Fungal Biotechnology Applications in Sustainable Oil Extraction
Abstract
1. Introduction
2. Biotechnological Interests of Filamentous Fungi and Their Key Associated Genera
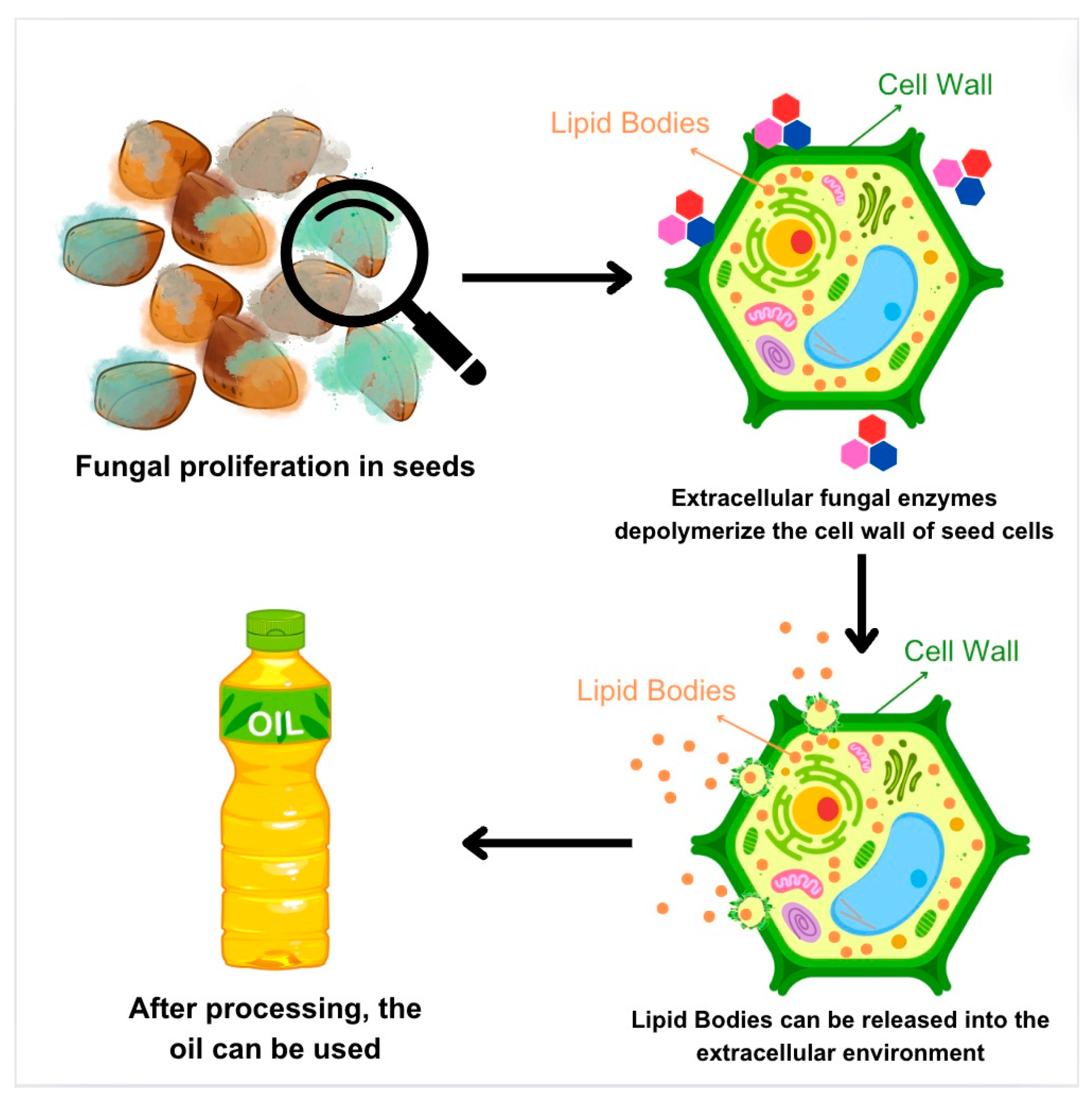
3. Exploring the Mechanisms and Implications of Fungal Seed Degradation for Oil Extraction
4. Exploration of Traditional Amazonian Techniques for Oil Extraction
5. Advantages and Disadvantages of Using Filamentous Fungi in Seed Degradation for Oil Extraction
6. Future Perspectives and Emerging Biotechnologies
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amazônia. Ministério do Meio Ambiente e Mudança do Clima. Available online: https://www.gov.br/mma/pt-br/assuntos/biodiversidade-e-biomas/biomas-e-ecossistemas/biomas/amazonia (accessed on 9 September 2024).
