Bacillus velezensis SM1: A Promising Biocontrol Solution for Phytophthora Durian Root Rot
Abstract
1. Introduction
2. Materials and Methods
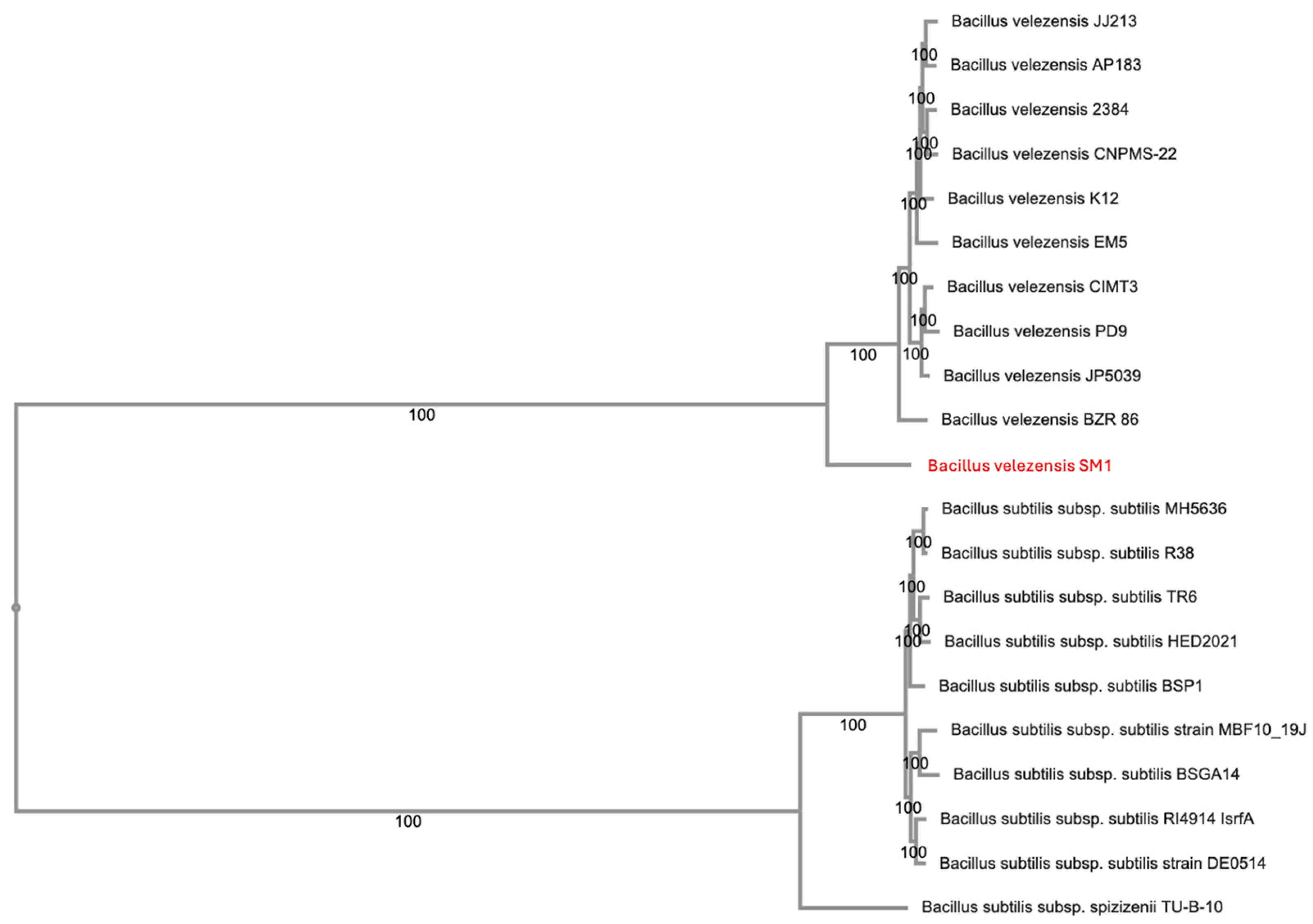
2.1. Isolation and Identification of a Bacterial Biocontrol Agent
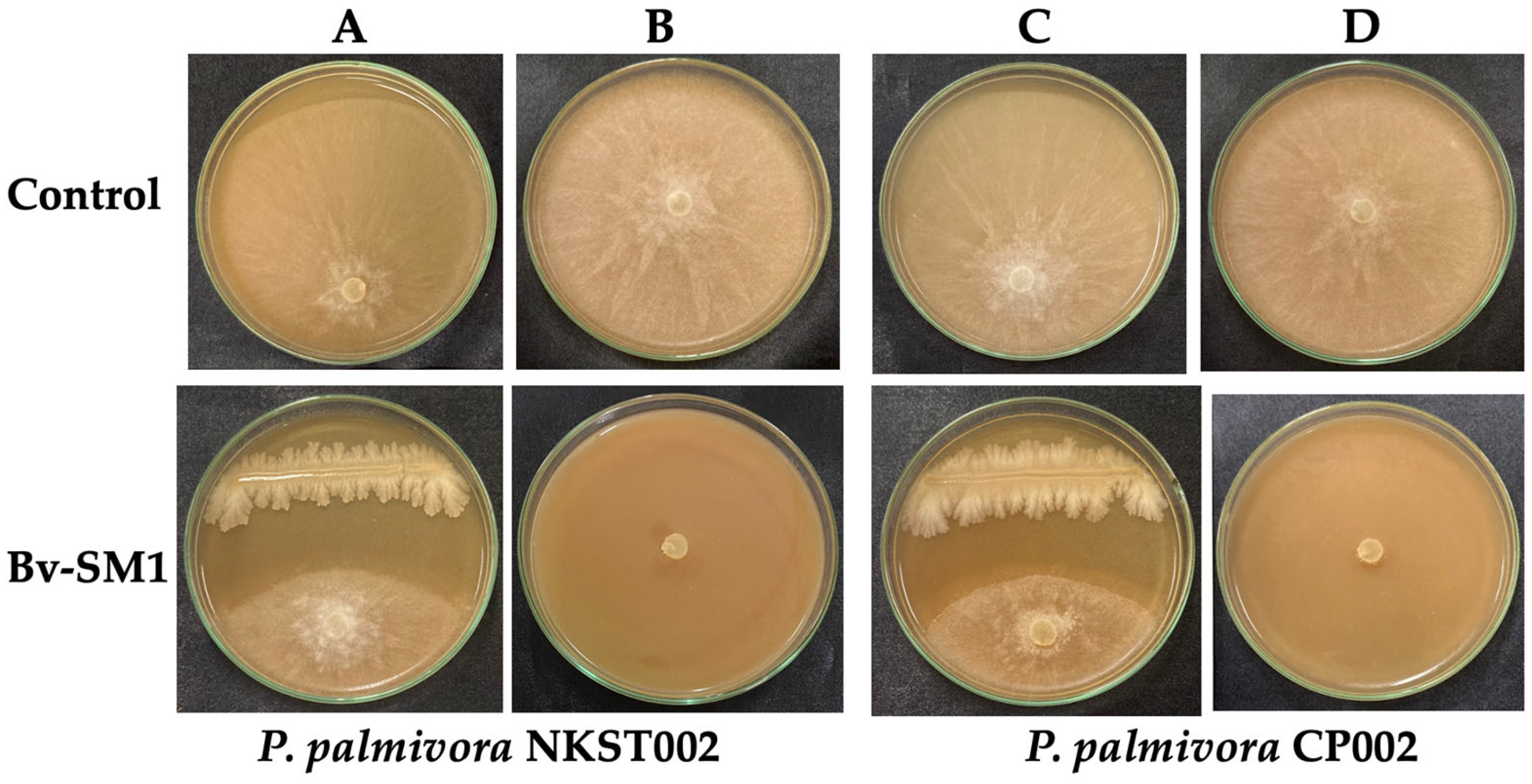
2.2. In Vitro Screening of Antifungal Activity Against P. palmivora Isolates NKST002 and CP002
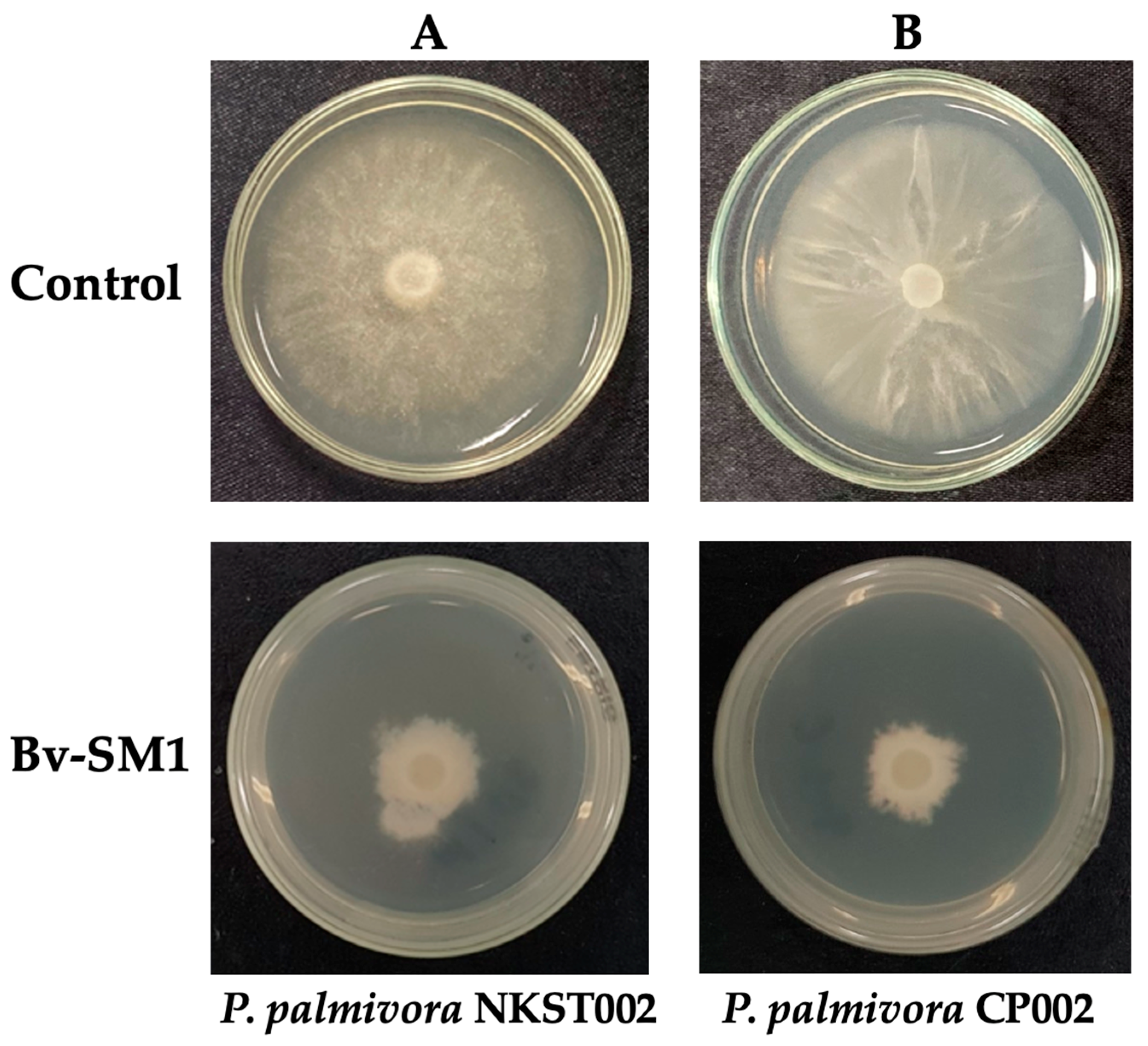
2.3. Antifungal Activity of Volatile Organic Compounds from Bv-SM1 on P. palmivora Isolates NKST002 and CP002
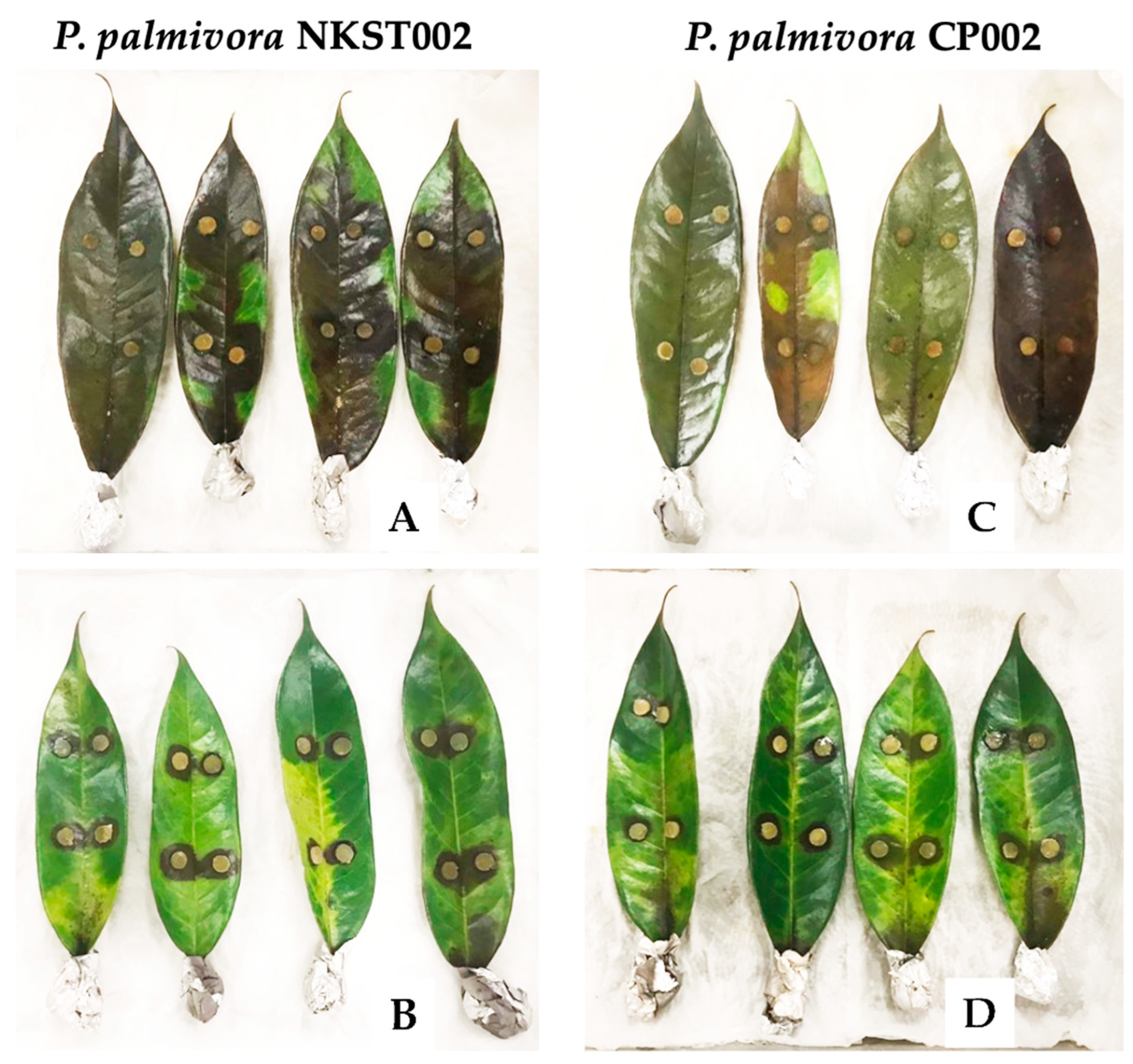
2.4. Assessing the Efficacy of Bv-SM1 in Controlling Phytophthora
2.5. Genome Sequencing and Annotation
3. Results
3.1. Bacterial Isolation and Identification
3.2. In Vitro Antagonistic Assays
3.3. Antifungal Activity of Volatile Organic Compounds
3.4. Efficacy of Bv-SM1 in Controlling Phytophthora on Durian Leaves
3.5. General Genomic Feature
3.6. Genes Associated with Antagonistic Features
3.6.1. Hydrolytic Enzymes
3.6.2. Siderophores
3.6.3. Secondary Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bacillus velezensis strain SM1 BGCs | Bv-SM1 Biosynthetic gene clusters |
| Phytophthora palmivora Phytophthora palmivora isolate NKST002 | P. palmivora NKST002 |
| Phytophthora palmivora isolate CP002 | CP002 |
References
- Subhadrabandhu, S.; Ketsa, S. Durian: King of Tropical Fruit; CABI publishing: New York, NY, USA, 2021. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Durian Global Trade Overview 2023; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Sichai, K.; Nianwichai, P.; Taraput, N.; Tongsri, V.; Songkumarn, P. Phytophthora palmivora RPA1, a Homolog of Phytophthora infestans RPA190, is Irrelevant to Metalaxyl Resistance in Phytophthora palmivora Causing Root and Stem Rot of Durian in Thailand. Korean J. Mycol. 2024, 52, 73–96. [Google Scholar]
- Somnuek, S.; Kongtragoul, P.; Jaenaksorn, T. Fungicide resistance of Phytophthora palmivora causing durian diseases in eastern and southern Thailand and the in vitro alternative control by cajeput leaf extracts. Int. J. Agric. Technol. 2023, 19, 703–720. [Google Scholar]
- Pongpisutta, R.; Sangchote, S. Morphological and host range variability. In Diversity and Management of Phytophthora in Southeast Asia; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 2004; pp. 53–58. [Google Scholar]
