, 106577. [Google Scholar] [CrossRef]
- Prajapati, N.; Patel, J.; Singh, S.; Yadav, V.K.; Joshi, C.; Patani, A.; Prajapati, D.; Sahoo, D.K.; Patel, A. Postbiotic production: Harnessing the power of microbial metabolites for health applications. Front. Microbiol. 2023, 14, 1306192. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, P.A. Recycling metchnikoff: Probiotics, the intestinal microbiome and the quest for long life. Front. Public Health 2013, 1, 52. [Google Scholar] [CrossRef] [PubMed]
- Lagier, J.C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Gimenez, G.; Maraninchi, M.; et al. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.; Dufour, J.C.; Lagier, J.C.; Cadoret, F.; Daoud, Z.; Dubourg, G.; Raoult, D. The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species. Microbiome 2018, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lucas, N.; Legrand, R.; Deroissart, C.; Dominique, M.; Azhar, S.; Le Solliec, M.-A.; Léon, F.; Rego, J.-C.D.; Déchelotte, P.; Fetissov, S.O. Hafnia alvei HA4597 strain reduces food intake and body weight gain and improves body composition, glucose, and lipid metabolism in a mouse model of hyperphagic obesity. Microorganisms 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, C.; Qin, X.; Zhou, B.; Liu, X.; Liu, T.; Xie, R.; Liu, J.; Wang, B.; Cao, H. Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol. Res. 2022, 181, 106291. [Google Scholar] [CrossRef] [PubMed]
- Pothoulakis, C. anti-inflammatory mechanisms of action of Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2009, 30, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Bischoff, S.C. Influence of Saccharomyces boulardii CNCM I-745on the gut-associated immune system. Clin. Exp. Gastroenterol. 2016, 13, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, A.; Kordowska-Wiater, M. Probiotic and potentially probiotic yeasts—Characteristics and food application. Foods 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of yeasts as potential probiotics: A review of gastrointestinal tract conditions and investigation methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V. Probiotic yeasts: A developing reality? J. Fungi 2024, 10, 489. [Google Scholar] [CrossRef] [PubMed]
- Tchamani Piame, L.; Kaktcham, P.M.; Kouam, E.M.F.; Techeu, U.D.F.; Ngouénam, R.J.; Ngoufack, F.Z. Technological characterisation and probiotic traits of yeasts isolated from Sha’a, a Cameroonian maize-based traditional fermented beverage. Heliyon 2022, 8, e10850. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef] [PubMed]
- Moslehi-Jenabian, S.; Pedersen, L.L.; Jespersen, L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients 2010, 2, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, P.; Djazayery, A.; Mostafavi, S.-A.; Javanbakht, M.H.; Derakhshanian, H.; Rahimiforoushani, A.; Djalali, M. Brewer’s yeast improves blood pressure in type 2 diabetes mellitus. Iran. J. Public Health 2013, 42, 602. [Google Scholar] [PubMed]
- Horn, P.A.; Zeni, A.L.B.; Herkenhoff, M.E.; Curbani, L.; Gonçalves, G.H.P.; Rutkoski, C.F.; Israel, N.G.; de Almeida, E.A. Brewer’s spent yeast improves human gut microbiota and ameliorates clinical blood parameters: A randomized, double-blind, placebo-controlled trial. Bioact. Carbohydr. Diet. Fibre 2024, 32, 100442. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Obaid, R.S.; Olaimat, A.N.; Liu, S.-Q.; Ayyash, M.M. In vitro characterization and identification of potential probiotic yeasts isolated from fermented dairy and non-dairy food products. J. Fungi 2022, 8, 544. [Google Scholar] [CrossRef] [PubMed]
- Chapot-Chartier, M.-P.; Kulakauskas, S. Cell wall structure and function in lactic acid bacteria. Microb. Cell Factories 2014, 13 (Suppl. 1), S9. [Google Scholar] [CrossRef] [PubMed]
- Hahn-Hägerdal, B.; Karhumaa, K.; Larsson, C.U.; Gorwa-Grauslund, M.; Görgens, J.; Van Zyl, W.H. Role of cultivation media in the development of yeast strains for large scale industrial use. Microb. Cell Factories 2005, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, J.; Mi, Z.; Mo, L.; Jin, H.; Yao, C.; Ren, D.; Menghe, B. A metabolomics approach uncovers differences between traditional and commercial dairy products in Buryatia (Russian Federation). Molecules 2018, 23, 735. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Sha, S.; Ghatani, K. Metabolomics of ethnic fermented foods and beverages: Understanding new aspects through omic techniques. Front. Sustain. Food Syst. 2023, 7, 1040567. [Google Scholar] [CrossRef]