- Macedo, G. Climate Security, the Amazon, and the Responsibility to Protect. Braz. Political Sci. Rev. 2021, 15, e0007. [Google Scholar] [CrossRef]
- Dias, K.K.B.; Cardoso, A.L.; da Costa, A.A.F.; Passos, M.F.; Costa, C.E.F.d.; Rocha Filho, G.N.d.; Andrade, E.H.d.A.; Luque, R.; Nascimento, L.A.S.d.; Noronha, R.C.R. Biological Activities from Andiroba (Carapa guianensis Aublet.) and Its Biotechnological Applications: A Systematic Review. Arab. J. Chem. 2023, 16, 104629. [Google Scholar] [CrossRef]
- Mendonça, A.P.; Ferraz, I.D.K. Óleo de Andiroba: Processo Tradicional Da Extração, Uso e Aspectos Sociais No Estado Do Amazonas, Brasil. Acta Amaz. 2007, 37, 353–364. [Google Scholar] [CrossRef]
- Lavenburg, V.M.; Rosentrater, K.A.; Jung, S. Extraction Methods of Oils and Phytochemicals from Seeds and Their Environmental and Economic Impacts. Processes 2021, 9, 1839. [Google Scholar] [CrossRef]
- Cravotto, C.; Claux, O.; Bartier, M.; Fabiano-Tixier, A.-S.; Tabasso, S. Leading Edge Technologies and Perspectives in Industrial Oilseed Extraction. Molecules 2023, 28, 5973. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.G.; Cohen, J.L.; Bell, J.M.L.N.d.M. Conversion of Agricultural Streams and Food-Processing By-Products to Value-Added Compounds Using Filamentous Fungi. Annu. Rev. Food Sci. Technol. 2018, 9, 503–523. [Google Scholar] [CrossRef]
- Sousa, D.; Salgado, J.M.; Cambra-López, M.; Dias, A.; Belo, I. Bioprocessing of Oilseed Cakes by Fungi Consortia: Impact of Enzymes Produced on Antioxidants Release. J. Biotechnol. 2023, 364, 5–12. [Google Scholar] [CrossRef]
- Griebeler, N.E.; Bortoli, V.d.; Astolfi, A.L.; Daronch, N.A.; Schumann, A.C.; Salazar, L.N.; Cansian, R.L.; Backes, G.T.; Zeni, J. Seleção de fungos filamentosos produtores de amilases, proteases, celulases e pectinases. Rev. Acadêmica Ciênc. Anim. 2015, 13, 13–22. [Google Scholar] [CrossRef]
- Payne, C.M.; Knott, B.C.; Mayes, H.B.; Hansson, H.; Himmel, M.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T. Fungal Cellulases. Chem. Rev. 2015, 115, 1308–1448. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Lima, G.D.S.; dos Santos, G.F.; Lyra, F.H.; da Silva-Hughes, A.F.; Goncalves, F.A. Filamentous Fungi and Chemistry: Old Friends, New Allies. Rev. Virtual Quím. 2017, 9, 2351–2382. [Google Scholar] [CrossRef]
- Fleming, A.; Wise, R.M.; Hansen, H.; Sams, L. The Sustainable Development Goals: A Case Study. Mar. Policy 2017, 86, 94–103. [Google Scholar] [CrossRef]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; De Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a Circular Economy with Fungal Biotechnology: A White Paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- CDC. About Fungal Diseases. Fungal Diseases. Available online: https://www.cdc.gov/fungal/about/index.html (accessed on 26 August 2024).
- Hussin, M.S.; Nuaman, R.S.; Al-Shammari, R.H.H. Fungal Importance in Our Environment. South Asian Res. J. Biol. Appl. Biosci. 2023, 5, 123–128. [Google Scholar] [CrossRef]
- Li, Y.; Steenwyk, J.L.; Chang, Y.; Wang, Y.; James, T.Y.; Stajich, J.E.; Spatafora, J.W.; Groenewald, M.; Dunn, C.W.; Hittinger, C.T.; et al. A Genome-Scale Phylogeny of the Kingdom Fungi. Curr. Biol. 2021, 31, 1653–1665.e5. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-N.; Guo, S.; Zhang, S. Effects of Solid-State Fermentation with Three Higher Fungi on the Total Phenol Contents and Antioxidant Properties of Diverse Cereal Grains. FEMS Microbiol. Lett. 2018, 365, fny163. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, X.; Zeng, J.; Chen, S. Feasibility of Filamentous Fungi for Biofuel Production Using Hydrolysate from Dilute Sulfuric Acid Pretreatment of Wheat Straw. Biotechnol. Biofuels 2012, 5, 50. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic Residues: Biodegradation and Bioconversion by Fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Madhavan, A.; Arun, K.; Sindhu, R.; Alphonsa Jose, A.; Pugazhendhi, A.; Binod, P.; Sirohi, R.; Reshmy, R.; Kumar Awasthi, M. Engineering Interventions in Industrial Filamentous Fungal Cell Factories for Biomass Valorization. Bioresour. Technol. 2022, 344, 126209. [Google Scholar] [CrossRef] [PubMed]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.K.; Krüger, C.; Silveira, M.A.D.; Piana, P.A.; Rodrigues, M.L.F.; Rosado, A.F.; da Silva de Lucca, R.A.; Fagundes-Klen, M.R.; da Silva, E.A.; Buzanello, C.V.; et al. Lipolytic Production from Solid-State Fermentation of the Filamentous Fungus Penicillium Polonicum and Its Applicability as Biocatalyst in the Synthesis of Ethyl Oleate. Environ. Sci. Pollut. Res. Int. 2024, 31, 28632–28643. [Google Scholar] [CrossRef] [PubMed]