- Kongtragoul, P.; Ishikawa, K.; Ishii, H. Metalaxyl resistance of Phytophthora palmivora causing durian diseases in Thailand. Horticulturae 2021, 7, 375. [Google Scholar] [CrossRef]
- Drenth, A.; Guest, D.I. Diversity and Management of Phytophthora in Southeast Asia; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 2004; pp. 7–93. [Google Scholar]
- Nguyen, M.C.; Huynh, V.T.; Diczbalis, Y.; Guest, D.I. Durian propagation and nursery practice. In Diversity and Management of Phytophthora in Southeast Asia; Australian Centre for International Agricultural Research (ACIAR): Canberra, ACT, Australia, 2004; pp. 200–205. [Google Scholar]
- Chaube, H.S.; Mishra, D.S.; Varshney, S.; Singh, U.S. Biocontrol of plant pathogens by fungal antagonists: Historical background, present status and future prospects. Ann. Rev. Plant. Pathol. 2004, 2, 1–42. [Google Scholar]
- Khabbaz, S.E.; Zhang, L.; Cáceres, L.A.; Sumarah, M.; Wang, A.; Abbasi, P.A. Characterisation of antagonistic Bacillus and Pseudomonas strains for biocontrol potential and suppression of damping-off and root rot diseases. Ann. Appl. Biol. 2015, 166, 456–471. [Google Scholar] [CrossRef]
- Yu, X.; Ai, C.; Xin, L.; Zhou, G. The siderophore-producing bacterium, Bacillus subtilis CAS15, has a biocontrol effect on Fusarium wilt and promotes the growth of pepper. Eur. J. Soil Biol. 2011, 47, 138–145. [Google Scholar] [CrossRef]
- Phae, C.G.; Shoda, M.; Kita, N.; Nakano, M.; Ushiyama, K. Biological control of crown and root rot and bacterial wilt of tomato by Bacillus subtilis NB22. Jpn. J. Phytopathol. 1992, 58, 329–339. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.; Chen, Y.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest. Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiradate, S.; Tsukamoto, T.; Hatakeda, K.; Shirata, A. Antimicrobial Activity of Culture Filtrate of Bacillus amyloliquefaciens RC-2 Isolated from Mulberry Leaves. Phytopathology 2001, 91, 181–187. [Google Scholar] [CrossRef]
- Yuan, J.; Raza, W.; Shen, Q.; Huang, Q. Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Appl. Environ. Microbiol. 2012, 78, 5942–5944. [Google Scholar] [CrossRef]