- De Miranda, N.M.Z.; De Souza, A.C.; de Souza Costa Sobrinho, P.; Dias, D.R.; Schwan, R.F.; Ramos, C.L. Novel yeasts with potential probiotic characteristics isolated from the endogenous ferment of artisanal Minas cheese. Braz. J. Microbiol. 2023, 54, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.W.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Abildinova, G.Z.; Benberin, V.V.; Vochshenkova, T.A.; Afshar, A.; Mussin, N.M.; Kaliyev, A.A.; Zhussupova, Z.; Tamadon, A. Global trends and collaborative networks in gut microbiota-insulin resistance research: A comprehensive bibliometric analysis (2000–2024). Front. Med. 2024, 11, 1452227. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Fujisawa, K.; Ito, E.; Idei, S.; Kawaguchi, N.; Kimoto, M.; Hiemori, M.; Tsuji, H. Improvement of obesity and glucose tolerance by acetate in Type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Biosci. Biotechnol. Biochem. 2007, 71, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Moffett, J.R.; Puthillathu, N.; Vengilote, R.; Jaworski, D.M.; Namboodiri, A.M. Acetate revisited: A key biomolecule at the nexus of metabolism, epigenetics and oncogenesis—Part 1: Acetyl-CoA, acetogenesis and acyl-CoA short-chain synthetases. Front. Physiol. 2020, 11, 580167. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.K.; Janowitz, C.; Metea, C.; Asquith, M.; Karstens, L.; Rosenbaum, J.T.; Lin, P. Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci. Rep. 2017, 7, 11745. [Google Scholar] [CrossRef] [PubMed]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Kucuksen, S.; Kucuk, A.; Cicekler, H. Serum ischemia-modified albumin and malondialdehyde levels and superoxide dismutase activity in patients with fibromyalgia. Clin. Lab. 2014, 60, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, M.S.; Stocker, C.J.; O’Dowd, J.; Davies, A.; Bellahcene, M.; Cawthorne, M.A.; Brown, A.J.; Smith, D.M.; Arch, J.R. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 2010, 584, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Ives, S.J.; Zaleski, K.S.; Slocum, C.; Escudero, D.; Sheridan, C.; Legesse, S.; Vidal, K.; Lagalwar, S.; Reynolds, T.H. The effect of succinic acid on the metabolic profile in high-fat diet-induced obesity and insulin resistance. Physiol. Rep. 2020, 8, e14630. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, L.; Li, Y.; Shi, X.; Li, C.; Chai, J.; Jiang, S.; Zheng, R. Succinic Acid Improves the Metabolism of High-Fat Diet-Induced Mice and Promotes White Adipose Browning. Nutrients 2024, 16, 3828. [Google Scholar] [CrossRef] [PubMed]
- Glastras, S.J.; Chen, H.; Teh, R.; McGrath, R.T.; Chen, J.; Pollock, C.A.; Wong, M.G.; Saad, S. Mouse models of diabetes, obesity and related kidney disease. PloS ONE 2016, 11, e0162131. [Google Scholar] [CrossRef] [PubMed]
- Muhamed, S.A.; Moussa, E.M.; Aboasy, N.K.; Gaweesh, Y.Y. Effect of 1% malic acid spray on diabetes mellitus-induced xerostomia: A randomized clinical trial. Oral Dis. 2024, 30, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Shavandi, A.; Mirdamadi, S.; Soleymanzadeh, N.; Motahari, P.; Mirdamadi, N.; Moser, M.; Subra, G.; Alimoradi, H.; Goriely, S. Bioactive peptides from yeast: A comparative review on production methods, bioactivity, structure-function relationship, and stability. Trends Food Sci. Technol. 2021, 118, 297–315. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, H.S.; Choi, J.W.; Ra, K.S.; Kim, M.R.; Suh, H.J. Glucose tolerance and antioxidant activity of Spent Brewer’s yeast hydrolysate with a high content of cyclo-his-pro (CHP). J. Food Sci. 2011, 76, C272–C278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Bai, L.; Wang, Y.; Yang, J.K. The benefits and risks of DPP 4-inhibitors vs. sulfonylureas for patients with type 2 diabetes: Accumulated evidence from randomised controlled trial. Int. J. Clin. Pract. 2016, 70, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.; Bhatt, S.; Schoenly, N.E.; Lee, A.Y.; Nislow, C.; Bobek, L.A. Chemical genomic screening of a Saccharomyces cerevisiae genomewide mutant collection reveals genes required for defense against four antimicrobial peptides derived from proteins found in human saliva. Antimicrob. Agents Chemother. 