- Venkatesagowda, B.; Ponugupaty, E.; Barbosa, A.M.; Dekker, R.F.H. Diversity of Plant Oil Seed-Associated Fungi Isolated from Seven Oil-Bearing Seeds and Their Potential for the Production of Lipolytic Enzymes. World J. Microbiol. Biotechnol. 2012, 28, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Abdul-Hadi, S.Y.; Darwish, A.G. Aspergillus: Biodiversity, Ecological Significances, and Industrial Applications. In Recent Advancement in White Biotechnology Through Fungi; Yadav, A.N., Mishra, S., Singh, S., Gupta, A., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 121–179. [Google Scholar] [CrossRef]
- Ilić, N.; Milić, M.; Beluhan, S.; Dimitrijević-Branković, S. Cellulases: From Lignocellulosic Biomass to Improved Production. Energies 2023, 16, 3598. [Google Scholar] [CrossRef]
- Squina, F.M.; Mort, A.J.; Decker, S.R.; Prade, R.A. Xylan Decomposition by Aspergillus Clavatus Endo-Xylanase. Protein Expr. Purif. 2009, 68, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Tewari, R.; Gupta, J.K.; Soni, R.; Soni, S.K. A Novel Strain of Aspergillus Niger Producing a Cocktail of Hydrolytic Depolymerising Enzymes for the Production of Second Generation Biofuels. BioResources 2010, 6, 552–569. [Google Scholar] [CrossRef]
- Renuka, J.S.; Shrivastava, D.; Singh, S. An Emerging Microbe for Food Enzyme Production in Biomanufacturing. Salud Cienc. Tecnol. 2023, 3, 410. [Google Scholar] [CrossRef]
- Tanaka, M.; Gomi, K. Induction and Repression of Hydrolase Genes in Aspergillus oryzae. Front. Microbiol. 2021, 12, 677603. [Google Scholar] [CrossRef]
- Gusakov, A.V.; Sinitsyn, A.P. Cellulases from Penicillium Species for Producing Fuels from Biomass. Biofuels 2012, 3, 463–477. [Google Scholar] [CrossRef]
- Santi, L.; Beys-da-Silva, W.O.; Berger, M.; Yates, J.R.; Brandelli, A.; Vainstein, M.H. Penicillium Oxalicum Secretomic Analysis Identify Plant Cell Wall Degrading Enzymes Important for Fruit Juice Extraction. J. Food Sci. Technol. 2021, 58, 1764–1775. [Google Scholar] [CrossRef]
- Barreiro, C.; Albillos, S.M.; García-Estrada, C. Penicillium Chrysogenum: Beyond the Penicillin. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; Volume 127, pp. 143–221. [Google Scholar] [CrossRef]
- Freitas, A.; Escaramboni, B.; Carvalho, A.; Lima, V.; Oliva-Neto, P. Production and Application of Amylases of Rhizopus oryzae and Rhizopus microsporus var. oligosporus from Industrial Waste in Acquisition of Glucose. Chem. Pap. 2014, 68, 442–450. [Google Scholar] [CrossRef]
- Londoño-Hernández, L.; Ramírez-Toro, C.; Ruiz, H.A.; Ascacio-Valdés, J.A.; Aguilar-Gonzalez, M.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Rhizopus oryzae—Ancient Microbial Resource with Importance in Modern Food Industry. Int. J. Food Microbiol. 2017, 257, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Reis, W.S.M.; Matias, A.B.; Mendes, A.A.; de Castro, H.F.; Pereira, E.B. Production and Characterization of Whole-Cell Rhizopus oryzae CCT3759 to Be Applied as Biocatalyst in Vegetable Oils Hydrolysis. Catal. Lett. 2022, 152, 1–11. [Google Scholar] [CrossRef]
- Denardi-Souza, T.; Massarolo, K.C.; Tralamazza, S.M.; Badiale-Furlong, E. Monitoring of Fungal Biomass Changed by Rhizopus oryzae in Relation to Amino Acid and Essential Fatty Acids Profile in Soybean Meal, Wheat and Rice. CyTA—J. Food 2018, 16, 156–164. [Google Scholar] [CrossRef]
- Sousa, D.; Salgado, J.M.; Cambra-López, M.; Dias, A.C.; Belo, I. Degradation of Lignocellulosic Matrix of Oilseed Cakes by Solid-State Fermentation: Fungi Screening for Enzymes Production and Antioxidants Release. J. Sci. Food Agric. 2022, 102, 1550–1560. [Google Scholar] [CrossRef]
- Keshavarz, B.; Khalesi, M. Trichoderma reesei, a Superior Cellulase Source for Industrial Applications. Biofuels 2016, 7, 713–721. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and Biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef] [PubMed]