- Salvatierra-Martinez, R.; Arancibia, W.; Araya, M.; Aguilera, S.; Olalde, V.; Bravo, J.; Stoll, A. Colonization ability as an indicator of enhanced biocontrol capacity—An example using two Bacillus amyloliquefaciens strains and Botrytis cinerea infection of tomatoes. J. Phytopathol. 2018, 166, 601–612. [Google Scholar] [CrossRef]
- Jangir, M.; Pathak, R.; Sharma, S.; Sharma, S. Biocontrol mechanisms of Bacillus sp., isolated from tomato rhizosphere, against Fusarium oxysporum f. sp. lycopersici. Biol. Control 2018, 123, 60–70. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, M.; Dang, S.; Shi, M.; Zhang, J.; Li, Y. Biocontrol potential of Bacillus amyloliquefaciens LYZ69 against anthracnose of alfalfa (Medicago sativa L.). Phytopathology 2021, 111, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, G.; Feliziani, E. Botrytis cinerea (gray mold). In Postharvest Decay; Academic Press: New York, NY, USA, 2014; pp. 131–146. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Corrigendum: Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2019, 10, 1279. [Google Scholar] [CrossRef]
- Stoica, R.; Moscovici, M.; Tomulescu, C.; Cășărică, A.; Băbeanu, N.; Popa, O.; Kahraman, H.A. Antimicrobial compounds of the genus Bacillus: A review. Rom. Biotechnol. Lett. 2019, 24, 1111–1119. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Dimkić, I.; Janakiev, T.; Petrović, M.; Degrassi, G.; Fira, D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—A review. Physiol. Mol. Plant Pathol. 2022, 117, 101754. [Google Scholar] [CrossRef]
- Wan, T.; Zhao, H.; Wang, W. Effect of biocontrol agent Bacillus amyloliquefaciens SN16-1 and plant pathogen Fusarium oxysporum on tomato rhizosphere bacterial community composition. Biol. Control 2017, 112, 1–9. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Wang, D.; Wang, F.; Shen, H.; Sun, G.; Feng, C.; Wang, X.; Chen, D.; Sun, X. Biocontrol efficacy of Bacillus siamensis LZ88 against brown spot disease of tobacco caused by Alternaria alternata. Biol. Control 2021, 154, 104508. [Google Scholar] [CrossRef]
- Saleh, H.A.; Kabary, H.A.; Zayed, K.M. Assessment of Bacillus aerius and Bacillus toyonensis extracts as biocontrol agents against Biomphalaria alexandrina snails. Aquacult. Res. 2022, 53, 5382–5397. [Google Scholar] [CrossRef]