2013, 57, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Viana, T.; Albergaria, H.; Arneborg, N. Antimicrobial peptides (AMPs) produced by Saccharomyces cerevisiae induce alterations in the intracellular pH, membrane permeability and culturability of Hanseniaspora guilliermondii cells. Int. J. Food Microbiol. 2015, 205, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Gier, S.; Lermen, M.; Schmitt, M.J.; Breinig, F. Substitution of cysteines in the yeast viral killer toxin K1 precursor reveals novel insights in heterodimer formation and immunity. Sci. Rep. 2019, 9, 13127. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Watcharawipas, A.; Runguphan, W. Red yeasts and their carotenogenic enzymes for microbial carotenoid production. FEMS Yeast Res. 2023, 23, foac063. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Yonekura, L.; Nagao, A. Effect of glycerophospholipid class on the β-carotene uptake by human intestinal Caco-2 cells. Biosci. Biotechnol. Biochem. 2010, 74, 209–211. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto carotenoids attenuate oxidative stress and inflammatory response after high-calorie meal in healthy subjects. Food Res. Int. 2017, 100, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, Z.; Hadjzadeh, M.-A.-R.; Nemati, H.; Hosseini, M.; Ahmadi, M.; Shafiee, S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J. Med. Food 2013, 16, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Duzguner, V.; Kucukgul, A.; Erdogan, S.; Celik, S.; Sahin, K. Effect of lycopene administration on plasma glucose, oxidative stress and body weight in streptozotocin diabetic rats. J. Appl. Anim. Res. 2008, 33, 17–20. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Xu, G.; Zheng, S.; Sun, S.; Wen, N.; Sheng, L.; Shi, Y.; Zhang, Y. Beneficial impact of crocetin, a carotenoid from saffron, on insulin sensitivity in fructose-fed rats. J. Nutr. Biochem. 2007, 18, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Muriach, M.; Bosch-Morell, F.; Alexander, G.; Blomhoff, R.; Barcia, J.; Arnal, E.; Almansa, I.; Romero, F.J.; Miranda, M. Lutein effect on retina and hippocampus of diabetic mice. Free. Radic. Biol. Med. 2006, 41, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Innocenti, M.; Turchetti, B.; Libkind, D.; van Broock, M.; Mulinacci, N. Carotenoid profiles of yeasts belonging to the genera Rhodotorula, Rhodosporidium, Sporobolomyces, and Sporidiobolus. Can. J. Microbiol. 2007, 53, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, Y.; Li, J.; Liu, C.; Qian, H.; Zhang, G. Torularhodin Alleviates Hepatic Dyslipidemia and Inflammations in High-Fat Diet-Induced Obese Mice via PPARα Signaling Pathway. Molecules 2022, 27, 6398. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, H.; Du, C.; Zhang, W.; Qian, H. Tentative identification of torulene cis/trans geometrical isomers isolated from Sporidiobolus pararoseus by high-performance liquid chromatography-diode array detection-mass spectrometry and preparation by column chromatography. Anal. Sci. 2013, 29, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Elfeky, N.; Elmahmoudy, M.; Zhang, Y.; Guo, J.; Bao, Y. Lipid and Carotenoid Production by Rhodotorula glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur, and Aluminium Stress. Appl. Sci. 2019, 9, 2444. [Google Scholar] [CrossRef]
- Promdonkoy, P.A.; Watcharawipas, S.; Bubphasawan, K.; Sansatchanon, N.; Suwanakitti; Kocharin, K.; Runguphan, W. Metabolic Engineering of Saccharomyces cerevisiae for Production of Canthaxanthin, Zeaxanthin, and Astaxanthin. J. Fungi 2024, 10, 433. [Google Scholar]
- Lobato, R.V.; Silva, V.O.; Andrade, E.F.; Orlando, D.R.; Zangeronimo, M.G.; de Souza, R.V.; Pereira, L.J. Metabolic effects of β-glucans (Saccharomyces cerevisae) per os administration in rats with streptozotocin-induced diabetes. Nutr. Hosp. 2015, 32, 256–264. [Google Scholar]
- Palacios-Garcia, A.A.; Yamamoto-Cuevas, J.V.; Abreu-Rosario, C.G.; Moreno-Higareda, R.; Ceron-Trujillo, B.; Ramirez-Ramirez, E. Systematic Review and Meta-Analysis of the Effects of a Polysaccharide-Rich Hydrolysate Derived from Saccharomyces cerevisiae on Body Weight Reduction. 2025. Available online: https://www.preprints.org/manuscript/202505.0491/v1 (accessed on 20 July 2025).