- De Paula, R.G.; Antoniêto, A.C.C.; Ribeiro, L.F.C.; Carraro, C.B.; Nogueira, K.M.V.; Lopes, D.C.B.; Silva, A.C.; Zerbini, M.T.; Pedersoli, W.R.; Costa, M.D.N.; et al. New Genomic Approaches to Enhance Biomass Degradation by the Industrial Fungus Trichoderma reesei. Int. J. Genom. 2018, 2018, 1974151. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of Mechanisms and Uses of Trichoderma Spp. Phytopathology® 2006, 96, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S. Enzymatic and saprophytic ability of Trichoderma species for biological control of fungal plant pathogens. Indian J. Sci. Res. 2023, 13, 33–42. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gené, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: Mais Que Um Nó Ou Célula Basal Em Forma de Pé. Stud. Mycol. 2021, 98, 100116. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.L.; Oliveira, H.S.S.; Uetanabaro, A.P.T.; Kamida, H.M. Biodegradação de celulose e lignina por fungos: Uma breve revisão. Sitientibus Sér. Ciênc. Biológicas 2009, 9, 35–40. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current Status of Fusarium oxysporum Formae Speciales and Races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef]
- Perincherry, L.; Urbaniak, M.; Pawłowicz, I.; Kotowska, K.; Waśkiewicz, A.; Stępień, Ł. Dynamics of Fusarium Mycotoxins and Lytic Enzymes during Pea Plants’ Infection. Int. J. Mol. Sci. 2021, 22, 9888. [Google Scholar] [CrossRef] [PubMed]
- Ben Taheur, F.; Kouidhi, B.; Al Qurashi, Y.M.A.; Ben Salah-Abbès, J.; Chaieb, K. Review: Biotechnology of Mycotoxins Detoxification Using Microorganisms and Enzymes. Toxicon 2019, 160, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Domracheva, L.I.; Fokina, A.I.; Skugoreva, S.G.; Ashikhmina, T.Y. Two Sides of Soil Fungi of the Genus Fusarium and Their Metabolites: Danger to Biota and the Possibility of Use in Biotechnology (Review). Theor. Appl. Ecol. 2021, 1, 6–15. [Google Scholar] [CrossRef]
- Pessôa, M.G.; Paulino, B.N.; Mano, M.C.R.; Neri-Numa, I.A.; Molina, G.; Pastore, G.M. Fusarium Species-a Promising Tool Box for Industrial Biotechnology. Appl. Microbiol. Biotechnol. 2017, 101, 3493–3511. [Google Scholar] [CrossRef] [PubMed]
- Kakde, R.; Chavan, A. Deteriorative Changes in Oilseeds Due to Storage Fungi and Efficacy of Botanicals. Curr. Bot. 2011, 2, 17–22. [Google Scholar]
- Sperb, J.G.C.; Costa, T.M.; Vaz, D.A.; Valle, J.A.B.; Valle, R.d.C.S.C.; Tavares, L.B.B. Análise qualitativa da produção de lipases e biossurfactantes por fungos isolados de resíduos oleosos. Engevista 2015, 17, 385–397. [Google Scholar] [CrossRef][Green Version]
- Gao, D.; Zeng, J.; Yu, X.; Dong, T.; Chen, S. Improved Lipid Accumulation by Morphology Engineering of Oleaginous Fungus Mortierella isabellina. Biotechnol. Bioeng. 2014, 111, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Hassane, A.M.A.; Eldiehy, K.S.H.; Saha, D.; Mohamed, H.; Mosa, M.A.; Abouelela, M.E.; Abo-Dahab, N.F.; El-Shanawany, A.-R.A. Oleaginous Fungi: A Promising Source of Biofuels and Nutraceuticals with Enhanced Lipid Production Strategies. Arch. Microbiol. 2024, 206, 338. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, M.; Hu, B. Endophytic Fungal Strains of Soybean for Lipid Production. Bioenergy Res. 2014, 7, 353–361. [Google Scholar] [CrossRef]
- Tian, M.; Bai, Y.; Tian, H.; Zhao, X. The Chemical Composition and Health-Promoting Benefits of Vegetable Oils—A Review. Molecules 2023, 28, 6393. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.-S.; Chemat, F. Vegetable Oils as Alternative Solvents for Green Oleo-Extraction, Purification and Formulation of Food and Natural Products. Mol. J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 1474. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.H. Essential Oils. Z. Naturforschung C 2020, 75, 177. [Google Scholar] [CrossRef]
- Amaro, H.T.R.; Araujo, E.F.; Araujo, R.F.; Dias, L.A.D.S.; David, A.M.S.D.S.; Silva, F.W.S. Secagem e Armazenamento de Sementes de Culturas Oleaginosas. Pesqui. Agropecuária Gaúcha 2019, 25, 105–119. [Google Scholar] [CrossRef]
- Pazzoti, G.S.D.O.; Veronezi, C.M.; Luzia, D.M.M.; Jorge, N. Caracterização de Sementes e de Óleos de Chia, Gergelim e Linhaça Extraídos Por Prensagem a Frio. Rev. Principia—Divulg. Científica E Tecnológica IFPB 2022, 59, 1187. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.-D.; Rose, R.J. Oil Body Biogenesis and Biotechnology in Legume Seeds. Plant Cell Rep. 2017, 36, 1519–1532. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic Acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B. Use of Palm Oil for Frying in Comparison with Other High-stability Oils. Eur. J. Lipid Sci. Technol. 2007, 109, 400–409. [Google Scholar] [CrossRef]
- Urugo, M.M.; Teka, T.A.; Teshome, P.G.; Tringo, T.T. Palm Oil Processing and Controversies over Its Health Effect: Overview of Positive and Negative Consequences. J. Oleo Sci. 2021, 70, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. Sci. World J. 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose Degradation: An Overview of Fungi and Fungal Enzymes Involved in Lignocellulose Degradation. Eng. Life Sci. 2018, 18, 768. [Google Scholar] [CrossRef] [PubMed]