- Kanjanamaneesathian, M.; Kusonwiriyawong, C.; Pengnoo, A.; Nilratana, L. Screening of potential bacterial antagonists for control of sheath blight in rice and development of suitable bacterial formulations for effective application. Australas. Plant Pathol. 1998, 27, 198–206. [Google Scholar] [CrossRef]
- Pengnoo, A.; Wiwattanapattapee, R.; Chumthong, A.; Kanjanamaneesathian, M. Bacterial antagonist as seed treatment to control leaf blight disease of bambara groundnut (Vigna subterranea). World J. Microbiol. Biotechnol. 2006, 22, 9–14. [Google Scholar] [CrossRef]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, A.M.; Dickinson, C.H. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]
- Dennis, C.; Webster, J. Antagonistic properties of species-groups of Trichoderma. II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971, 57, 41–48. [Google Scholar] [CrossRef]
- Vawdrey, L.L.; Martin, T.M.; De Faveri, J. A detached leaf bioassay to screen Durian cultivars for susceptibility to Phytophthora palmivora. Australas. Plant Pathol. 2005, 34, 251–253. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J.; Dempsey, D.M.; Dickerman, A.; Dietrich, E.M.; Kenyon, R.W. Introducing the bacterial and viral bioinformatics resource center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Jin, Z.; Zhou, W.; Wang, Y.; Wang, Y.; Li, W.; Shi, C.; Liu, Y.; Wan, Y. Antibacterial effect and cytotoxicity of beta-1,3-1, 4-glucanase from endophytic Bacillus subtilis SWB8. Wei Sheng Wu Xue Bao 2011, 51, 1527–1537. [Google Scholar]
- Müller, J.J.; Thomsen, K.K.; Heinemann, U. Crystal structure of barley 1,3-1,4-beta-glucanase at 2.0-A resolution and comparison with Bacillus 1,3-1,4-beta-glucanase. J. Biol. Chem. 1998, 273, 3438–3446. [Google Scholar] [CrossRef]
- Wang, J.; Niu, C.; Liu, X.; Chen, X.; Li, Q. Characterization of a new 1,3-1,4-β-glucanase gene from Bacillus tequilensis CGX5-1. Appl. Biochem. Biotechnol. 2014, 173, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, T.; Li, S. β-1,3-1,4-glucanase gene from Bacillus velezensis ZJ20 exerts antifungal effect on plant pathogenic fungi. World J. Microbiol. Biotechnol. 2016, 32, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zhang, Y.; Yu, Z.; Zhuang, W.; Zeng, Z. Bacillus velezensis BV01 Has Broad-Spectrum Biocontrol Potential and the Ability to Promote Plant Growth. Microorganisms 2023, 11, 2627. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ye, C.; Dong, X.; Chen, C.; Zou, D.; Huang, K.; Wei, X. Genetic identification and expression optimization of a novel protease HapR from Bacillus velezensis. Front. Bioeng. Biotechnol. 2024, 12, 1383083. [Google Scholar] [CrossRef]
- Zhang, D.; Kang, Y.; Zhan, S.; Zhao, Z.; Jin, S.; Chen, C.; Zhang, L.; Shen, J.; Wang, C.; Wang, G.; et al. Effect of Bacillus velezensis on Aeromonas veronii-Induced Intestinal Mucosal Barrier Function Damage and Inflammation in Crucian Carp (Carassius auratus). Front. Microbiol. 2019, 10, 2663. [Google Scholar] [CrossRef]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Han, D.M.; Baek, J.H.; Chun, B.H.; Jeon, C.O. Fermentative features of Bacillus velezensis and Leuconostoc mesenteroides in doenjang-meju, a Korean traditional fermented soybean brick. Food Microbiol. 2023, 110, 104186. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, C.; Zuo, Y.; Qiao, J.; Shen, J.; Yin, X.; Liu, Y. Characterization of Siderophores Produced by Bacillus velezensis YL2021 and Its Application in Controlling Rice Sheath Blight and Rice Blast. Phytopathology 2024, 114, 2491–2501. [Google Scholar] [CrossRef]
- Nalli, Y.; Singh, S.; Gajjar, A.; Mahizhaveni, B.; Dusthackeer, V.N.A.; Shinde, P.B. Bacillibactin class siderophores produced by the endophyte Bacillus subtilis NPROOT3 as antimycobacterial agents. Lett. Appl. Microbiol. 2023, 76, ovac026. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Wei, Z.; Guan, Z.; Cai, Y.; Liao, X. Antifungal Activity of Isolated Bacillus amyloliquefaciens SYBC H47 for the Biocontrol of Peach Gummosis. PLoS ONE 2016, 11, e0162125. [Google Scholar] [CrossRef] [PubMed]
- Elbouazaoui, A.; Sijilmassi, B.; Maafa, I.; Allal, D.; Ahmed, S. Biocontrol activity of Bacillus, Paenibacillus and Pseudomonas against Fusarium wilt of chickpea in Morocco. Acta Agric. Scandinavica.Sect. B Soil Plant Sci. 2022, 72, 847–859. [Google Scholar] [CrossRef]
- Wan, T.; Zhao, H.; Wang, W. Effects of the biocontrol agent Bacillus amyloliquefaciens SN16-1 on the rhizosphere bacterial community and growth of tomato. J. Phytopathol. 2018, 166, 324–332. [Google Scholar] [CrossRef]