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Hoving, L.R.; van der Zande, H.J.; Pronk, A.; Guigas, B.; van Dijk, K.W.; van Harmelen, V. Dietary yeast-derived mannan oligosaccharides have immune-modulatory properties but do not improve high fat diet-induced obesity and glucose intolerance. PloS ONE 2018, 13, e0196165. [Google Scholar] [CrossRef] [PubMed]
- Tanihiro, R.; Sakano, K.; Oba, S.; Nakamura, C.; Ohki, K.; Hirota, T.; Sugiyama, H.; Ebihara, S.; Nakamura, Y. Effects of yeast mannan which promotes beneficial Bacteroides on the intestinal environment and skin condition: A randomized, double-blind, placebo-controlled study. Nutrients 2020, 12, 3673. [Google Scholar] [CrossRef] [PubMed]
- Günal-Köroğlu, D.; Karabulut, G.; Mohammadian, F.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Production of yeast cell wall polysaccharides-β-glucan and chitin by using food waste substrates: Biosynthesis, production, extraction, and purification methods. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70161. [Google Scholar] [CrossRef] [PubMed]
- Chioru, A.; Chirsanova, A.; Dabija, A.; Avrămia, I.; Boiştean, A.; Chetrariu, A. Extraction Methods and Characterization of β-Glucans from Yeast Lees of Wines Produced Using Different Technologies. Foods 2024, 13, 3982. [Google Scholar] [CrossRef] [PubMed]
- Duysburgh, C.; Miclotte, L.; Green, J.B.; Watts, K.T.; Sardi, M.I.; Chakrabarti, A.; Khafipour, E.; Marzorati, M. Saccharomyces cerevisiae derived postbiotic alters gut microbiome metabolism in the human distal colon resulting in immunomodulatory potential in vitro. Front. Microbiol. 2024, 15, 1358456. [Google Scholar] [CrossRef] [PubMed]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Hart, A.N.; Schauss, A.G. An antiinflammatory immunogen from yeast culture induces activation and alters chemokine receptor expression on human natural killer cells and B lymphocytes in vitro. Nutr. Res. 2007, 27, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Robinson, L.E.; Zawada, E.T., Jr.; Kittelsrud, J.M.; Chen, D.-G.; Reeves, S.G.; Weaver, S.E. Effects of a modified yeast supplement on cold/flu symptoms. Urol. Nurs. 2008, 28, 50–55. [Google Scholar] [PubMed]
- Moyad, M.A.; Robinson, L.E.; Zawada, E.T., Jr.; Kittelsrud, J.; Chen, D.-G.; Reeves, S.G.; Weaver, S. Immunogenic yeast-based fermentate for cold/flu-like symptoms in nonvaccinated individuals. J. Altern. Complement. Med. 2010, 16, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A.; Robinson, L.E.; Kittelsrud, J.M.; Reeves, S.G.; Weaver, S.E.; Guzman, A.I.; Bubak, M.E. Immunogenic yeast-based fermentation product reduces allergic rhinitis-induced nasal congestion: A randomized, double-blind, placebo-controlled trial. Adv. Ther. 2009, 26, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A. Brewer’s/baker’s yeast (Saccharomyces cerevisiae) and preventive medicine: Part II. Urol. Nurs. 2008, 28, 73–75. [Google Scholar] [PubMed]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchamani Piame, L. Bioactive Metabolites from Yeasts Presumptively Qualified as Safe as Functional Agents in the Management of Type 2 Diabetes. Appl. Microbiol. 2025, 5, 84. https://doi.org/10.3390/applmicrobiol5030084
Tchamani Piame L. Bioactive Metabolites from Yeasts Presumptively Qualified as Safe as Functional Agents in the Management of Type 2 Diabetes. Applied Microbiology. 2025; 5(3):84. https://doi.org/10.3390/applmicrobiol5030084
Chicago/Turabian StyleTchamani Piame, Laverdure. 2025. "Bioactive Metabolites from Yeasts Presumptively Qualified as Safe as Functional Agents in the Management of Type 2 Diabetes" Applied Microbiology 5, no. 3: 84. https://doi.org/10.3390/applmicrobiol5030084
APA StyleTchamani Piame, L. (2025). Bioactive Metabolites from Yeasts Presumptively Qualified as Safe as Functional Agents in the Management of Type 2 Diabetes. Applied Microbiology, 5(3), 84. https://doi.org/10.3390/applmicrobiol5030084