- Inforsato, F.J.; Porto, A.L.M. Atividade enzimática de celulases pelo método dns de fungos isolados de sementes em germinação. Rev. Bras. Energ. Renov. 2016, 5, 444–465. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic Acids: Old Metabolites, New Themes. J. Chem. Technol. Biotechnol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- El-Bakry, M.; Abraham, J.; Cerda, A.; Barrena, R.; Ponsá, S.; Gea, T.; Sánchez, A. From Wastes to High Value Added Products: Novel Aspects of SSF in the Production of Enzymes. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1999–2042. [Google Scholar] [CrossRef]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal Bioconversion of Lignocellulosic Residues; Opportunities & Perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [CrossRef]
- Lima, L.G.R.; Gonçalves, M.M.M.; Couri, S.; Melo, V.F.; Sant’Ana, G.C.F.; Costa, A.C.A.D. Lipase Production by Aspergillus Niger C by Submerged Fermentation. Braz. Arch. Biol. Technol. 2019, 62, e19180113. [Google Scholar] [CrossRef]
- Costa, T.M.; Hermann, K.L.; Garcia-Roman, M.; Valle, R.D.C.S.C.; Tavares, L.B.B. Lipase production by aspergillus niger grown in different agro-industrial wastes by solid-state fermentation. Braz. J. Chem. Eng. 2017, 34, 419–427. [Google Scholar] [CrossRef]
- Vovk, H.; Karnpakdee, K.; Ludwig, R.; Nosenko, T. Enzymatic Pretreatment of Plant Cells for Oil Extraction. Food Technol. Biotechnol. 2023, 61, 160–178. [Google Scholar] [CrossRef]
- Do-Myoung, K.; Eun, J.C.; Ji, W.K.; Yong-Woog, L.; Hwa-Jee, C. Production of Cellulases by Penicillium sp. in a Solid-State Fermentation of Oil Palm Empty Fruit Bunch. Afr. J. Biotechnol. 2014, 13, 145–155. [Google Scholar] [CrossRef]
- Chepchak, T.P.; Kurchenko, I.; Iur’eva, E.M. Biodegradation of Agricultural Plant Residues by Fusarium oxysporum Strains. Mikrobiol. Zh. 2014, 76, 41–46. [Google Scholar]
- Hou, R.; Hu, J.; Wang, Y.; Wei, H.; Gao, M.-T. Simultaneous Production of Cellulase and Ferulic Acid Esterase by Penicillium Decumbens with Rice Straw as the Sole Carbon Source. J. Biosci. Bioeng. 2020, 129, 276–283. [Google Scholar] [CrossRef]
- De Souza, N.M.; Bellini, J.R.; Sousa, D.D.A.; Brod, F.C.A.; Mariotto, S.; Da Silva, R.O. Eficiência da hidrólise ácida e explosão a vapor na liberação de pentoses e hexoses de resíduos agroindustriais. Rev. Contemp. 2023, 3, 12078–12095. [Google Scholar] [CrossRef]
- Noyola, T.P.; Avalos, O.P. Celulasas y Xilanasas En La Industria. Av. Y Perspect. 2002, 21, 273–277. [Google Scholar]
- Coffman, A.M.; Li, Q.; Ju, L.-K. Effect of Natural and Pretreated Soybean Hulls on Enzyme Production by Trichoderma reesei. J. Am. Oil Chem. Soc. 2014, 91, 1331–1338. [Google Scholar] [CrossRef]
- Park, H.-S.; Jun, S.-C.; Han, K.-H.; Hong, S.-B.; Yu, J.-H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 100, pp. 161–202. [Google Scholar] [CrossRef]
- Santi, L.; Berger, M.; Silva, W.D. Pectinases e pectina: Aplicação comercial e potencial biotecnológico. Cad. Pedagógico 2014, 11, 130–139. [Google Scholar]
- Dhillon, S.S.; Gill, R.K.; Gill, S.S.; Singh, M. Studies on the Utilization of Citrus Peel for Pectinase Production Using Fungus Aspergillus niger. Int. J. Environ. Stud. 2004, 61, 199–210. [Google Scholar] [CrossRef]
- Sharma, P.; Rath, S.K. Potential Applications of Fungi in the Remediation of Toxic Effluents from Pulp and Paper Industries. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 193–211. [Google Scholar] [CrossRef]
- Shankar, A.; Saini, S.; Sharma, K.K. Fungal-Integrated Second-Generation Lignocellulosic Biorefinery: Utilization of Agricultural Biomass for Co-Production of Lignocellulolytic Enzymes, Mushroom, Fungal Polysaccharides, and Bioethanol. Biomass Convers. Biorefinery 2024, 14, 1117–1131. [Google Scholar] [CrossRef]
- Fernandes, A. Avaliação do Potencial Enzimático de Fungos Filamentosos Isolados de Diferentes Fontes. Master’s Dissertation, Universidade Federal de Lavras (UFLA), Lavras, Brazil, 2014. [Google Scholar]
- Jakovljevic, V.; Stojanovic, J.; Vrvic, M. The Potential Application of Fungus Trichoderma Harzianum Rifai in Biodegradation of Detergent and Industry. Chem. Ind. Chem. Eng. Q. 2015, 21, 131–139. [Google Scholar] [CrossRef]