- Stumbriene, K.; Gudiukaite, R.; Semaskiene, R.; Svegzda, P.; Jonaviciene, A.; Suproniene, S. Screening of new bacterial isolates with antifungal activity and application of selected Bacillus sp. cultures for biocontrol of Fusarium graminearum under field conditions. Crop Prot. 2018, 113, 22–28. [Google Scholar] [CrossRef]
- Volynchikova, E.; Kim, K.D. Biological control of oomycete soilborne diseases caused by Phytophthora capsici, Phytophthora infestans, and Phytophthora nicotianae in solanaceous crops. Mycobiology 2022, 50, 269–293. [Google Scholar] [CrossRef]
- Guo, D.; Yuan, C.; Luo, Y.; Chen, Y.; Lu, M.; Chen, G.; Ren, G.; Cui, C.; Zhang, J.; An, D. Biocontrol of tobacco black shank disease (Phytophthora nicotianae) by Bacillus velezensis Ba168. Pestic. Biochem. Physiol. 2020, 165, 104523. [Google Scholar] [CrossRef]
- Torres, M.; Llamas, I.; Torres, B.; Toral, L.; Sampedro, I.; Bejar, V. Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Pu, Y.; Zhang, B.; Wang, B.; Xing, L.; Li, Y.; Zhang, Y.; Gu, R.; Jia, F. Antagonistic Strain Bacillus velezensis JZ Mediates the Biocontrol of Bacillus altitudinis m-1, a Cause of Leaf Spot Disease in Strawberry. Int. J. Mol. Sci. 2024, 25, 8872. [Google Scholar] [CrossRef]
- Zhou, J.; Xie, Y.; Liao, Y.; Li, X.; Li, Y.; Li, S.; Ma, X.; Lei, S.; Lin, F.; Jiang, W. Characterization of a Bacillus velezensis strain isolated from Bolbostemmatis Rhizoma displaying strong antagonistic activities against a variety of rice pathogens. Front. Microbiol. 2022, 13, 983781. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, Y.; Li, Y.; Dong, J.; Liu, X.; Li, C. Biocontrol of Rhizoctonia solani via induction of the defense mechanism and antimicrobial compounds produced by Bacillus subtilis SL-44 on pepper (Capsicum annuum L.). Front. Microbiol. 2019, 10, 2676. [Google Scholar] [CrossRef]
- Petkova, M.; Dimova, M. Biological control of lettuce drop (Sclerotinia minor Jagger) using antagonistic Bacillus species. Appl Microbiol 2024, 4, 1283–1293. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Zhang, F.; Zheng, D.; Chang, Y.; Xu, L.; Huang, L. Biocontrol activity of Bacillus velezensis D4 against apple Valsa canker. Biol. Control 2021, 163, 104760. [Google Scholar] [CrossRef]