- Wang, J.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keutgen, A.J.; Wolf, G.A. Impact of the Fungal Protease Produced by Fusarium Culmorum on the Protein Quality and Breadmaking Properties of Winter Wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.B.; Shivakumar, S.B.; Devappa, S. Fermentação Em Estado Sólido de Torta de Semente de Jatropha Para Otimização de Lipase, Protease e Desintoxicação de Antinutrientes Em Torta de Semente de Jatropha Usando Aspergillus versicolor CJS-98. J. Biosci. Bioeng. 2014, 117, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Athoillah, A.Z.; Ahmad, F.B. Biodiesel Production from Bioremediation of Palm Oil Mill Effluent via Oleaginous Fungi. CLEAN—Soil Air Water 2022, 50, 2200025. [Google Scholar] [CrossRef]
- Hasni, M.H.; Ahmad, F.B.; Athoillah, A.Z. The Production of Microbial Biodiesel from Cellulose-Derived Fungal Lipid via Consolidated Bioprocessing. Environ. Technol. Innov. 2023, 30, 103123. [Google Scholar] [CrossRef]
- Riyadi, F.A.; Alam, M.Z.; Salleh, M.N.; Salleh, H.M. Optimization of Thermostable Organic Solvent-Tolerant Lipase Production by Thermotolerant Rhizopus Sp. Using Solid-State Fermentation of Palm Kernel Cake. 3 Biotech 2017, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Ayinla, Z.A.; Ademakinwa, A.N.; Agboola, F.K. Studies on the Optimization of Lipase Production by Rhizopus sp. ZAC3 Isolated from the Contaminated Soil of a Palm Oil Processing Shed. J. Appl. Biol. Biotechnol. 2017, 5, 30–37. [Google Scholar] [CrossRef]
- Daba, G.M.; Mostafa, F.A.; Elkhateeb, W.A. The Ancient Koji Mold (Aspergillus oryzae) as a Modern Biotechnological Tool. Bioresour. Bioprocess. 2021, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.R.; Mendes, T.P.S.; Miranda, A.C.D.A.; Costa, D.N.; Figueroa, G.M.; Soares, V.D.M.; Valasques Junior, G.L.; Cedro, P.É.P. Produção e Caracterização Da Amilase Obtida de Rhizopus Microsporus Var. Oligosporus. Res. Soc. Dev. 2020, 9, e694974810. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, V.K.; Fitter, J.; Polen, T.; Kayastha, A.M. α-Amilase de Sementes de Soja Em Germinação (Glycine Max)—Purificação, Caracterização e Similaridade Sequencial de Resíduos de Aminoácidos Conservados e Catalíticos. Phytochemistry 2010, 71, 1657–1666. [Google Scholar] [CrossRef]
- Okunwaye, T.; Uadia, P.O.; Okogbenin, B.O.; Okogbenin, E.A.; Onyia, D.C.; Obibuzor, J.U. Amylase-Producing Fungi and Bacteria Associated with Some Food Processing Wastes. Niger. J. Biotechnol. 2021, 38, 74–82. [Google Scholar] [CrossRef]
- Ben Salah, R.; Ghamghui, H.; Miled, N.; Mejdoub, H.; Gargouri, Y. Production of Butyl Acetate Ester by Lipase from Novel Strain of Rhizopus oryzae. J. Biosci. Bioeng. 2007, 103, 368–372. [Google Scholar] [CrossRef]
- Cong, S.; Tian, K.; Zhang, X.; Lu, F.; Singh, S.; Prior, B.; Wang, Z.-X. Synthesis of Flavor Esters by a Novel Lipase from Aspergillus Niger in a Soybean-Solvent System. 3 Biotech 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium Chrysogenum, a Vintage Model with a Cutting-Edge Profile in Biotechnology. Microorganisms 2022, 10, 573. [Google Scholar] [CrossRef] [PubMed]
- López-Fernández, J.; Benaiges, M.D.; Valero, F. Rhizopus oryzae Lipase, a Promising Industrial Enzyme: Biochemical Characteristics, Production and Biocatalytic Applications. Catalysts 2020, 10, 1277. [Google Scholar] [CrossRef]
- Najjar, A.; Abdullah, N.; Saad, W.Z.; Ahmad, S.; Oskoueian, E.; Gherbawy, Y. Removal of Phorbol Esters Present in Jatropha Curcas Kernel by Fungal Isolates. Int. J. Agric. Biol. 2014, 16, 871–878. [Google Scholar]
- Nardi, M.; Lira-Guedes, A.C.; Albuquerque Cunha, H.F.; Guedes, M.C.; Mustin, K.; Gomes, S.C.P. Artisanal Extraction and Traditional Knowledge Associated with Medicinal Use of Crabwood Oil (Carapa guianensis Aublet.) in a Peri-Urban Várzea Environment in the Amazon Estuary. Evid. Based Complement. Altern. Med. 2016, 2016, 5828021. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, J.A.; Ferreira, N.R.; Da Silva, S.H.M.; Oliveira, G.; Monteiro, R.C.; Alves, Y.F.M.; Lopes, A.S. Filamentous Fungi Diversity in the Natural Fermentation of Amazonian Cocoa Beans and the Microbial Enzyme Activities. Ann. Microbiol. 2019, 69, 975–987. [Google Scholar] [CrossRef]
- Lira, G.B.; Lopes, A.S.D.C.; Nascimento, F.C.D.A.; Conceição, G.D.S.; Brasil, D.D.S.B. Processos de Extração e Usos Industriais de Óleos de Andiroba e Açaí: Uma Revisão. Res. Soc. Dev. 2021, 10, e229101220227. [Google Scholar] [CrossRef]
- Brito, A.D.; Coelho, R.d.F.R.; Rosal, L.F. Os extrativistas de andiroba em projetos de assentamentos agroextrativistas (paex) da várzea de igarapé-miri, pará, Brasil. Rev. Agroecossistemas 2020, 11, 82. [Google Scholar] [CrossRef]
- Altieri, M.A. Linking Ecologists and Traditional Farmers in the Search for Sustainable Agriculture. Front. Ecol. Environ. 2004, 2, 35–42. [Google Scholar] [CrossRef]
- Archer, D.B.; Peberdy, J.F. The Molecular Biology of Secreted Enzyme Production by Fungi. Crit. Rev. Biotechnol. 1997, 17, 273–306. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.A.; Alnoch, R.C.; Silva Dias, G.; Santos Reis, N.D.; Tavares, I.M.d.C.; Ruiz, H.A.; Bilal, M.; de Oliveira, J.R.; Krieger, N.; Franco, M. Production of a Fermented Solid Containing Lipases from Penicillium roqueforti ATCC 10110 and Its Direct Employment in Organic Medium in Ethyl Oleate Synthesis. Biotechnol. Appl. Biochem. 2022, 69, 1284–1299. [Google Scholar] [CrossRef]
- Souza Dos Santos, P.; Santos Solidade, L.; Gomes Barreto Souza, J.; Sampaio, G.; Ricardo Braga, A.C., Jr.; Do Val De Assis, F.G.; Lopes Leal, P. Fermentação em estado sólido em resíduos agroindustriais para a produção de enzimas: Uma revisão sistemática. J. Eng. Exact Sci. 2018, 4, 0181–0188. [Google Scholar] [CrossRef]
- Wösten, H.A.B. Filamentous Fungi for the Production of Enzymes, Chemicals and Materials. Curr. Opin. Biotechnol. 2019, 59, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; El-Ghonemy, D.H. Optimization of Culture Conditions for the Highest Lipid Production from Some Oleaginous Fungi for Biodiesel Preparation. Asian J. Appl. Sci. 2014, 2, 600–609. [Google Scholar]
- Lau, E.V.; Gan, S.; Ng, H.K. Extraction of Phenanthrene and Fluoranthene from Contaminated Sand Using Palm Kernel and Soybean Oils. J. Environ. Manag. 2012, 107, 124–130. [Google Scholar] [CrossRef]
- Moscato, G.; Bonavita, S.; Regina, T.M.R. Assessing Olive Oil Quality Using Different DNA-Based Methods. Plants 2024, 13, 3220. [Google Scholar] [CrossRef] [PubMed]
- Gutarowska, B.; Skóra, J.; Stępień, L.; Twarużek, M.; Błajet-Kosicka, A.; Otlewska, A.; Grajewski, J. Estimation of Fungal Contamination and Mycotoxin Production at Workplaces in Composting Plants, Tanneries, Archives and Libraries. World Mycotoxin J. 2014, 7, 345–356. [Google Scholar] [CrossRef]
- Cornelison, C.T.; Stubblefield, B.; Gilbert, E.; Crow, S.A. Recurrent Aspergillus Contamination in a Biomedical Research Facility: A Case Study. J. Ind. Microbiol. Biotechnol. 2012, 39, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.G.; Westrick, N.M.; Baldwin, T.T. Fungal Biotechnology: From Yesterday to Tomorrow. Front. Fungal Biol. 2023, 4, 1135263. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Huang, H.; Ramarao, B.V. (Eds.) Separation and Purification Technologies in Biorefineries, 1st ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef]
- Nahle, S.; El Khoury, A.; Savvaidis, I.; Chokr, A.; Louka, N.; Atoui, A. Detoxification Approaches of Mycotoxins: By Microorganisms, Biofilms and Enzymes. Int. J. Food Contam. 2022, 9, 3. [Google Scholar] [CrossRef]
- Bhanja, A.; Minde, G.; Magdum, S.; Kalyanraman, V. Comparative Studies of Oleaginous Fungal Strains (Mucor circinelloides and Trichoderma reesei) for Effective Wastewater Treatment and Bio-Oil Production. Biotechnol. Res. Int. 2014, 2014, 479370. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Agger, J.W.; Meyer, A.S. Fungal Biotechnology: Unlocking the Full Potential of Fungi for a More Sustainable World. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Grand Challenges in Biology and Biotechnology; Springer International Publishing: Cham, Switzerland, 2020; pp. 3–32. [Google Scholar] [CrossRef]
- Wakai, S.; Arazoe, T.; Ogino, C.; Kondo, A. Future Insights in Fungal Metabolic Engineering. Bioresour. Technol. 2017, 245, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Kun, R.S.; Gomes, A.C.S.; Hildén, K.S.; Salazar Cerezo, S.; Mäkelä, M.R.; De Vries, R.P. Developments and Opportunities in Fungal Strain Engineering for the Production of Novel Enzymes and Enzyme Cocktails for Plant Biomass Degradation. Biotechnol. Adv. 2019, 37, 107361. [Google Scholar] [CrossRef] [PubMed]
- Zorn, S.M.F.E.; Reis, C.E.R.; Silva, M.B.; Hu, B.; De Castro, H.F. Consortium Growth of Filamentous Fungi and Microalgae: Evaluation of Different Cultivation Strategies to Optimize Cell Harvesting and Lipid Accumulation. Energies 2020, 13, 3648. [Google Scholar] [CrossRef]
- El Amrani, A.; Dumas, A.-S.; Wick, L.Y.; Yergeau, E.; Berthomé, R. “Omics” Insights into PAH Degradation toward Improved Green Remediation Biotechnologies. Environ. Sci. Technol. 2015, 49, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ‘Omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Muggia, L.; Ametrano, C.G.; Sterflinger, K.; Tesei, D. An Overview of Genomics, Phylogenomics and Proteomics Approaches in Ascomycota. Life 2020, 10, 356. [Google Scholar] [CrossRef]