- Lin, Y.; Du, D.; Si, C.; Zhao, Q.; Li, Z.; Li, P. Potential biocontrol Bacillus sp. strains isolated by an improved method from vinegar waste compost exhibit antibiosis against fungal pathogens and promote growth of cucumbers. Biol. Control 2014, 71, 7–15. [Google Scholar] [CrossRef]
- Devi, S.; Kiesewalter, H.T.; Kovács, R.; Frisvad, J.C.; Weber, T.; Larsen, T.O.; Kovács, Á.T.; Ding, L. Depiction of secondary metabolites and antifungal activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019, 4, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Jabiri, S.; Legrifi, I.; Benhammou, M.; Laasli, S.; Mokrini, F.; Amraoui, M.B.; Lahlali, R. Screening of rhizobacterial isolates from apple rhizosphere for their biocontrol and plant growth promotion activity. Appl Microbiol 2023, 3, 948–967. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, X.; Wang, Y.; Zhu, M. Biocontrol potential of Bacillus pumilus HR10 against Sphaeropsis shoot blight disease of pine. Biol. Control 2021, 152, 104458. [Google Scholar] [CrossRef]
- Wu, L.; Shang, H.; Gu, H.; Zheng, J. Bacterial iturins mediate biocontrol activity of Bacillus sp. against postharvest pear fruit-rotting fungi. J. Phytopathol. 2019, 167, 501–509. [Google Scholar] [CrossRef]
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Zhou, H.; Luo, C.; Fang, X.; Xiang, Y.; Wang, X.; Zhang, R.; Chen, Z. Loss of GltB Inhibits Biofilm Formation and Biocontrol Efficiency of Bacillus subtilis Bs916 by Altering the Production of γ-Polyglutamate and Three Lipopeptides. PLoS ONE 2016, 11, e0156247. [Google Scholar] [CrossRef]

| Isolate | Growth of Isolate NKST002 (cm) | Growth of Isolate CP002 (cm) | ||
|---|---|---|---|---|
| Day 7 | Day 14 | Day 7 | Day 14 | |
| Bv-SM1 | 0.88 ± 0.09 | 1.06 ± 0.06 | 1.06 ± 0.06 | 1.20 ± 0.05 |
| Control | 3.01 ± 0.03 | 5.02 ± 0.07 | 3.00 ± 0.04 | 5.00 ± 0.12 |
| Isolate | Growth of Isolate NKST002 (cm) | Growth of Isolate CP002 (cm) |
|---|---|---|
| Bv-SM1 | 2.20 ± 0.20 | 2.11 ± 0.09 |
| Control | 5.00 ± 0.00 | 5.00 ± 0.00 |
| Isolate | Growth of Isolate NKST002 (cm) | Growth of Isolate CP002 (cm) | ||||
|---|---|---|---|---|---|---|
| Day 3 | Day 5 | Day 7 | Day 3 | Day 5 | Day 7 | |
| Bv-SM1 | 0.00 ± 0.00 | 0.62 ± 0.17 | 3.78 ± 0.41 | 0.03 ± 0.02 | 0.36 ± 0.18 | 2.16 ± 0.32 |
| Control | 0.09 ± 0.09 | 1.32 ± 0.16 | 5.06 ± 0.59 | 0.30 ± 0.02 | 1.37 ± 0.24 | 4.73 ± 0.29 |