- Wikandari, R.; Hasniah, N.; Taherzadeh, M.J. The Role of Filamentous Fungi in Advancing the Development of a Sustainable Circular Bioeconomy. Bioresour. Technol. 2022, 345, 126531. [Google Scholar] [CrossRef] [PubMed]
| Enzyme | Function | Target Component | Fungi | Biotechnologycal Aplication | Oil-Bearing Seeds Treated | Reference |
|---|---|---|---|---|---|---|
| Cellulase | Degrades cellulose into glucose by breaking β-1,4-glycosidic bonds | Cellulose | Trichoderma reesei, Aspergillus niger, Penicillium oxalicum, Penicillium sp. | Production of biofuels, bioconversion of lignocellulosic biomass, textile industry for fabric processing | Soybean, rice, sunflower seeds | [70,74,75,76,77,78,79,80] |
| Hemicellulase | Hydrolyzes hemicellulose, releasing xylose and other simple sugars | Hemicellulose | Aspergillus niger, Penicillium chrysogenum, Trichoderma reesei | Bioconversion of plant biomass, biofuel production, paper industry for pulp processing | Soybean | [74,81,82,83] |
| Pectinase | Breaks down pectin into galacturonic acids, facilitating cell wall breakdown | Pectin | Aspergillus oryzae, Aspergillus niger, Penicillium notatum, Rhizopus oryzae | Food industry for fruit juice clarification, wine production, textile industry for plant fiber processing | Citrus seeds | [37,84,85,86] |
| Ligninase | Oxidizes lignin, facilitating the decomposition of the lignocellulosic matrix | Lignin | Aspergillus fumigatus | Pulp and paper industry, bioremediation of contaminated sites, biofuel production | Cotton seeds, sunflower seeds | [47,79,87,88] |
| Protease | Breaks down proteins into peptides and amino acids | Structural Proteins | Aspergillus oryzae, Rhizopus oryzae, Trichoderma harzianum, Fusarium calmorum | Detergent industry, leather processing, food industry, waste management | Wheat seed, jatropha seed | [37,89,90,91,92] |
| Lipase | Catalyzes the hydrolysis of triacylglycerols into free fatty acids and glycerol | Stored lipids (lipid droplets) | Rhizopus oryzae, Aspergillus niger | Oil extraction processes, biodiesel production, food industry, pharmaceutical industry | Soybean, palm seed | [54,93,94,95,96] |
| Amylase | Degrades starch into simple sugars like maltose and glucose | Starch | Aspergillus oryzae, Aspergillus niger, Rhizopus oryzae | Food industry for brewing, baking, and high-fructose corn syrup production, textile industry for desizing | Soybean, shea seed | [84,97,98,99,100] |
| Esterase | Breaks down plant esters, contributing to the release of fatty acids | Lipid and polysaccharide esters | Penicillium chrysogenum, Aspergillus niger, Rhizopus oryzae | Synthesis of esters for flavor and fragrance industry, bioremediation, pharmaceutical industry | Jatropha seed | [54,101,102,103,104,105] |
| Fungal Strain | Oily Seed Substrate | Enzyme Activity | Application | Reference |
|---|---|---|---|---|
| Aspergillus sp. | Soybean | Lipase, cellulase | Enhancing oil extraction efficiency through cell wall breakdown | [75,76,77] |
| Rhizopus oryzae | Palm seed | Lipase | Improving lipid release during fermentation | [95,96] |
| Trichoderma reesei | Soybean | Cellulase, hemicellulase | Breakdown of lignocellulosic barriers to release oil | [83] |
| Penicillium sp. | Rice | Pectinase, cellulase | Optimizing oil release with pectin and celluase degradation | [78] |
| Fusarium oxysporum | Sunflower | Cellulase, ligninase | Biodegradation of lignin to improve oil recovery | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, M.B.; Corrêa Junior, D.; Frases, S. Fungal Biotechnology Applications in Sustainable Oil Extraction. Appl. Microbiol. 2025, 5, 8. https://doi.org/10.3390/applmicrobiol5010008
Barbieri MB, Corrêa Junior D, Frases S. Fungal Biotechnology Applications in Sustainable Oil Extraction. Applied Microbiology. 2025; 5(1):8. https://doi.org/10.3390/applmicrobiol5010008
Chicago/Turabian StyleBarbieri, Mariana B., Dario Corrêa Junior, and Susana Frases. 2025. "Fungal Biotechnology Applications in Sustainable Oil Extraction" Applied Microbiology 5, no. 1: 8. https://doi.org/10.3390/applmicrobiol5010008
APA StyleBarbieri, M. B., Corrêa Junior, D., & Frases, S. (2025). Fungal Biotechnology Applications in Sustainable Oil Extraction. Applied Microbiology, 5(1), 8. https://doi.org/10.3390/applmicrobiol5010008