| Contigs | 20 (https://www.bv-brc.org/view/Genome/2.14291#view_tab=sequences, accessed on 5 January 2025) |
| Genome Length | 3,964,444 |
| GC Content | 46.19 |
| Contig N50 | 2,988,422 |
| tRNA | 86 |
| rRNA | 27 |
| CDS | 4100 |
| Hypothetical CDS | 1129 |
| Metabolite | % Similarity with a Bacterium |
|---|---|
| fengycin | 93% Bacillus velezensis FZB42 |
| bacillaene | 100% Bacillus velezensis FZB42 |
| macrolactin H | 100% Bacillus velezensis FZB42 |
| butirosin A | 7% Bacillus circulans |
| surfactin | 82% Bacillus velezensis FZB42 |
| bacilysin | 100% Bacillus velezensis FZB42 |
| bacillibactin | 100% Bacillus subtilis subsp. subtilis str. 168 |
| type III polyketide synthase (T3PKS) | No known cluster |
| plipastatin | 30% Bacillus subtilis subsp. subtilis |
| terpene | No known cluster identified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pengnoo, A.; Lohlaeh, U.; Maduerehand, F.; Kaewmano, C.; Krualee, S.; Wongpisal, P.; Homhaul, W.; Boonyapipat, P.; Saeng-ngam, S.; Äkbärjan, A.; et al. Bacillus velezensis SM1: A Promising Biocontrol Solution for Phytophthora Durian Root Rot. Appl. Microbiol. 2025, 5, 21. https://doi.org/10.3390/applmicrobiol5010021
Pengnoo A, Lohlaeh U, Maduerehand F, Kaewmano C, Krualee S, Wongpisal P, Homhaul W, Boonyapipat P, Saeng-ngam S, Äkbärjan A, et al. Bacillus velezensis SM1: A Promising Biocontrol Solution for Phytophthora Durian Root Rot. Applied Microbiology. 2025; 5(1):21. https://doi.org/10.3390/applmicrobiol5010021
Chicago/Turabian StylePengnoo, Ashara, Usman Lohlaeh, Fadila Maduerehand, Chuthamard Kaewmano, Sudanai Krualee, Pimchana Wongpisal, Wipa Homhaul, Pawika Boonyapipat, Sukhumaporn Saeng-ngam, Abbas Äkbärjan, and et al. 2025. "Bacillus velezensis SM1: A Promising Biocontrol Solution for Phytophthora Durian Root Rot" Applied Microbiology 5, no. 1: 21. https://doi.org/10.3390/applmicrobiol5010021
APA StylePengnoo, A., Lohlaeh, U., Maduerehand, F., Kaewmano, C., Krualee, S., Wongpisal, P., Homhaul, W., Boonyapipat, P., Saeng-ngam, S., Äkbärjan, A., & Phuntumart, V. (2025). Bacillus velezensis SM1: A Promising Biocontrol Solution for Phytophthora Durian Root Rot. Applied Microbiology, 5(1), 21. https://doi.org/10.3390/applmicrobiol5010021






